Abstract
Triclabendazole is reported to be highly effective in treatment of human fascioliasis. We present 7 of 19 selected cases of human fascioliasis referred to our center in the Cusco region of Peru that failed to respond to triclabendazole. These were mostly symptomatic adults of both sexes that continued passing Fasciola eggs in the stool despite multiple treatments with 2 doses of triclabendazole at 10 mg/kg per dose. We documented the presence of eggs by rapid sedimentation and Kato Katz tests after each treatment course. We found that repeated triclabendazole courses were not effective against fascioliasis in this group of people. These findings suggest that resistance to triclabendazole may be an emerging problem in the Andes.
Author Summary
Fascioliasis is a zoonotic food borne trematode infection with a wide distribution. The complex epidemiology of this infection makes control efforts difficult. The paucity of drugs available for treatment may further hinder their success. Triclabendazole, the only first line drug for Fasciola, has been used for many years in the livestock industry. Resistant livestock Fasciola infections have emerged in developing and developed countries. However, most human trials report triclabendazole efficacies close to 100% after a few doses. Only a few cases of triclabendazole treatment failure have been published. We document 7 patients infected with Fasciola hepatica in Cusco–Peru that failed several treatment courses with triclabendazole. This raises concerns regarding preparedness to address resistant parasite infections and calls for more research to find new medications and tools to evaluate resistance.
Introduction
Fascioliasis is a worldwide zoonotic infection caused by the trematode parasite Fasciola hepatica. Livestock infection causes large economic losses in developed and developing countries.[1] Even in some wealthy countries, up to 50% of the dairy and meat herds may be infected; but data from resource-poor countries are limited.[2–4] Heavily infected cattle have significantly decreased milk (≥ 1.5 L daily) and meat (≥ 3 kg) production.[5,6] Human infection has been reported in more than 70 countries, but the highest burden occurs in the Andes and parts of the Middle East.[7] School-age children have the highest prevalence of fascioliasis and bear most of its severe consequences. Lopez et al. described a threefold increase in anemia risk among children with fascioliasis compared with children without infection.[8] Significant weight loss during the acute and chronic infections has been described in other studies.[9,10] Thus, the long term effects of fascioliasis complications have motivated significant efforts to tackle livestock and human infection.
Triclabendazole is the most effective drug for fascioliasis based on safety and cure rates reported in small mostly uncontrolled studies.[11] Mass treatment with triclabendazole has been proposed as a strategy to control fascioliasis in livestock and humans. In developed countries cattle and sheep herds are treated with triclabendazole under professional supervision. However, in resource-poor countries, mass livestock treatment is often inconsistent.[12] Mass treatment and inconsistent dosing of triclabendazole may select resistant parasites.[13] The emergence of triclabendazole resistance has been described among sheep and cattle herds in Scotland, Northern Ireland, and Australia and has been associated with decreased beef and dairy production.[12,14] Increasing resistance has also been reported in Cajamarca, Peru, where only 31% of cattle treated with 12 mg/kg of triclabendazole were cured after 14 days.[15] Triclabendazole resistance in humans has only rarely been noted.[16,17] Reports of resistance are of concern given that triclabendazole is the only highly effective treatment available. In this report, we describe 7 patients with fascioliasis with persistent infection despite multiple treatment courses with triclabendazole.
Methods
The Cusco region in the south highlands of Peru is an endemic area for fascioliasis. In rural areas of this region the prevalence of Fasciola hepatica infection among children is 11%.[8] The Universidad Peruana Cayetano Heredia and University of Texas Medical Branch Collaborative Research Center in Cusco is a referral center for research and management of Fasciola infection. Patients referred to us with diagnosed or suspected fascioliasis are evaluated with up to three Lumbreras rapid sedimentation and Kato Katz stool tests. Subjects with negative stool tests and significant eosinophilia are evaluated with Fas2 ELISA for serum antibodies against Fasciola hepatica. Except when noted, treatment courses for patients with stool or serologic evidence of fascioliasis consisted of 2 doses of triclabendazole at 10 mg/kg every 12 hours preceded by a meal rich in fat. All subjects received counseling on avoidance of vegetables that might put them at risk for reinfection. Treatment response was assessed with Lumbreras rapid sedimentation and Kato Katz stool tests between 1 and 3 months after treatment.[18,19] Those found to remain infected received repeated courses of triclabendazole.
