Abstract
Hepatitis C virus (HCV) poses a global threat to public health. HCV envelop protein E2 is the major component on the virus envelope, which plays an important role in virus entry and morphogenesis. Here, for the first time, we affinity purified E2 complex formed in HCV-infected human hepatoma cells and conducted comparative mass spectrometric analyses. 85 cellular proteins and three viral proteins were successfully identified in three independent trials, among which alphafetoprotein (AFP), UDP-glucose: glycoprotein glucosyltransferase 1 (UGT1) and HCV NS4B were further validated as novel E2 binding partners. Subsequent functional characterization demonstrated that gene silencing of UGT1 in human hepatoma cell line Huh7.5.1 markedly decreased the production of infectious HCV, indicating a regulatory role of UGT1 in viral lifecycle. Domain mapping experiments showed that HCV E2-NS4B interaction requires the transmembrane domains of the two proteins. Altogether, our proteomics study has uncovered key viral and cellular factors that interact with E2 and provided new insights into our understanding of HCV infection.
Introduction
HCV is an important human pathogen that primarily infects human hepatocytes and causes chronic liver diseases [1]. This deadly RNA virus encodes ten viral proteins to complete its life cycle. In order to establish a productive infection, HCV structural proteins (core, E1, and E2) and nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, NS5B) form complex interaction networks (interactomes) with a myriad of host cellular factors. Viral glycoproteins E1 and E2 together form spikes on the viral envelope, which then engage with cell surface molecules[2–7], including CD81[6], scavenger receptor BI (SR-BI) [7], claudin-1 (CLDN1) [8], occludin (OCLN) [9, 10], epidermal growth factor receptor (EGFR)[8], and cholesterol-uptake receptor Niemann-Pick C1-like 1 (NPC1L1) [9], and trigger the endocytosis of the viral particle[10, 11]. The ectodomain of E2 is the primary ligand that binds aforementioned receptors, whereas transmembrane domain (TMD) of E2 functions in the membrane anchoring, heterodimerization with E1, and ER retention [12–16]. A hydrophobic sequence locating in the TMD of E2 is important for E2 translocation to ER lumen where the glycosylation occurs [14]. Besides mediating viral entry, E2 also interacts with HCV nonstructural protein 2 (NS2) and plays an important role in virus morphogenesis [17]. However, much of E2 biogenesis as well as its role in viral morphogenesis have yet to be understood.
To fill this knowledge gap, we developed a strategy to purify intact E2 complex formed in HCV infected human hepatoma cells and reproducibly identified 85 HCV E2 binding proteins. Our comparative proteomics and functional analyses revealed an important interaction between HCV E2 and the endoplasmic reticulum (ER) protein UGT1, which regulates the production of infectious HCV. Interestingly, another viral protein, NS4B, was also found to interact with E2. Multiple domains of HCV NS4B coprecipitated with HCV E2 and this interaction was abolished when E2 transmembrane domain was removed. Characterizing these interactions in detail would provide a deeper understanding of HCV infection and also potentially present targets for antivirals to disrupt virus biology.
Materials and Methods
Cells, reagents, and constructs
The human kidney epithelial cell line Lenti-X 293T was purchased from Clontech. The human liver cell line Huh7.5.1 was provided by Dr. Francis Chisari (Scripps Research Institute) [18]. All cell lines were maintained in DMEM supplemented with 5% penicillin and streptomycin, 1% NEAA, and 10% fetal bovine serum (FBS) (Gemini Bio-Products). Anti-Flag M2 antibody and Rabbit anti-UGGT1 antibody (HPA015127) were purchased from Sigma. Secondary antibodies are purchased from Jackson ImmunoResearch Laboratories.
JFH1-Flag-E2, which expresses Flag-tagged JFH1 E2, has been described previously[19]. pLVX-Flag-UGT1 was generated by replacing the EcoRI-XhoI fragment of pLVX-DFT with PCR fragments generated using forward primer UGT1-FP (5′-gcgaattctgggctgcaagggagacgcgag-3′) and reverse primers UGT1-RP (5′-atatagctcgagtcatttcttacccttgatga-3′). Individual HCV protein was PCR amplified from the JFH1 clone and subcloned in frame after a Flag tag vector (pMIR-DFT).
Affinity purification of HCV E2 complex
Huh-7.5.1 cells (2 x 108) were infected by JFH1-AM2 and JFH1-Flag-E2-AM2 viruses. On day 3 postinfection, cells were trypsinized and washed with ice-cold phosphate-buffered saline and then Dounce homogenized in 10 ml of immunoprecipitation (IP) buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 0.5% NP-40, 5 mM β-glycerophosphate) supplemented with a protease inhibitor cocktail. Centrifugation-cleared lysates were then subjected to IP with 50 μl anti-Flag M2 affinity resin and rotated 4 hours at 4°C. After four washes with the IP buffer, bound proteins were eluted with Flag peptide (100μg/ml, Sigma) in 100 μl Tris-buffered saline and resolved on SDS/PAGE, and the proteins were visualized by SYRO Ruby staining. Roughly 20 gel slices were excised from either JFH1-AM2 or JFH1-Flag-E2-AM2 lane and subjected for LC-MS/MS.
LC-MS/MS and data analysis
Gel bands of interest were subjected to in-gel digestion according to established protocols [20]. Briefly, gel bands were destained in 50% acetonitrile in 50 mM NH4HCO3, pH 8.4 and vacuum dried. Trypsin (20 μg/mL in 25 mM NH4HCO3, pH 8.4) was added and samples were allowed to incubate on ice for 45 minutes. The supernatant was removed and the gel bands were covered with 25 mM NH4HCO3, pH 8.4, and incubated at 37°C overnight. Tryptic peptides were extracted from the gel pieces with 70% acetonitrile, 5% formic acid, lyophilized to dryness and resuspended in 10 μL of 0.1% formic acid prior to MS analysis.
