Abstract
In the south of France, Leishmania infantum is responsible for numerous cases of canine leishmaniasis (CanL), sporadic cases of human visceral leishmaniasis (VL) and rare cases of cutaneous and muco-cutaneous leishmaniasis (CL and MCL, respectively). Several endemic areas have been clearly identified in the south of France including the Pyrénées-Orientales, Cévennes (CE), Provence (P), Alpes-Maritimes (AM) and Corsica (CO). Within these endemic areas, the two cities of Nice (AM) and Marseille (P), which are located 150 km apart, and their surroundings, concentrate the greatest number of French autochthonous leishmaniasis cases. In this study, 270 L. infantum isolates from an extended time period (1978–2011) from four endemic areas, AM, P, CE and CO, were assessed using Multi-Locus Microsatellite Typing (MLMT). MLMT revealed a total of 121 different genotypes with 91 unique genotypes and 30 repeated genotypes. Substantial genetic diversity was found with a strong genetic differentiation between the Leishmania populations from AM and P. However, exchanges were observed between these two endemic areas in which it seems that strains spread from AM to P. The genetic differentiations in these areas suggest strong epidemiological structuring. A model-based analysis using STRUCTURE revealed two main populations: population A (consisting of samples primarily from the P and AM endemic areas with MON-1 and non-MON-1 strains) and population B consisting of only MON-1 strains essentially from the AM endemic area. For four patients, we observed several isolates from different biological samples which provided insight into disease relapse and re-infection. These findings shed light on the transmission dynamics of parasites in humans. However, further data are required to confirm this hypothesis based on a limited sample set. This study represents the most extensive population analysis of L. infantum strains using MLMT conducted in France.
Author Summary
In the south of France, the parasite Leishmania infantum is responsible for diseases that primarily affect dogs but can also impact humans. Several endemic areas have been clearly identified in the south of France including the Pyrénées-Orientales, Cévennes (CE), Provence (P), Alpes-Maritimes (AM) and Corsica (CO). In this study, 270 L. infantum isolates from four endemic areas, AM, P, CE and CO, were assessed using Multi-Locus Microsatellite Typing (MLMT), a tool applied for population genetic studies. MLMT revealed a strong genetic differentiation between the Leishmania populations from AM and P with exchanges observed between these two endemic areas. For four patients, the occurrence of disease relapses and re-infections was examined. These findings shed light on the transmission dynamics of parasites in humans. This study represents the most extensive population analysis of L. infantum isolates using MLMT conducted in France.
Introduction
Leishmaniases are a group of diseases caused by obligatory intracellular protozoan parasites of the genus Leishmania. Among the species of Leishmania, Leishmania infantum is mostly responsible for canine leishmaniasis (CanL), although it also causes sporadic cases of human visceral leishmaniasis (VL), and rare cases of cutaneous and muco-cutaneous leishmaniasis (CL and MCL) throughout the Mediterranean basin [1]. Transmission to humans is caused by the bite of infected phlebotomine sandflies, and dogs are considered to be the principal domestic reservoir. In France, the parasite is currently only endemic in the south of France, along the Mediterranean coast, where several foci have been clearly identified: Pyrénées-Orientales, Cévennes (CE), Provence (P), Alpes-Maritimes (AM) and Corsica (CO) [2]. In the Provence-Alpes-Côtes d’Azur (PACA) region, which comprises the AM and P endemic areas, transmission has been reported for 100 years [3,4]. The two cities of Nice and Marseille, which are located 150 km apart, and their surroundings concentrate the greatest number of French autochthonous leishmaniasis cases [2,5]. Although the same species of L. infantum (primarily zymodeme MON-1), the same predominant vector (Phlebotomus perniciosus) and the same and unique reservoir (dog) are found in both regions, the transmission environment of VL is heterogeneous in these two foci [5]. Disease transmission in Nice and the surrounding area is associated with scattered habitation and mixed forest in the foothills [5]. In contrast, around Marseille, VL transmission is associated with an urban environment [5]. Regarding the main vector; Phlebotomus pernicious; the population is quite homogeneous and belongs mainly to the same haplogroup (for 88% pern01) in Provence, France [6]. The isolates of L. infantum from AM and P endemic foci have been characterized using Multi-Locus Enzyme Electrophoresis (MLEE), which is the current reference method. However, MLEE based analyses are limited at the intrinsic level of polymorphisms. Thus, differentiating between isolates in PACA region is impossible using the MLEE method [7]. Epidemiological studies on L. infantum require the use of highly discriminative techniques that can differentiate between MON-1 strains. Multi-Locus Microsatellite Typing (MLMT) has been shown to be a powerful tool for population genetics and epidemiological studies of Leishmania spp. [8]. This tool has been already applied to genotype L. infantum isolates from healthy blood donors, sandflies, dogs and human patients in Southern France [9]. Genetic differentiations were evidenced between asymptomatic carrier strains and non-asymptomatic carrier strains and especially between asymptomatic carrier and HIV+ populations [9]. However, due to the weak sample size, these results must be confirmed on a larger sample set [9].