Cases report
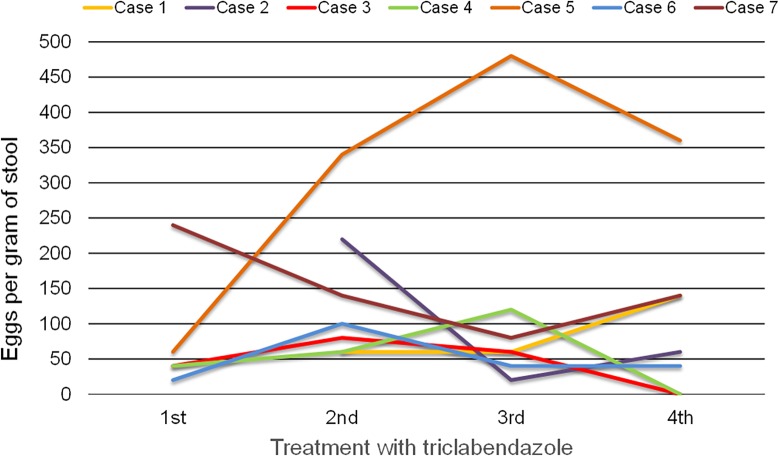
Between January 2014 and April 2015, 7 out of 19 patients with Fasciola hepatica infection referred to our center for evaluation and treatment failed to respond to multiple courses of triclabendazole (Egaten 250 mg tablets, Novartis Pharma AG, Basel, Switzerland, expiration date December 2015) 2 doses at 10 mg/d every 12 h with a fatty meal. Three of these were males, 2 were younger than 18 years old, and all but one were born in Cusco City. Four patients had acute presentations with delayed diagnosis, severe symptoms requiring prolonged hospital admissions for hypereosinophilia. All patients had a history of eating fresh watercress and other green leafy vegetables and self-medicated at least once with triclabendazole for veterinary use without response. In an attempt to improve their clinical response, all were prescribed triclabendazole and nitazoxanide (Colufase, Roemmers SA, Lima, Peru) 500mg PO every 12 hours for 7 days in combination after failing courses of treatment with triclabendazole monotherapy. Only one patient had consecutive negative stool tests and was deemed cured. The characteristics of the patients are shown in Table 1. The cases and their clinical course are briefly described below. Fig 1 shows the egg counts 1 to 3 months after each treatment course with triclabendazole.
Table 1. Characteristics of the patients with fascioliasis that failed triclabendazole treatment.
| Case | Age | Sex | Clinical presentation | Eosinophilia | Times treated* | Days of admission | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 51 | F | Abdominal pain, weight loss, fever | Yes | 4 | 30 | No |
| 2 | 36 | F | Abdominal pain, weight loss, multiple liver lesions on CT scan | Yes | 3 | 15 | No |
| 3 | 43 | F | Asymptomatic | No | 3 | None | Subcutaneous nodule and hypereosinophilia |
| 4 | 42 | M | Abdominal pain, weight loss, fever, rash, multiple liver lesions on CT scan | Yes | 4 | 10 | No |
| 5 | 15 | M | Abdominal pain, pleural effusion, ascitis, fever, multiple liver lesions on CT scan | Yes | 4 | 60 | No |
| 6 | 12 | M | Asymptomatic | No | 4 | None | Mild abdominal pain and vomiting |
| 7 | 36 | F | Asymtomatic | No | 4 | No | Severe abdominal pain and vomiting |
* Times treated: Number of times the patient was prescribed treatment. The use of veterinary formulation of triclabendazole is not included as it was impossible to ascertain the dose received.