Nanoflow reversed-phase liquid chromatography (RPLC) was performed using a Dionex Ultimate 3000 LC system (Dionex Corporation, Sunnyvale, CA) coupled online to an LTQ-Orbitrap XL mass spectrometer (ThermoFisher Scientific, San Jose, CA). Separations were performed using 75 μm i.d. x 360 o.d. x 20 cm long fused silica capillary columns (Polymicro Technologies, Phoenix, AZ) that were slurry packed in house with 5 μm, 300 Å pore size C-18 silica-bonded stationary phase (Jupiter, Phenomenex, Torrance, CA). Following sample injection onto a C-18 trap column (Dionex), the column was washed for 3 min with mobile phase A (2% acetonitrile, 0.1% formic acid in water) at a flow rate of 0.3 μL/min. Peptides were eluted using a linear gradient of 0.34% mobile phase B (0.1% formic acid in acetonitrile) / min for 117 minutes, then to 95% B in an additional 10 min, all at a constant flow rate of 0.2 μL/min. Column washing was performed at 95% B for 20 minutes, after which the column was re-equilibrated in mobile phase A prior to subsequent injections.
The LIT-MS was operated in a data dependent MS/MS mode in which each full MS scan was followed by seven MS/MS scans where the seven most abundant peptide molecular ions are selected for collision-induced dissociation (CID), using a normalized collision energy of 35%. Data were collected over a broad mass to charge (m/z) precursor ion selection scan range of 300–1800, utilizing dynamic exclusion to minimize redundant selection of peptides previously selected for CID. Tandem mass spectra were searched against a combined UniProt human protein database (03/2011) from the European Bioinformatics Institute (http://www.ebi.ac.uk/integr8) and the Hepatitis C virus genotype 2a (isolate JFH-1) protein sequence (UniProt Accession Q99IB8) using SEQUEST (ThermoFisher Scientific). For a fully tryptic peptide to be considered legitimately identified, it had to achieve stringent charge state and proteolytic cleavage-dependent cross correlation (Xcorr) scores of 1.9 for [M+H]1+, 2.2 for [M+2H]2+ and 3.5 for [M+3H]3+, and a minimum delta correlation (ΔCn) of 0.08. The false discovery rate cutoff was set as < 1%. Additionally, peptides were searched for methionine oxidation and cysteine carboxyamidomethylation with a mass addition of 15.99492 and 57.02416, respectively. The obtained proteins were further filtered by removing proteins identified by less than two unique peptides. Finally, those proteins identified from the JFH1-AM2 lane were subtracted from the total proteins that were identified in the JFH1-Flag-E2-AM2 lane (comparative or subtractive approach) in order to obtain the real E2 interacting partners. The purification, LC-MS/MS and subtractive analyses were done three times independently.
Pathway and network analysis on changed heart tissue proteins was performed using Ingenuity Pathway Analysis software (Redwood City, CA).
shRNA knockdown
Four shRNA clones targeting human UGT1 and the pLKO.1 control plasmid were purchased through TRC consortium from Sigma. To generate lentivirus, 3 μg of shRNA clone, 3 μg of pCMV8.2ΔR, and 1.5 μg VSV-G expression plasmid were transfected into 293T cells in 6-cm plates by lipofectamine 2000 (Invitrogen). Viruses were collected at 48 hours post-transfection and cleared through filtration. 500 μl viruses were added to Huh7.5.1 cells in a 12-well plate and selected by puromycin (0.6 μg/ml). To verify the knockdown efficiency, cell lysates were prepared and analyzed by Western blotting.
Western Blotting
Briefly, 30 μg of proteins were run on 4–20% precast polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes. Membranes were blocked with Odyssey Blocking Buffer (LI-COR, Lincoln, NE) followed by incubation with primary antibodies at a 1:1000 dilutions. Membranes were washed three times with 1X TBS, incubated with IRDye secondary antibodies (LI-COR, Lincoln, NE) for 1 h and washed again to remove unbound antibody. Western blotting images were taken using the ODYSSEY CLx (LI-COR, Lincoln, NE).
Statistical analysis
Bar graphs were plotted to show mean ± standard deviation (SD) of at least two independent experiments. Statistical analyses were performed using Graphpad Prism 5. A p value of <0.05 in the Student's test was considered statistically significant.
Results
Reproducible Identification of HCV E2 Interacting Proteins
The urgent need of prophylactic vaccines and alternative therapies demands for better understanding of the virus life cycle [21–23]. However, previously efforts in depicting the global HCV-Host protein-protein interactome have relied on the yeast two-hybrid assay [24], in which protein-protein interactions form beyond the context of infection. With recent development of cell culture systems supporting the entire HCV life cycle (HCVcc), which can be propagated in human liver cell line Huh7 and its derivatives [2, 18, 25], we are now poised to depict the virus-host interaction networks under physiologically relevant condition.
We have previously engineered a Japanese fulminant hepatitis 1 (JFH-1) clone that expresses a FLAG tag in fusion with its glycoprotein E2 (Fig 1A). This clone efficiently infects human Huh7.5 cells and produces infectious virions [19]. Using cell lysates made from the infected Huh7.5 cells, we conducted affinity pull down assays using anti-FLAG agarose resin and identified proteins that associate with E2 (Fig 1B and 1C). The experiments and mass spectrometric analyses were done three times under identical conditions. For database search and protein identification, the set criteria included that a protein must be identified in all three immunoaffinity pull downs with at least two unique peptide matches. In addition, those proteins identified from the control IP samples were subtracted out. Ultimately only those proteins that were identified in all three trials (listed in S1 Table) were subsequently categorized according to cellular distributions and biological functions (Fig 2 and S2 and S3 Tables). The majority of the identified proteins are localized to cytoplasm or membrane. Of note, viral proteins E1, NS2, and NS4B were also pulled down in all three trials.
