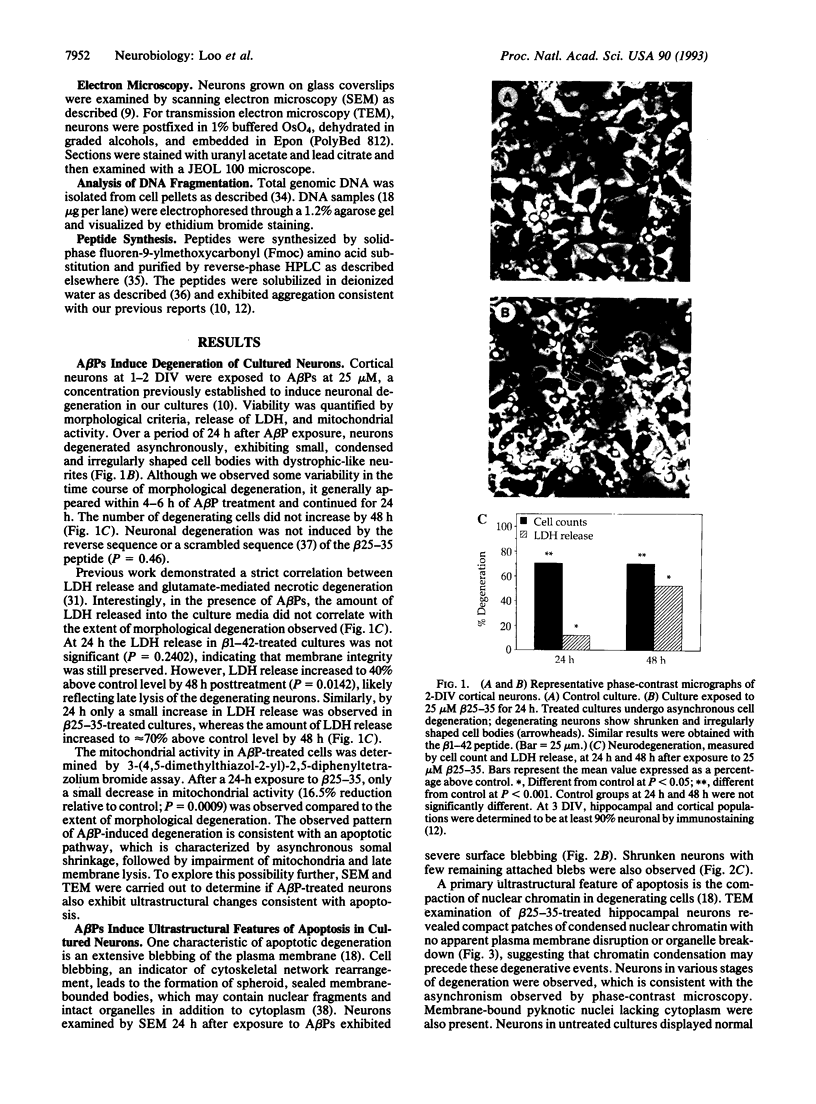
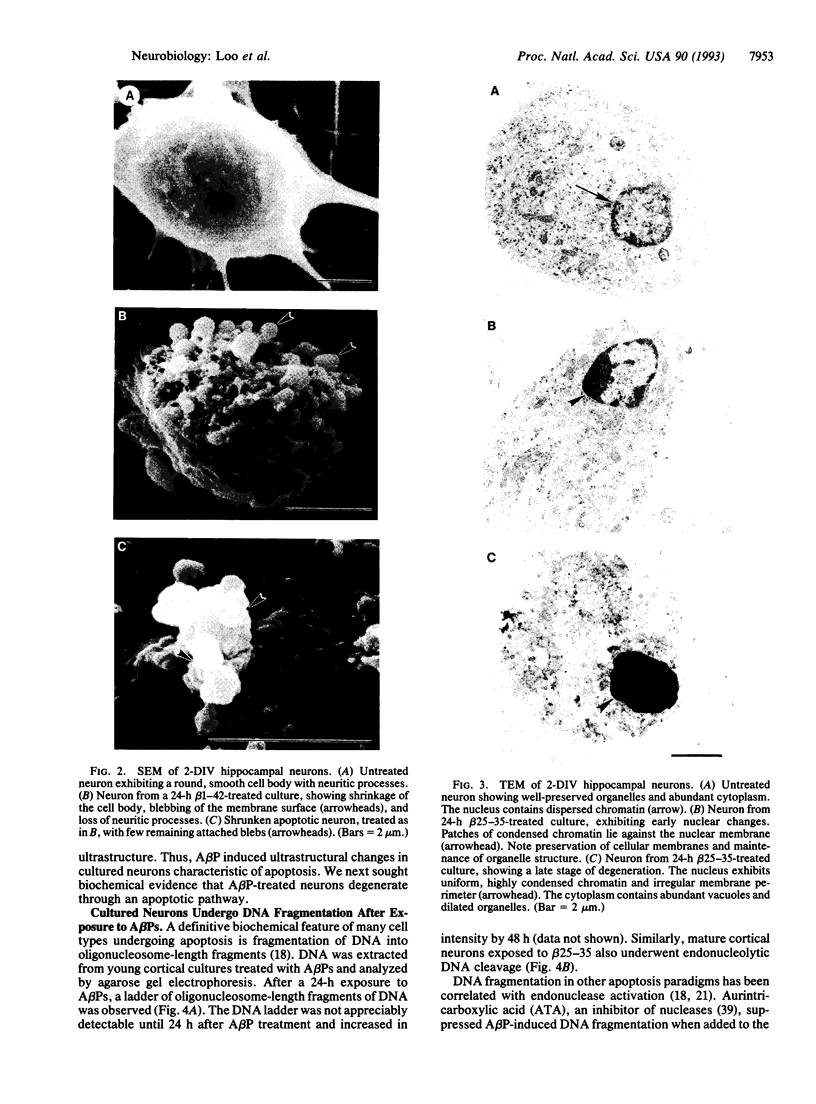
Abstract
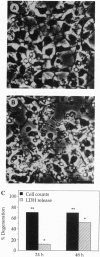
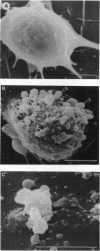
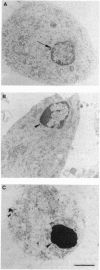
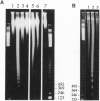
The molecular mechanism responsible for the neurodegeneration in Alzheimer disease is not known; however, accumulating evidence suggests that beta-amyloid peptide (A beta P) contributes to this degeneration. We now report that synthetic A beta Ps trigger the degeneration of cultured neurons through activation of an apoptotic pathway. Neurons treated with A beta Ps exhibit morphological and biochemical characteristics of apoptosis, including membrane blebbing, compaction of nuclear chromatin, and internucleosomal DNA fragmentation. Aurintricarboxylic acid, an inhibitor of nucleases, prevents DNA fragmentation and delays cell death. Our in vitro results suggest that apoptosis may play a role in the neuronal loss associated with Alzheimer disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Programmed cell death: the paths to suicide. Trends Neurosci. 1992 Aug;15(8):278–280. doi: 10.1016/0166-2236(92)90076-k. [DOI] [PubMed] [Google Scholar]
- Batistatou A., Greene L. A. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991 Oct;115(2):461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing W. C., Mufson E. J., Armstrong D. M. Alzheimer's disease-like dystrophic neurites characteristically associated with senile plaques are not found within other neurodegenerative diseases unless amyloid beta-protein deposition is present. Brain Res. 1993 Mar 19;606(1):10–18. doi: 10.1016/0006-8993(93)91563-8. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. J., Cotman C. W. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res. 1989 Aug 7;494(1):65–74. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Bursch W., Oberhammer F., Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci. 1992 Jun;13(6):245–251. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Lorenzo A., Yankner B. A. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol Aging. 