Fig 1. F. hepatica egg counts 1 to 3 months after triclabendazole treatment.
Case 1 was a 51 years old female farmer with 16 kg weight loss, right upper quadrant abdominal pain, night sweats, anorexia, malaise, fever, and hypereosinophilia. She was first treated with a single dose of 10 mg/kg triclabendazole with improvement of symptoms. However, after 8 months her symptoms returned and she was again noted to be shedding Fasciola eggs. The patient was treated 2 additional times with triclabendazole (each with 2 doses of 10 mg/kg every 12 hours) and then with 2 doses of triclabendazole 10 mg/kg every 12 hours followed by nitaxozanide 500 mg every 12 hours for 7 days with improvement of symptoms. However, continued to pass Fasciola eggs on stool tests.
Case 2 was a 36 years old female with right upper quadrant abdominal pain, jaundice, severe joint pain, fatigue, 8 kg weight loss, and hypereosinophilia. Her stool tests and Fas-2 ELISA were positive for Fasciola. After failing a treatment course with triclabendazole (2 doses of 10 mg/kg every 12 hours), she was referred to our center. Over 10 months she received 1 additional course of triclabendazole treatment (2 doses of 10 mg/kg every 12 hours), a course of 3 doses of triclabendazole 10 mg/kg every 12 hours, and a course of 2 doses of triclabendazole 10 mg/kg every 12 hours followed by nitazoxanide (500 mg every 12 hours for 7 days) with marked improvement of symptoms but persistence of Fasciola eggs in the stools.
Case 3 was a 43 years old female who was asymptomatic. She was tested for Fasciola hepatica ova after her husband (case 4) was diagnosed with the infection. Both Fas2 ELISA and stool tests were positive for Fasciola infection. She failed a course of triclabendazole (2 doses of 10 mg/kg every 12 hours) and was prescribed 2 doses of triclabendazole 10 mg/kg every 12 hours followed by nitaxozanide 500 mg every 12 hours for 7 days after which she developed intrahepatic bile obstruction with removal of 3 adult Fasciola by endoscopic retrograde cholangiopancreatography. Also a migratory subcutaneous nodule due to Fasciola developed despite self-medication with a veterinary formulation of triclabendazole in 2 occasions. She was prescribed triclabendazole (2 doses of 10 mg/kg every 12 hours) with negative stool tests for Fasciola at 5–6 weeks follow up.
Case 4 is a 42 years old male born in the jungle of Cusco state married with case 3. He was admitted to the hospital with fever, diffuse abdominal pain, cough, severe fatigue, 5 kg weight loss, rash in the lower extremities and buttocks, and hypereosinophilia (eosinophil count > 30,000/dL). Lumbreras rapid sedimentation and Fas2 ELISA tests were positive for Fasciola hepatica infection. A few days after receiving triclabendazole treatment (2 doses of 10 mg/kg every 12 hours) his symptoms disappeared, but continue passing Fasciola ova. He was prescribed 2 additional treatment courses with triclabendazole (2 doses of 10 mg/kg every 12 hours) followed by a course of 2 doses of triclabendazole 10 mg/kg every 12 hours combined with nitaxozanide 500 mg every 12 hours for 7 days and 2 courses of triclabendazole veterinary formulation turning his Kato Katz tests negative. However, the Lumbreras rapid sedimentation test has remained positive.
Case 5 was a 15 years old male who presented with epigastric pain, fever, shortness of breath, chest pain, 5 kg weight loss, hypereosinophilia, ascites, and pleural effusion. The stool tests for Fasciola were initially negative, but the Fas2 ELISA was positive. He was treated with a single dose of veterinary formulation triclabendazole with partial improvement of symptoms. His follow up stool tests were positive and remained positive since then despite 3 courses of triclabendazole (2 doses of 10 mg/kg every 12 hours each), and a course of 2 doses of triclabendazole 10 mg/kg every 12 hours in combination with nitaxozanide 500 mg every 12 hours for 7 days.