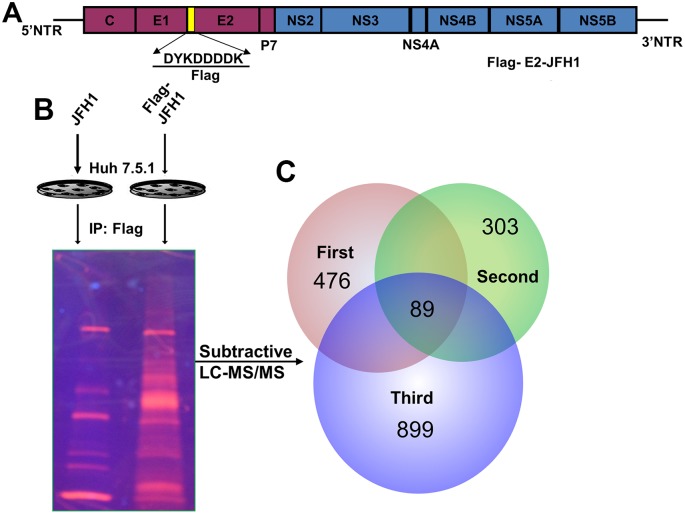
Fig 1. Identification of cellular proteins from HCV E2 complex.
Details can be found in Experimental Procedures. (A), Genomic organization of the Flag-E2-JFH1 virus. (B), Schematic of the purification strategy. The infection efficiency was nearly 100% in all three replicates. (C), Venn diagram of 89 proteins (including 4 viral proteins) that were identified in all three trials.
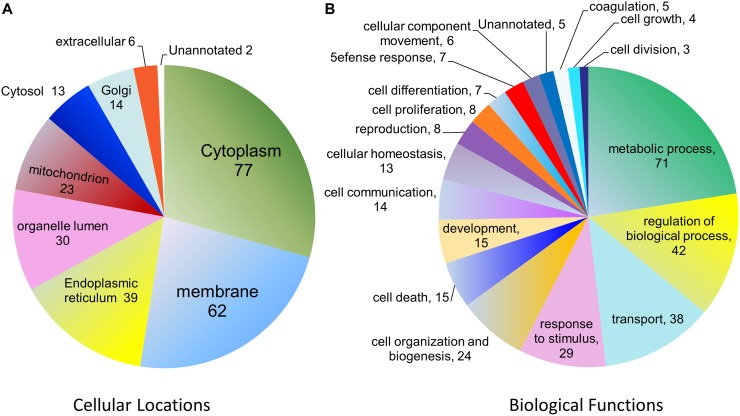
Fig 2. Pie charts showing the frequency of functional groups (analyzed by ProteinCenter, Thermo Scientific) from 85 cellular proteins.
Shown in (A) are proteins categorized according to cellular locations. Shown in (B) are proteins categorized according to biological functions. Notably some proteins are classified into more than one location or biological function.
Network analysis of HCV E2 associated factors
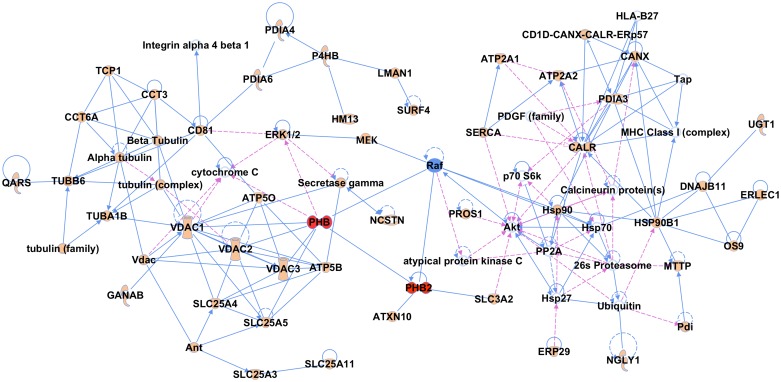
To depict the networks to which the 85 factors are clustered, we used Ingenuity Pathway Analysis (IPA) software to curate information on protein-protein interactions (PPIs) and molecular pathways. Indeed, many of the identified proteins interact with each other according to the database and can be linked to the same protein network, which further validates the success of our affinity purification. Well-represented networks include molecular transport and cell signaling; carbohydrate metabolism, lipid metabolism; cell-to-cell signaling and interaction (S1 Fig). PHB1 and 2 caught our attention due to their abundance in E2 complex and their known roles in regulating Ras-CRaf-MEK-ERK pathway [26] (Fig 3). Subsequent characterization showed that PHB1/2 are essential HCV entry factors and candidate drug targets [27].
Fig 3. Protein network analysis (using Ingenuity Analysis Software) for PHB1/2 network.
PHB1 (PHB) and PHB2 are highlighted in red; proteins labeled in solid shapes were identified in this study except Raf and MEK, which were added according to published data; proteins shown in empty circles were from Ingenuity database. The solid line represents direct interaction; the dashed line represents indirect interaction.