In the current study, microsatellite markers were used to analyze the genetic diversity of L. infantum parasites from Southeast France, with a focus on the PACA region. We assessed an extensive panel of isolates from an extended time period (1978–2011), from the two endemic regions of AM and P. The geographical and temporal distributions of genotypes were examined. The microsatellite profiles were used to assess relapse and re-infection among patients as well as the association between genotype and the various clinical forms of the disease.
Materials and Methods
Parasites
The L. infantum isolates used in this study were obtained from the collection of the Centre National de Référence des Leishmania (Leishmania collection, BRC-Leish, Montpellier, France, BioBank N° BB-0033-00052). All human and animal samples had been isolated from patients and animals as part of routine diagnosis and treatment with no unnecessary invasive procedures. A total of 270 L. infantum isolates from the south of France were included in this study (Table 1). This panel included 247 human isolates from 239 patients (four patients harbored more than one isolate), 20 from CanL, one from feline leishmaniasis and two from sandflies. Among the 239 patients, there were 154 adult VL cases, 58 infant VL cases, 13 CL cases (five infants, seven adults and one unknown), three MCL adult cases, nine asymptomatic carriers (in adults) and two unknown cases (one adult and one unknown). Among the 247 human samples, 139 were isolated from immunocompetent patients, 95 from immunocompromised HIV+ patients, 11 from immunocompromised patients other than HIV+ (e.g., renal transplantation, lymphoma, auto immunity disease and cancer) and two from unknown cases. The location of isolates based on their position relative to the Vars River (S1 Table) was used for genetic differentiation analysis.
Table 1. Designation and characteristics of Leishmania infantum isolates used in this study.