Case 6 was a 12 years old male brother of case 5 diagnosed with asymptomatic F. hepatica infection. He failed the initial treatment with a single dose of triclabendazole. He was prescribed and failed 2 additional triclabendazole courses (2 doses of 10 mg/kg every 12 hours). He was subsequently treated with 2 doses of triclabendazole 10 mg/kg every 12 hours followed by nitaxozanide 500 mg every 12 hours for 7 days but his stool tests have remained positive for Fasciola eggs.
Case 7 was a 36 years old woman mother of case 5 diagnosed with chronic F. hepatica infection. She received 3 courses of triclabendazole (2 doses of 10 mg/kg every 12 hours) followed by a course of 2 doses of triclabendazole 10 mg/kg every 12 hours combined with nitaxozanide 500 mg every 12 hours for 7 days with persistently positive stool tests.
Ethics statement
The study was reviewed by the Institutional Ethics Committee from Universidad Peruana Cayetano Heredia in Lima, Peru. Written informed consent was obtained from the subjects.
Results/Discussion
Although triclabendazole resistance in veterinary medicine is well known, resistant human infections have only rarely been reported. In this manuscript, we report 7 cases of Fasciola hepatica infection that failed to respond to multiple treatment courses with recommended doses of triclabendazole. Two other case reports of failure of triclabendazole treatment for fascioliasis have been published. In 2012, Winkelhagen et al. from the Netherlands reported a single case of multiple treatment failures with triclabendazole and nitazoxanide.[16] In 2014, Gil et al. reported 4 cases of triclabendazole failure in Chile.[17] However, in 3 of those cases the timeline between symptoms, treatment, and evaluation of response suggest reinfection rather than treatment failures. Of note, none of the reported cases had quantitative tests to evaluate the response of egg burden to treatment. Most of our patients had low egg burdens and the egg counts did not showed significant reductions after treatment.
The microscopic detection of Fasciola eggs in human stool after the ingestion of metacercaria takes approximately 12 weeks. Our cases were followed up and tested for cure between 1 and 3 months after treatment with triclabendazole. This approach to monitoring was chosen to distinguish the treatment response from reinfection. Failure of anthelmintic treatment may be due to a number of factors. Quality control of the medications is essential. In these cases, all treatment failures received Egaten 250 mg tablets (Novartis Pharma AG, Basel, Switzerland) stored according to the manufacturer recommendations with an expiration date in December 2015 (well after treatment). Treatment failure may also result from inadequate drug absorption. Food has significant effects on the absorption of triclabendazole. It is recommended that patients with fascioliasis ingest a fatty meal before each triclabendazole dose to increase medication absorption in the intestine, as was done in all of our cases. The impact in cure rates of not following this recommendation has not been studied. However, uncontrolled clinical trials with single dose triclabendazole report efficacies around 90% despite absence of fat ingestion before treatment.[11] Reduced triclabendazole conversion to triclabendazole sulfoxide and triclabendazole sulfone in the presence of severe liver impairment have been proposed as a cause of treatment failure.[20] All our cases had relatively low Fasciola egg counts suggesting mild infections and probably minimal liver damage. Of note, most of our patients presented with symptoms of acute Fasciola infection. Whether this was associated with the initial treatment failure cannot be ascertained. In early stages of infection with Schistosoma sp. the parasite has reduced susceptibility to praziquantel as demonstrated in vitro and in vivo.[20,21] Some in vitro studies with Fasciola hepatica infection suggest reduced triclabendazole susceptibility among juvenile parasites compared to adults.[22] This has not been rigorously documented in case series of patients with acute infections.[23,24] Marcos et al. reported the resolution of eosinophilia after a single dose of triclabendazole in 10 patients with acute Fasciola infection. However, the authors failed to report the absence of eggs in the stools during the follow up.[25] Thus, the clinical evidence gathered suggests the presence of triclabendazole resistant Fasciola hepatica infection in our cases.