Initial Validation
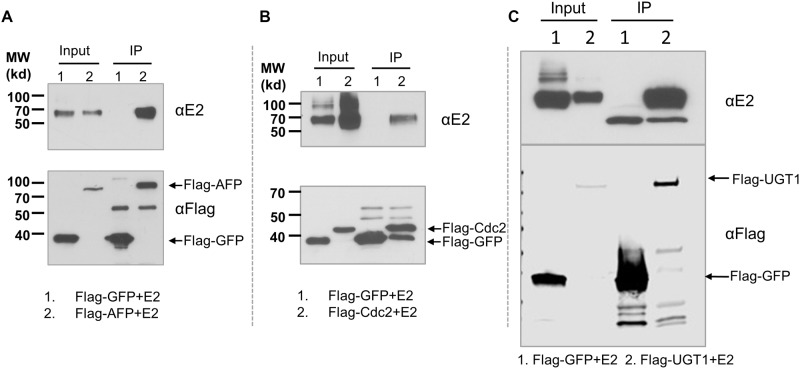
Among the 85 cellular proteins, endoplasmic reticulum chaperones such as calnexin [28] and calreticulin [29] have been known to interact with HCV glycoproteins and affect their folding; CD81 is a known HCV co-receptor; Cdc2 was previously reported to affect HCV entry[8]. The identification of alpha-fetoprotein (AFP) is interesting because for decades it has been the most widely used biochemical blood test for liver cancer [30]. To further validate the mass spectrometry results and potential interactions between E2 and cellular proteins, we performed reverse immunoprecipitations against selected cellular targets. To this end, we subcloned AFP, UDP-glucose:glycoprotein glucosyltransferase 1 (UGT1), and Cdc2 in a Flag-tagged expression plasmid and co-transfected with a HCV E2 expression plasmid into 293T cells. Whole cell lysates (input) and eluates (IP) from anti-Flag affinity resin were separated by 1D SDS-PAGE. The presence of E2 in three immunoprecipitates was confirmed by Western Blotting using a specific antibody against HCV E2 (Fig 4).
Fig 4. Co-immunoprecipitations of AFP (A), Cdc2 (B), and UGT1 (C) with HCV E2.
293T cells were transfected with indicated plasmids. Whole cell lysates were prepared for immunoprecipitation followed by western blotting. Representative data of three independent experiments are shown.
UGT1 is required for HCV Production
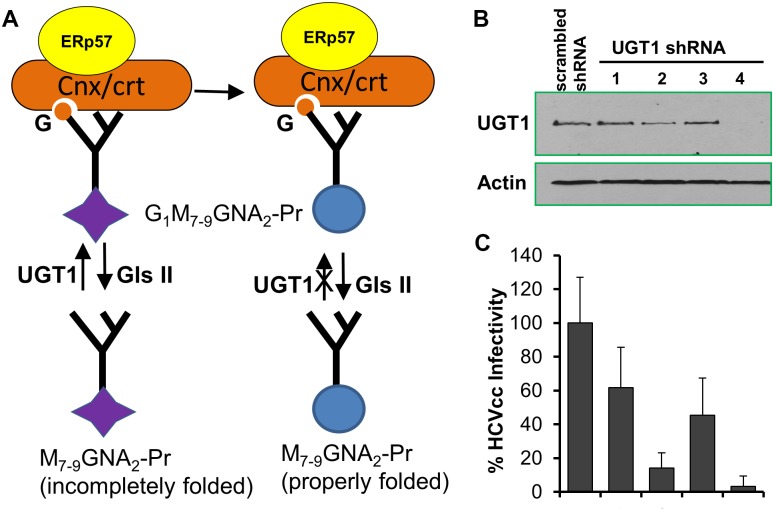
HCV E2 is synthesized at rough endoplasmic reticulum (ER) and then glycosylated at eleven sites before secretion. It is known that the eleven N-glycans of HCV E2 greatly influence its function in E2 ER-localization, dimerization, maturation, binding to CD81, protein folding, virus entry, assembly, and protection against neutralization, etc [31–34]. One of the E2 interacting partner that was identified in this study, UGT1, is the enzyme that adds a glucose residue from UDP-glucose to an N-linked Man(9)GlcNAc(2)oligosaccharide and thus plays a critical role in repairing folding defects of glycoproteins (Fig 5A).
Fig 5. UGT1 knockdown inhibited the production of infectious HCV particles.
(A) The UGT1-dependent glycoprotein repair and refolding. Incompletely folded proteins carrying N-glycans (M7-9GNA2-Pr) are sensed and re-glucosylated by UGT1, which results in binding to the ER lectin chaperones, calnexin and calreticulin. This prevents the proteins from degradation and allows longer ER retention for further folding. Properly folded proteins are released from Cal/Crt by de-glucosylation by glucosidase II. Abbreviations: GNA,N-acetylglucosamine; M, mannose; G, glucose; Pr, protein; Gls II, glucosidase II;G,glucose;Cnx/crt, calnexin/calreticulin. (B) Western blotting results of UGT1 knockdown in Huh7.5.1 cells. 4 different shRNA lentiviral clones (Purchased from Sigma, clones TRCN0000004520-23) were used to silence UGT1, with clone #4 showing the best silencing effect. (C) Cells from (B) were infected with HCVcc-Luc (MOI 1) and supernatant viruses were collected 48 hrs post infection and tiered for infectivity. Data shown are representatives of three independent experiments.