| Endemic area | Patients | Sample WHO code | Y | C F / H | HIV status | Zymo | G | P | Sub-Pop A | Sub-Pop A | Sub-Pop B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| K = 6 | K = 4 | K = 2 | |||||||||
| P | Patient 1 | MHOM/FR/94/LPM135 | 1994 | VL | HIV | MON-1 | 1 | A | 6 | 3 | |
| AM | Patient 2 | MHOM/FR/90/LPN63 | 1990 | VL | HIV | MON-24 | 2 | A | 6 | 3 | |
| AM | Patient 3 | MHOM/FR/2002/LPN202 | 2002 | IVL | MON-1 | 3 | A | 4 | 1 | ||
| P | MCAN/FR/2000/LPM206 | 2000 | CanL | MON-1 | 4 | A | 4 | 1 | |||
| AM | Patient 4 | MHOM/FR/2003/LPN212 | 2003 | VL | HIV | MON-1 | 4 | A | 4 | 1 | |
| AM | Patient 4 | MHOM/FR/2004/LPN243 | 2004 | RVL | HIV | MON-1 | 4 | A | 4 | 1 | |
| AM | Patient 5 | MHOM/FR/90/LPN67 | 1990 | VL | HIV | MON-1 | 5 | A | 4 | 1 | |
| AM | Patient 6 | MHOM/FR/96/LPN126 | 1996 | IVL | MON-1 | 6 | A | 4/3 | 1 | ||
| P | Patient 7 | MHOM/FR/93/LPM110 | 1993 | VL | HIV | MON-11 | 7 | A | 4/6 | 1/3 | |
| AM | Patient 8 | MHOM/FR/90/LPN62 | 1990 | VL | MON-1 | 8 | B | 1 | |||
| AM | Patient 9 | MHOM/FR/98/LPN163 | 1998 | IVL | MON-1 | 9 | B | 1 | |||
| P | Patient 10 | MHOM/FR/96/LPM150 | 1996 | VL | HIV | MON-1 | 10 | A | 4/5 | 1/2 | |
| AM | Patient 11 | MHOM/FR/2001/LPN192 | 2001 | IVL | MON-1 | 11 | B/A | ||||
| CE | MCAN/FR/2006/LPN278 | 2006 | CanL | MON-1 | 12 | A | 4/5 | 1/2 | |||
| P | Patient 12 | MHOM/FR/97/LPM180 | 1997 | VL | HIV | MON-1 | 13 | A | 5 | 2 | |
| P | MCAN/FR/2000/LPM207 | 2000 | CanL | MON-1 | 13 | A | 5 | 2 | |||
| P | Patient 13 | MHOM/FR/2009/LPM262 | 2009 | VL | HIV | MON-1 | 13 | A | 5 | 2 | |
| P | Patient 14 | MHOM/FR/2000/LPM197 | 2000 | VL | HIV | MON-1 | 14 | A | 5 | 2 | |
| AM | Patient 15 | MHOM/FR/97/LPN161 | 1997 | CL | MON-1 | 15 | A | 1 | 4 | ||
| CO | Patient 16 | MHOM/FR/97/LPN154 | 1997 | VL | MON-1 | 16 | A | 6 | 3 | ||
| P | Patient 17 | MHOM/FR/2002/LPM225 | 2002 | VL | HIV | MON-1 | 17 | A | 5 | 2 | |
| P | Patient 18 | MHOM/FR/2002/LPM215 | 2002 | VL | HIV | MON-1 | 17 | A | 5 | 2 | |
| P | Patient 19 | MHOM/FR/2000/LPM 195 | 2000 | VL | HIV | MON-1 | 18 | A | 2/5/3 | 1/3/2 | |
| AM | Patient 20 | MHOM/FR/86/LPN30 | 1986 | VL | HIV | MON-1 | 19 | A/B | |||
| AM | Patient 21 | MHOM/FR/88/LPN50 | 1988 | VL | HIV | MON-1 | 20 | A | 5 | 2 | |
| AM | Patient 29 | MHOM/FR/84/LPN23 | 1984 | VL | MON-1 | 21 | A | 5 | 2 | ||
| AM | Patient 30 | MHOM/FR/85/LPN25a | 1985 | VL | MON-1 | 21 | A | 5 | 2 | ||
| AM | Patient 31 | MHOM/FR/94/LPN112 | 1994 | VL | MON-1 | 21 | A | 5 | 2 | ||