The mechanisms by which the fluke can become resistant to triclabendazole remain to be elucidated. The β-tubulin gene mutations that cause benzimidazole resistance in nematode parasites does not seem to explain triclabendazole resistance in Fasciola hepatica.[26] Changes in drug uptake and parasite drug metabolism seem to play a bigger role. The uptake of the drug by the fluke is influenced by a P-glycoprotein linked efflux pump. Experiments have shown that inhibition of this pump leads to potentiation of triclabendazole activity.[27] In addition, triclabendazole resistant flukes have been shown to metabolize triclabendazole sulfoxide to sulfone to a greater extent than susceptible flukes.[28]. Thus, the combined effect of reduced drug uptake and more active drug metabolism could reduce the effective concentrations of triclabendazole.
Triclabendazole has been used in livestock to treat Fasciola for many years. Inconsistent dosing and schedules have led to widespread resistance in cattle rearing areas in the last decade. Human infections with triclabendazole resistant Fasciola in areas with zoonotic transmission is a potential problem. In contrast to veterinary medicine in which other treatment options for Fasciola exist, in humans triclabendazole is the only first line medication with reported high efficacy. Thus, the emergence of triclabendazole resistance in fascioliasis among humans is an important clinical and public health concern as no alternative drugs are available to treat the infection. In our case series, subjects were treated with nitaxozanide (500 mg twice daily for 7 days) after 2 doses of triclabendazole. This approach was based on a double blind placebo controlled trial of nitazoxanide for the treatment of fascioliasis. Although the trials showed limited efficacy in children (40%), the efficacy was slightly higher in adults (60%).[29,30] The development of biliary colic in some of the cases could have suggested response to the medication, but none were cured by combination treatment.
This study has some limitations that made difficult the assessment of resistance. Most of the information was collected retrospectively and the number of cases was small. We were not able to recover live flukes from the subjects after treatment or generate metacercariae from the eggs collected for susceptibility testing in vitro. Nevertheless, our clinical observations suggest the presence of triclabendazole resistant Fasciola infections in a selected group of patients from Cusco. Resistant infection in livestock has already been reported in the northern highlands of Peru. Although, this report does not reflect in any way the community prevalence of triclabendazole resistance among humans in Cusco, triclabendazole resistance appears to be an emerging problem deserving attention in Peru and probably other highly endemic areas. Research on new drugs and methods to evaluate drug resistance is urgently needed to control Fasciola.
Data Availability
Identifiable patient information and medical records will not be available. The data collected in CRFs from the participating patients are available from the Universidad Peruana Cayetano Heredia Institutional Ethics Review Board (Telephone # +51 3190000 e-mail: duict.cieh@oficinas-upch.pe).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Elliott TP, Kelley JM, Rawlin G, Spithill TW. High prevalence of fasciolosis and evaluation of drug efficacy against Fasciola hepatica in dairy cattle in the Maffra and Bairnsdale districts of Gippsland, Victoria, Australia. Vet Parasitol. 2015. April 15;209(1–2):117–24. 10.1016/j.vetpar.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RM. Fasciola hepatica: a review of the economic impact in cattle and considerations for control. Vet Ther Res Appl Vet Med. 2001;2(1):40–50. [PubMed] [Google Scholar]