To investigate the functional role of UGT1 in HCV life cycle, we tested four short-hairpin interfering RNA (shRNA) clones that target human UGT1. Western blotting showed that #2 and #4 shRNA markedly reduced the endogenous level of UGT1 in Huh7.5.1 cells (Fig 5B). Concomitantly, UGT1 knockdown Huh 7.5.1 cells produced significantly less infectious virus (Fig 5C). Presently the exact mechanism for UGT1 to regulate HCV production remains to be determined. However, UGT1 knockdown did not affect HCV entry (data not shown), suggesting that UGT1 is modulating a later step of the viral lifecycle. As an extremely sensitive sensor of the tertiary structure of glycoproteins, UGT1 catalyzes the addition of monoglucose to the defective glycoprotein for repair through an unconventional pathway. Of note, minor defects often occur during glycosylation and can significantly affect the protein folding, maturation and function. Improperly folded glycoproteins will be degraded by proteasomes [35]. Therefore we suspect that UGT1 is required for the proper folding of glycosylated E2, and hence affects its biological function. In fact, a recent study based on HCVcc further demonstrated that several glycans potentially influence HCVcc assembly and infectivity [34]. Given that UGT1is the central enzyme that modifies N-linked glycans for proper folding of glycoproteins, the decreased virus infectivity in UGT1 knockdown cells can be the result of decreased assembly, secretion, decreased specific infectivity of virions as a result of altered E2 glycosylation. Ongoing investigations are dissecting these possibilities.
HCV NS4B Interacts with E2
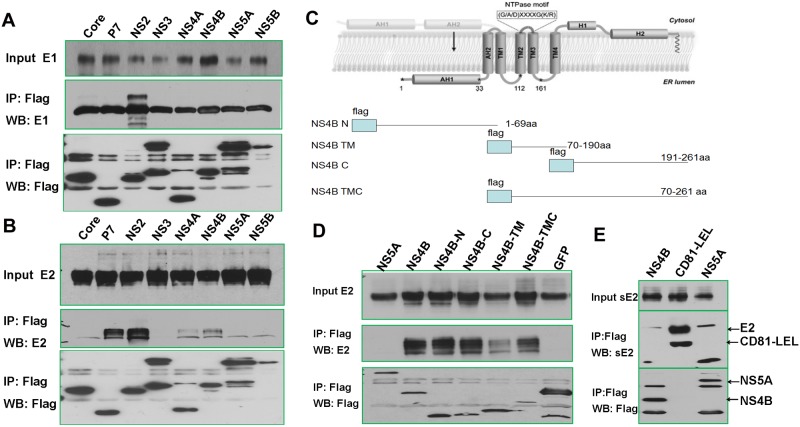
The main function of E2 is to bind cellular cell surface molecules, namely the tetraspannin CD81 and scavenger receptor SR-BI [2–7], and trigger the endocytosis of the viral particle [10, 11]. In addition, E2 is believed to play an important role in virus morphogenesis [17]. Interestingly, our affinity pull-down and mass spectrometric analyses identified HCV E1, NS2, and NS4B in E2 complex (S1 Table). To confirm this finding, we expressed individual viral protein with HCV E2 in 293T cells and performed co-IP experiments. It is clear that all of identified proteins co-precipitated with E2 or E1. By contrast, individually expressed NS3, 5A, 5B failed to precipitate with E2 (Fig 6).
Fig 6. HCV E2 interacts with NS4B.
A) 293T cells were co-transfected with indicated flag-tagged HCV protein DNA constructs and a HCV E1 construct. Input cell lysates and immunoprecipitates were blotted with antibodies against HCV E1and flag. Only NS2 robustly interacted with HCV E1. B) Lysates of 293T cells transfected with indicated flag-tagged HCV constructs together with an E2 expressing plasmid were immunoprecipitated with anti-Flag affinity resin. It was observed that p7, NS2, NS4A, and NS4B were able to individually precipitate E2. C) Mapping the domains of NS4B that interact with E2. Topology of HCV NS4B and mutants (modified from ref [36]). D) All NS4B deletion mutants except NS4B-TM robustly precipitated with E2. E) NS4B failed to precipitate soluble E2 (sE2). 293T cells were co-transfected with indicated plasmids expressing flag-NS4B or flag-NS5A or CD81 LEL fused with human Fc (hFc) and soluble E2. CD81-LEL efficiently pulled down sE2, whereas NS4B and NS5A failed to do so. The sE2 (aa 384–661 from H77 clone) lacks the entire TMD and is commonly used in binding studies as E2 substitute [37]. The CD81-LEL contains the large extracellular loop that interacts with sE2 in direct binding assays [38, 39].
Emerging evidence has indicated that p7, NS2, and NS3-NS4A participate in virus particle assembly. In particular, NS2 has been shown to interact with both E1 and E2 during viral assembly and plays an important role in the assembly process [40, 41]. The association between NS4B and E2, on the other hand, has not been reported. HCV NS4B is a 27 kDa ER membrane-associated protein [42] that is conserved in Flaviviridae family and is able to induce alteration of ER membrane and formation of a ‘membranous web’ structure, which provides a platform for the HCV replication complex [43, 44], [43], [45]. The topology of NS4B includes an N-terminal portion (aa 1–69), a central transmembrane part (aa 70–190) with five predicted transmembrane domains and a C-terminal portion (aa 191–261) (Fig 6C).
To characterize the molecular determinants of E2-NS4B interaction, we created deletion constructs containing the N-, C-, TM, TMC domain of NS4B to express Flag tagged proteins. Domain mapping study revealed that the N-terminus and C-terminus regions of NS4B efficiently precipitated with E2 (Fig 6D). The transmembrane domain of NS4B, by contrast, showed reduced capability in co-precipitating E2. Both domains are known to contain the amphipathic helix (AH) which tethers NS4B to ER-membrane [46]. Furthermore, soluble E2 (sE2) that is deprived of its TMD failed to precipitate NS4B, implying the TMD domain of E2 is required for this interaction (Fig 6E). Recent studies have hinted that assembly of infectious HCV may require transient physical interactions between structural and non-structural proteins [47]. NS4B is known to interact with NS5A and 5B in the replication complex [48–52]. Thus it is possible that NS4B serves as the bridge between structural protein E2 and the viral replication complex. In support, HCV NS3, 5A, 5B do not interact with E2, but NS4B is known to interact with NS3, 5A, and 5B to form the viral replication complex [48–52]. NS4B also contains a nucleotide binding motif that may bind viral RNA [53]. It is therefore not hard to envision that NS4B serves as the platform to bring structural proteins to the replication complex where newly synthesized viral RNA can be found for final assembly.