1992 Sep-Oct;13(5):609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991 Oct 31;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Copani A., Koh J. Y., Cotman C. W. Beta-amyloid increases neuronal susceptibility to injury by glucose deprivation. Neuroreport. 1991 Dec;2(12):763–765. doi: 10.1097/00001756-199112000-00008. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Pike C. J., Copani A. beta-Amyloid neurotoxicity: a discussion of in vitro findings. Neurobiol Aging. 1992 Sep-Oct;13(5):587–590. doi: 10.1016/0197-4580(92)90060-b. [DOI] [PubMed] [Google Scholar]
- Dipasquale B., Marini A. M., Youle R. J. Apoptosis and DNA degradation induced by 1-methyl-4-phenylpyridinium in neurons. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1442–1448. doi: 10.1016/0006-291x(91)92101-o. [DOI] [PubMed] [Google Scholar]
- Edwards S. N., Buckmaster A. E., Tolkovsky A. M. The death programme in cultured sympathetic neurones can be suppressed at the posttranslational level by nerve growth factor, cyclic AMP, and depolarization. J Neurochem. 1991 Dec;57(6):2140–2143. doi: 10.1111/j.1471-4159.1991.tb06434.x. [DOI] [PubMed] [Google Scholar]
- Frautschy S. A., Baird A., Cole G. M. Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8362–8366. doi: 10.1073/pnas.88.19.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaido M. L., Cidlowski J. A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J Biol Chem. 1991 Oct 5;266(28):18580–18585. [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Ignatowicz E., Vezzani A. M., Rizzi M., D'Incalci M. Nerve cell death induced in vivo by kainic acid and quinolinic acid does not involve apoptosis. Neuroreport. 1991 Nov;2(11):651–654. doi: 10.1097/00001756-199111000-00004. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987 May;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Cotman C. W. Programmed cell death: its possible contribution to neurotoxicity mediated by calcium channel antagonists. Brain Res. 1992 Aug 7;587(2):233–240. doi: 10.1016/0006-8993(92)91002-v. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Koike T., Martin D. P., Johnson E. M., Jr Role of Ca2+ channels in the ability of membrane depolarization to prevent neuronal death induced by trophic-factor deprivation: evidence that levels of internal Ca2+ determine nerve growth factor dependence of sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6421–6425. doi: 10.1073/pnas.86.16.