- 3.Mezo M, González-Warleta M, Castro-Hermida JA, Muiño L, Ubeira FM. Association between anti-F. hepatica antibody levels in milk and production losses in dairy cows. Vet Parasitol. 2011. August 25;180(3–4):237–42. 10.1016/j.vetpar.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 4.Kuerpick B, Schnieder T, Strube C. Seasonal pattern of Fasciola hepatica antibodies in dairy herds in Northern Germany. Parasitol Res. 2012. May 8;111(3):1085–92. 10.1007/s00436-012-2935-5 [DOI] [PubMed] [Google Scholar]
- 5.Charlier J, Duchateau L, Claerebout E, Williams D, Vercruysse J. Associations between anti-Fasciola hepatica antibody levels in bulk-tank milk samples and production parameters in dairy herds. Prev Vet Med. 2007. January;78(1):57–66. [DOI] [PubMed] [Google Scholar]
- 6.Sargison ND, Wilson DJ, Penny CD, Bartley DJ. Unexpected production loss caused by helminth parasites in weaned beef calves. Vet Rec. 2010. November 6;167(19):752–4. 10.1136/vr.c5428 [DOI] [PubMed] [Google Scholar]
- 7.Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012. March;12(3):210–21. 10.1016/S1473-3099(11)70294-8 [DOI] [PubMed] [Google Scholar]
- 8.Lopez M, White AC, Cabada MM. Burden of Fasciola hepatica infection among children from Paucartambo in Cusco, Peru. Am J Trop Med Hyg. 2012. March 1;86(3):481–5. 10.4269/ajtmh.2012.11-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabada MM, Goodrich MR, Graham B, Villanueva-Meyer PG, Lopez M, Arque E, et al. Fascioliasis and eosinophilia in the highlands of Cuzco, Peru and their association with water and socioeconomic factors. Am J Trop Med Hyg. 2014. November;91(5):989–93. 10.4269/ajtmh.14-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Shazly AM, El-Nahas HA, Abdel-Mageed AA, Beshbishi SN El, Azab MS, Abou El Hasan M, et al. Human fascioliasis and anaemia in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2005. August;35(2):421–32. [PubMed] [Google Scholar]
- 11.Keiser J, Engels D, Büscher G, Utzinger J. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin Investig Drugs. 2005. December;14(12):1513–26. [DOI] [PubMed] [Google Scholar]
- 12.Sargison ND, Scott PR. Diagnosis and economic consequences of triclabendazole resistance in Fasciola hepatica in a sheep flock in south-east Scotland. Vet Rec. 2011. February 12;168(6):159 10.1136/vr.c5332 [DOI] [PubMed] [Google Scholar]
- 13.Sargison N. Diagnosis of triclabendazole resistance in Fasciola hepatica. Vet Rec. 2012. August 11;171(6):151–2. 10.1136/vr.e5357 [DOI] [PubMed] [Google Scholar]
- 14.Gordon D, Zadoks R, Skuce P, Sargison N. Confirmation of triclabendazole resistance in liver fluke in the UK. Vet Rec. 2012. August 11;171(6):159–60. 10.1136/vr.e5381 [DOI] [PubMed] [Google Scholar]
- 15.Ortiz P, Scarcella S, Cerna C, Rosales C, Cabrera M, Guzmán M, et al. Resistance of Fasciola hepatica against Triclabendazole in cattle in Cajamarca (Peru): a clinical trial and an in vivo efficacy test in sheep. Vet Parasitol. 2013. July 1;195(1–2):118–21. 10.1016/j.vetpar.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Winkelhagen AJS, Mank T, de Vries PJ, Soetekouw R. Apparent Triclabendazole-resistant human Fasciola hepatica infection, the Netherlands. Emerg Infect Dis. 2012. June;18(6):1028–9. 10.3201/eid1806.120302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil LC, Díaz A, Rueda C, Martínez C, Castillo D, Apt W. Resistant human fasciolasis: Report of four patients. Rev Médica Chile. 2014. October;142(10):1330–3. [DOI] [PubMed] [Google Scholar]