Discussion
Identifying which host proteins and complexes come into physical contact with the viral proteins is crucial for a comprehensive understanding of how HCV usurps the host's cellular machinery during the course of infection. To our knowledge, this is the first detailed interactome analysis of HCV E2 in the context of infection, which reduces identifying false interactions that only occur in the artificial yeast two-hybrid assay or when a singly overexpressed viral protein is used as a bait in immunoprecipitation. Three independent trials plus stringent criteria for protein identification yielded highly reproducible data that warrant future mechanistic investigations. In a recent study [54], Ramage and colleagues performed tandem affinity purification using individually overexpressed HCV viral protein as bait and combined with siRNA knockdown to generate a map of 139 high-confidence HCV-host protein-protein interactions. Our method differs significantly from that in the referenced study in that ours was performed in an infection setting and the Flag-E2 tagged HCV molecular clone is fully infectious. It is known N-terminally tagged HCV core protein simply leads to a dead virus [55, 56]; hence using individual viral protein tagged at its N-terminus as bait may not preserve authentic viral-host interactions. We believe this explains why several most characterized HCV E2 binding proteins, including its co-receptor CD81, was reproducibly identified in our study but not in the referenced study.
The 85 cellular factors identified in our experiment may not be binding to E2 directly, because we were using a fully infectious molecule clone. HCV E2 interacts with E1 and NS2 as other have published [57–60], it is therefore possible that some of the identified proteins were indirectly associating with E2 through their interactions with these viral proteins. Nevertheless, we subsequently performed co-IP to confirm the interaction with several proteins with E2 in the absence of other viral proteins. When we performed pathway analyses, the majority of the 85 identified cellular proteins can be clustered in same protein networks, supporting the success of our study.
The immediate application of use of the identified interactions is to develop better understanding of the molecular biology of HCV envelope protein E2. Heterodimers between E2 and E1 viral glycoprotein together make up the virus envelop spikes that mediate viral attachment and entry into host cells, and the assembly of infectious virus particles [47]. The function of E2 is influenced by its eleven N-linked glycans. Our study revealed the presence of UDP-glucose: glycoprotein glucosyltransferase 1 (UGT1 or UGCGL1) and HCV NS4B in the same complex. Initial functional characterization now presents preliminary evidence that gene silencing of UGT1 in human hepatoma cell line Huh7.5.1 markedly decreased HCV infectivity of the supernatant virus. UGT1 is the enzyme that catalyzes the addition of a glucose residue from UDP-glucose to an N-linked Man(9)GlcNAc(2)oligosaccharide and thus plays an important role in repairing minor defects in glycoprotein folding [35]. Based on these data and the literatures, it is possible that UGT1 modulates the production of infectious virus particles by affecting the proper folding of HCV E2. Here we also show that multiple regions of HCV NS4B co-precipitated with HCV E2 and the interaction was abolished when E2 transmembrane domain was removed. Previous studies have reported that various genetic interactions exist between structural and non-structural sequences [47]. The NS4B-E2 interaction may contribute to the assembly of infectious HCV particles. Future characterization will be needed to address these possibilities.
Our E2 interaction map now provides an excellent opportunity to look for druggable targets. In a separate publication, we described a detailed validation and functional characterization of E2-prohibitin interaction [27]. In the study, we demonstrated that not only the two newly identified E2-interacting proteins, prohibitins, are essential to viral entry, but serve as cellular targets for a novel class of small molecules to block HCV infection. Overall, our study revealed important virus-host interactions that regulate HCV E2 biogenesis and function and serve as potential targets for drug intervention.
Supporting Information
(TIF)
(XLS)
(XLS)
(XLS)
Acknowledgments
This work was supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (NIH R01DK088787 grant to T.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH R01DK088787 grant to TW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kontorinis N, Agarwal K, Dieterich DT. Current status of the use of growth factors and other adjuvant medications in patients receiving peginterferon and ribavirin. Rev Gastroenterol Disord. 2004;4 Suppl 1:S39–47. . [PubMed] [Google Scholar]
- 2.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309(5734):623–6. . [DOI] [PubMed] [Google Scholar]
- 3.Kato T, Date T, Miyamoto M, Zhao Z, Mizokami M, Wakita T. Nonhepatic cell lines HeLa and 293 support efficient replication of the hepatitis C virus genotype 2a subgenomic replicon. J Virol. 2005;79(1):592–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Randall G, Higginbottom A, Monk P, Rice CM, McKeating JA. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J Virol. 2004;78(3):1448–55. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cormier EG, Tsamis F, Kajumo F, Durso RJ, Gardner JP, Dragic T. CD81 is an entry coreceptor for hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(19):7270–4. Epub 2004/05/05. 10.1073/pnas.0402253101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197(5):633–42. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petracca R, Falugi F, Galli G, Norais N, Rosa D, Campagnoli S, et al. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J Virol. 2000;74(10):4824–30. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nature medicine. 