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F., Busciglio J., Duffy L. K., Yankner B. A. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., McKee A. C., Yankner B. A., Beal M. F. In vivo neurotoxicity of beta-amyloid [beta(1-40)] and the beta(25-35) fragment. Neurobiol Aging. 1992 Sep-Oct;13(5):537–542. doi: 10.1016/0197-4580(92)90053-z. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters J. N., Finch C. E., Sapolsky R. M. Glucocorticoid endangerment of hippocampal neurons does not involve deoxyribonucleic acid cleavage. Endocrinology. 1989 Jun;124(6):3083–3088. doi: 10.1210/endo-124-6-3083. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Murrell J., Farlow M., Ghetti B., Benson M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Perry G., Cras P., Siedlak S. L., Tabaton M., Kawai M. Beta protein immunoreactivity is found in the majority of neurofibrillary tangles of Alzheimer's disease. Am J Pathol. 1992 Feb;140(2):283–290. [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Cummings B. J., Cotman C. W. beta-Amyloid induces neuritic dystrophy in vitro: similarities with Alzheimer pathology. Neuroreport. 1992 Sep;3(9):769–772. doi: 10.1097/00001756-199209000-00012. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991 Nov 1;563(1-2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rawson C. L., Loo D. T., Duimstra J. R., Hedstrom O. R., Schmidt E. E., Barnes D. W. Death of serum-free mouse embryo cells caused by epidermal growth factor deprivation. J Cell Biol. 1991 May;113(3):671–680. doi: 10.1083/jcb.113.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush D. K., Aschmies S., Merriman M. C. Intracerebral beta-amyloid(25-35) produces tissue damage: is it neurotoxic? Neurobiol Aging. 1992 Sep-Oct;13(5):591–594. doi: 10.1016/0197-4580(92)90061-2. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Waite J., Cole G. M., Frautschy S. A., Connor D. J., Thal L. J. Solvent effects on beta protein toxicity in vivo. Neurobiol Aging. 1992 Sep-Oct;13(5):595–599. doi: 10.1016/0197-4580(92)90062-3. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]