- 18.Raunelli F, Gonzalez S. Strategic control and prevalence of Fasciola hepatica in Cajamarca, Peru. A pilot study. Intern J Appl Res Vet Med 2009:7;145–52. Accessed 24 November 2015. Available at: http://www.jarvm.com/articles/Vol7Iss4/Gonzalez.pdf [Google Scholar]
- 19.Identification of intestinal parasites Bench Aids for the Diagnosis of Intestinal Parasites, World Health Organization; 2012. Accessed 24 November 2015. Available at: http://apps.who.int/iris/bitstream/10665/37323/1/9789241544764_eng.pdf [Google Scholar]
- 20.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol 2004;34(4):527–33 [DOI] [PubMed] [Google Scholar]
- 21.Grandière-Pérez L, Ansart S, Paris L, Faussart A, Jaureguiberry S, Grivois J- P, et al. Efficacy of praziquantel during the incubation and invasive phase of Schistosoma haematobium schistosomiasis in 18 travelers. Am J Trop Med Hyg. 2006. May 1;74(5):814–8. [PubMed] [Google Scholar]
- 22.Duthaler U, Smith TA, Keiser J. In vivo and in vitro sensitivity of Fasciola hepatica to triclabendazole combined with artesunate, artemether, or OZ78. Antimicrob Agents Chemother 2010;54(11):4596–604 10.1128/AAC.00828-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JX, Chen MX, Ai L, Xu XN, Jiao JM, Zhu TJ, Su HY, Zang W, Luo JJ, Guo YH, Lv S, Zhou XN. An Outbreak of Human Fascioliasis gigantica in Southwest China. PLoS One. 2013. August 8;8(8):e71520 10.1371/journal.pone.0071520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu W, Su HY, Zou J, Li QC, Chen BY, Lin CS, Jiao JM. Clinical diagnosis and treatment in an outbreak of Fasciola gigantica infection in Yunnan Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2012;30(6):455–9 [Abstract] [PubMed] [Google Scholar]
- 25.Marcos LA, Tagle M, Terashima A, Bussalleu A, Ramirez C, Carrasco C, Valdez L, Huerta-Mercado J, Freedman DO, Vinetz JM, Gotuzzo E. Natural history, clinicoradiologic correlates, and response to triclabendazole in acute massive fascioliasis. Am J Trop Med Hyg 2008;78(2):222–7 [PubMed] [Google Scholar]
- 26.Fairweather I. Reducing the future threat from (liver) fluke: realistic prospect or quixotic fantasy? Vet Parasitol. 2011. August 4;180(1–2):133–43. 10.1016/j.vetpar.2011.05.034 [DOI] [PubMed] [Google Scholar]
- 27.Savage J, Meaney M, Brennan GP, Hoey E, Trudgett A, Fairweather I. Effect of the P-glycoprotein inhibitor, R(+)-verapamil on the drug susceptibility of a triclabendazole-resistant isolate of Fasciola hepatica. Vet Parasitol. 2013. July 1;195(1–2):72–86. 10.1016/j.vetpar.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 28.Robinson MW, Lawson J, Trudgett A, Hoey EM, Fairweather I. The comparative metabolism of triclabendazole sulphoxide by triclabendazole-susceptible and triclabendazole-resistant Fasciola hepatica. Parasitol Res. 2004. February;92(3):205–10. [DOI] [PubMed] [Google Scholar]
- 29.Favennec L, Jave Ortiz J, Gargala G, Lopez Chegne N, Ayoub A, Rossignol JF. Double-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru. Aliment Pharmacol Ther. 2003. January;17(2):265–70. [DOI] [PubMed] [Google Scholar]
- 30.Zumaquero-Ríos JL, Sarracent-Pérez J, Rojas-García R, Rojas-Rivero L, Martínez-Tovilla Y, Valero MA, et al. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: epidemiology and treatment with nitazoxanide. PLoS Negl Trop Dis. 2013. November;7(11):e2553 10.1371/journal.pntd.0002553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Identifiable patient information and medical records will not be available. The data collected in CRFs from the participating patients are available from the Universidad Peruana Cayetano Heredia Institutional Ethics Review Board (Telephone # +51 3190000 e-mail: duict.cieh@oficinas-upch.pe).