2011;17(5):589–95. Epub 2011/04/26. 10.1038/nm.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sainz B Jr, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nature medicine. 2012;18(2):281–5. Epub 2012/01/11. 10.1038/nm.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol. 2006;80(23):11571–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7271–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocquerel L, Meunier JC, Pillez A, Wychowski C, Dubuisson J. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J Virol. 1998;72(3):2183–91. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocquerel L, Duvet S, Meunier JC, Pillez A, Cacan R, Wychowski C, et al. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J Virol. 1999;73(4):2641–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocquerel L, Wychowski C, Minner F, Penin F, Dubuisson J. Charged residues in the transmembrane domains of hepatitis C virus glycoproteins play a major role in the processing, subcellular localization, and assembly of these envelope proteins. J Virol. 2000;74(8):3623–33. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocquerel L, Voisset C, Dubuisson J. Hepatitis C virus entry: potential receptors and their biological functions. J Gen Virol. 2006;87(Pt 5):1075–84. . [DOI] [PubMed] [Google Scholar]
- 16.Bartosch B, Cosset FL. Cell entry of hepatitis C virus. Virology. 2006;348(1):1–12. . [DOI] [PubMed] [Google Scholar]
- 17.Selby MJ, Glazer E, Masiarz F, Houghton M. Complex processing and protein:protein interactions in the E2:NS2 region of HCV. Virology. 1994;204(1):114–22. Epub 1994/10/01. S0042-6822(84)71515-7 [pii] 10.1006/viro.1994.1515 . [DOI] [PubMed] [Google Scholar]
- 18.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, et al. Robust hepatitis C virus infection in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9294–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Yang W, Shen L, Turner JR, Coyne CB, Wang T. Tight Junction Proteins Claudin-1 and Occludin Control Hepatitis C Virus Entry and are Downregulated during Infection to Prevent Superinfection. J Virol. 2009;83(4):2011–4. Epub 2008/12/03. JVI.01888-08 [pii] 10.1128/JVI.01888-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, et al. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379(6564):466–9. Epub 1996/02/01. 10.1038/379466a0 . [DOI] [PubMed] [Google Scholar]
- 21.Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436(7053):930–2. . [DOI] [PubMed] [Google Scholar]
- 22.Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168(3):477–88. Epub 2005/01/26. jcb.200407113 [pii] 10.1083/jcb.200407113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao B, Hong F, Radaeva S. Host factors and failure of interferon-alpha treatment in hepatitis C virus. Hepatology. 2004;39(4):880–90. . [DOI] [PubMed] [Google Scholar]
- 24.de Chassey B, Navratil V, Tafforeau L, Hiet MS, Aublin-Gex A, Agaugue S, et al. Hepatitis C virus infection protein network. Mol Syst Biol. 2008;4:230 Epub 2008/11/06. 10.1038/msb.2008.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nature medicine. 2005;11(7):791–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polier G, Neumann J, Thuaud F, Ribeiro N, Gelhaus C, Schmidt H, et al. The natural anticancer compounds rocaglamides inhibit the Raf-MEK-ERK pathway by targeting prohibitin 1 and 2. Chem Biol. 2012;19(9):1093–104. Epub 2012/09/25. 10.1016/j.chembiol.2012.07.012 . [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Wang W, Brown LE, Qiu C, Lajkiewicz N, Zhao T, et al. A Novel Class of Small Molecule Compounds that Inhibit Hepatitis C Virus Infection by Targeting the Prohibitin-CRaf Pathway. EBioMedicine. 2015. Epub September 17, 2015. 10.1016/j.ebiom.2015.09.018. [DOI] [PMC free article] [PubMed]
- 28.Dubuisson J, Rice CM. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J Virol. 1996;70(2):778–86. Epub 1996/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choukhi A, Ung S, Wychowski C, Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol. 1998;72(5):3851–8. Epub 1998/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. The New England journal of medicine. 1993;328(25):1797–801. Epub 1993/06/24. 10.1056/NEJM199306243282501 . [DOI] [PubMed] [Google Scholar]
- 31.Falkowska E, Kajumo F, Garcia E, Reinus J, Dragic T. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. J Virol. 2007;81(15):8072–9. Epub 2007/05/18. JVI.00459-07 [pii] 10.1128/JVI.00459-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flint M, Maidens C, Loomis-Price LD, Shotton C, Dubuisson J, Monk P, et al. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73(8):6235–44. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helle F, Goffard A, Morel V, Duverlie G, McKeating J, Keck ZY, et al. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81(15):8101–11. Epub 2007/05/25. JVI.00127-07 [pii] 10.1128/JVI.00127-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helle F, Vieyres G, Elkrief L, Popescu CI, Wychowski C, Descamps V, et al. Role of N-linked glycans in the functions of HCV envelope proteins incorporated into infectious virions. J Virol. 2010. Epub 2010/09/17. JVI.01548-10 [pii] 10.1128/JVI.01548-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parodi AJ. Protein glucosylation and its role in protein folding. Annu Rev Biochem. 2000;69:69–93. Epub 2000/08/31. 69/1/69 [pii] 10.1146/annurev.biochem.69.1.69 . [DOI] [PubMed] [Google Scholar]
- 36.Gouttenoire J, Penin F, Moradpour D. Hepatitis C virus nonstructural protein 4B: a journey into unexplored territory. Reviews in medical virology. 2010;20(2):117–29. Epub 2010/01/14. 10.1002/rmv.640 . [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Kuo W, Yang W, Liu W, Gibson GA, Dorko K, et al. The second extracellular loop dictates Occludin-mediated HCV entry. Virology. 2010;407(1):160–70. Epub 2010/09/09. 10.1016/j.virol.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cocquerel L, Kuo CC, Dubuisson J, Levy S. CD81-dependent binding of hepatitis C virus E1E2 heterodimers. Journal of virology. 2003;77(19):10677–83. Epub 2003/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flint M, Maidens C, Loomis-Price LD, Shotton C, Dubuisson J, Monk P, et al. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. Journal of virology. 1999;73(8):6235–44. Epub 1999/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan T, Beran RK, Peters C, Lorenz IC, Lindenbach BD. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J Virol. 2009;83(17):8379–95. Epub 2009/06/12. JVI.00891-09 [pii] 10.1128/JVI.00891-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi M, Ma Y, Yates J, Lemon SM. Trans-complementation of an NS2 defect in a late step in hepatitis C virus (HCV) particle assembly and maturation. PLoS Pathog. 2009;5(5):e1000403 Epub 2009/05/05. 10.1371/journal.ppat.1000403 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugle T, Fehrmann F, Bieck E, Kohara M, Krausslich HG, Rice CM, et al. The hepatitis C virus nonstructural protein 4B is an integral endoplasmic reticulum membrane protein. Virology. 2001;284(1):70–81. Epub 2001/05/16. 10.1006/viro.2001.0873 . [DOI] [PubMed] [Google Scholar]
- 43.Egger D, Wolk B, Gosert R, Bianchi L, Blum HE, Moradpour D, et al. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. Journal of virology. 2002;76(12):5974–84. Epub 2002/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundin M, Monne M, Widell A, Von Heijne G, Persson MA. Topology of the membrane-associated hepatitis C virus protein NS4B. Journal of virology. 2003;77(9):5428–38. Epub 2003/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welsch C, Albrecht M, Maydt J, Herrmann E, Welker MW, Sarrazin C, et al. Structural and functional comparison of the non-structural protein 4B in flaviviridae. J Mol Graph Model. 2007;26(2):546–57. Epub 2007/05/18. 10.1016/j.jmgm.2007.03.012 . [DOI] [PubMed] [Google Scholar]
- 46.Gouttenoire J, Penin F, Moradpour D. Hepatitis C virus nonstructural protein 4B: a journey into unexplored territory. Rev Med Virol. 2010;20(2):117–29. Epub 2010/01/14. 10.1002/rmv.640 . [DOI] [PubMed] [Google Scholar]
- 47.Murray CL, Jones CT, Rice CM. Architects of assembly: roles of Flaviviridae non-structural proteins in virion morphogenesis. Nat Rev Microbiol. 2008;6(9):699–708. Epub 2008/07/01. nrmicro1928 [pii] 10.1038/nrmicro1928 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimitrova M, Imbert I, Kieny MP, Schuster C. Protein-protein interactions between hepatitis C virus nonstructural proteins. J Virol. 2003;77(9):5401–14. Epub 2003/04/15. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao L, Aizaki H, He JW, Lai MM. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J Virol. 2004;78(7):3480–8. Epub 2004/03/16. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin C, Wu JW, Hsiao K, Su MS. The hepatitis C virus NS4A protein: interactions with the NS4B and NS5A proteins. J Virol. 1997;71(9):6465–71. Epub 1997/09/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paredes AM, Blight KJ. A genetic interaction between hepatitis C virus NS4B and NS3 is important for RNA replication. J Virol. 2008;82(21):10671–83. Epub 2008/08/22. JVI.00875-08 [pii] 10.1128/JVI.00875-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piccininni S, Varaklioti A, Nardelli M, Dave B, Raney KD, McCarthy JE. Modulation of the hepatitis C virus RNA-dependent RNA polymerase activity by the non-structural (NS) 3 helicase and the NS4B membrane protein. J Biol Chem. 2002;277(47):45670–9. Epub 2002/09/18. 10.1074/jbc.M204124200 M204124200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 53.Einav S, Elazar M, Danieli T, Glenn JS. A nucleotide binding motif in hepatitis C virus (HCV) NS4B mediates HCV RNA replication. J Virol. 2004;78(20):11288–95. Epub 2004/09/29. 10.1128/JVI.78.20.11288-11295.2004 78/20/11288 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramage HR, Kumar GR, Verschueren E, Johnson JR, Von Dollen J, Johnson T, et al. A combined proteomics/genomics approach links hepatitis C virus infection with nonsense-mediated mRNA decay. Mol Cell. 2015;57(2):329–40. Epub 2015/01/24. 10.1016/j.molcel.2014.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coller KE, Heaton NS, Berger KL, Cooper JD, Saunders JL, Randall G. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS pathogens. 2012;8(1):e1002466 Epub 2012/01/14. 10.1371/journal.ppat.1002466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Counihan NA, Rawlinson SM, Lindenbach BD. Trafficking of hepatitis C virus core protein during virus particle assembly. PLoS pathogens. 2011;7(10):e1002302 Epub 2011/10/27. 10.1371/journal.ppat.1002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vieyres G, Thomas X, Descamps V, Duverlie G, Patel AH, Dubuisson J. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. Journal of virology. 2010;84(19):10159–68. Epub 2010/07/30. 10.1128/JVI.01180-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Popescu CI, Callens N, Trinel D, Roingeard P, Moradpour D, Descamps V, et al. NS2 protein of hepatitis C virus interacts with structural and non-structural proteins towards virus assembly. PLoS pathogens. 2011;7(2):e1001278 Epub 2011/02/25. 10.1371/journal.ppat.1001278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stapleford KA, Lindenbach BD. Hepatitis C virus NS2 coordinates virus particle assembly through physical interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. Journal of virology. 2011;85(4):1706–17. Epub 2010/12/15. 10.1128/JVI.02268-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douam F, Dao Thi VL, Maurin G, Fresquet J, Mompelat D, Zeisel MB, et al. Critical interaction between E1 and E2 glycoproteins determines binding and fusion properties of hepatitis C virus during cell entry. Hepatology. 2014;59(3):776–88. Epub 2013/09/17. 10.1002/hep.26733 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLS)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.