| P | Patient 22 | MHOM/FR/96/LPM151 | 1996 | VL | HIV | MON-1 | 21 | A | 5 | 2 | |
| AM | Patient 32 | MHOM/FR/96/LPN132 | 1996 | VL | MON-1 | 21 | A | 5 | 2 | ||
| P | Patient 23 | MHOM/FR/97/LPM169 | 1997 | VL | HIV | MON-1 | 21 | A | 5 | 2 | |
| P | Patient 24 | MHOM/FR/97/LPM172 | 1997 | VL | HIV | MON-1 | 21 | A | 5 | 2 | |
| P | Patient 35 | MHOM/FR/2004/LPN228 | 2004 | IVL | MON-1 | 21 | A | 5 | 2 | ||
| P | Patient 25 | MHOM/FR/2005/LPM242 | 2005 | IVL | MON-1 | 21 | A | 5 | 2 | ||
| P | Patient 26 | MHOM/FR/2005/LPM247 | 2005 | IVL | MON-1 | 21 | A | 5 | 2 | ||
| P | Patient 27 | MHOM/FR/2009/LPM261 | 2009 | IVL | MON-1 | 21 | A | 5 | 2 | ||
| AM | MCAN/FR/95/LPN122* | CanL | MON-1 | 21 | A | 5 | 2 | ||||
| AM | MCAN/FR/95/LPN123* | CanL | MON-1 | 21 | A | 5 | 2 | ||||
| AM | MCAN/FR/95/LPN124* | CanL | MON-1 | 21 | A | 5 | 2 | ||||
| AM | Patient 33 | MHOM/FR/96/LPN136* | 1996 | AC | MON-1 | 21 | A | 5 | 2 | ||
| AM | Patient 34 | MHOM/FR/96/LPN131* | 1996 | AC | MON-1 | 21 | A | 5 | 2 | ||
| AM | Patient 28 | MHOM/FR/81/LPN5 | 1981 | IVL | MON-1 | 21 | A | 5 | 2 | ||
| AM | Patient 36 | n. d. | 2009 | IVL | MON-1 | 21 | A | 5 | 2 | ||
| AM | Patient 37 | MHOM/FR/90/LPN61 | 1990 | IVL | MON-1 | 22 | A/B | ||||
| AM | Patient 38 | MHOM/FR/2002/LPN199 | 2002 | VL | MON-1 | 23 | A | 3 | 1 | ||
| P | Patient 39 | MHOM/FR/2007/LPM255 | 2007 | IVL | MON-1 | 24 | A | 4/5/2 | 1 | ||
| P | Patient 40 | MHOM/FR/2002/LPM216 | 2002 | VL | HIV | MON-1 | 25 | A | 4/3/2 | 1 | |
| P | Patient 41 | MHOM/FR/2002/LPM217 | 2002 | VL | MON-1 | 25 | A | 4/3/2 | 1 | ||
| P | Patient 42 | MHOM/FR/98/LPM185 | 1998 | VL | HIV | MON-1 | 26 | A | 4/5/6 | 1 | |
| CO | Patient 43 | MHOM/FR/2006/LPM251 | 2006 | IVL | MON-24 | 27 | A | 2 | 1 | ||
| AM | Patient 44 | MHOM/FR/2001/LPN195 | 2001 | VL | MON-1 | 28 | A | 5/2/4 | 1/2 | ||
| AM | Patient 45 | MHOM/FR/2007/LPN312 | 2007 | IVL | MON-1 | 29 | A | 3 | 1 | ||
| AM | MCAN/FR/86/LPN28 | 1986 | CanL | MON-1 | 30 | A | 3 | 1 | |||
| P | Patient 46 | MHOM/FR/2003/LPN221 | 2003 | CL | MON-1 | 31 | A | 3/2 | 1 | ||
| AM | Patient 47 | MHOM/FR/97/LPN159 | 1997 | VL | MON-1 | 32 | B/A | ||||
| AM | Patient 48 | MHOM/FR/90/LPN64 | 1990 | VL | HIV | MON-1 | 33 | A | 3 | 1 | |
| AM | Patient 49 | MHOM/FR/89/LPN54 | 1989 | VL | MON-1 | 34 | B | 1 | |||
| AM | Patient 50 | MHOM/FR/91/LPN70 | 1991 | IVL | MON-1 | 34 | B | 1 | |||
| AM | Patient 51 | MHOM/FR/92/LPN78 | 1992 | VL | MON-1 | 34 | B | 1 | |||
| AM | Patient 52 | MHOM/FR/95/LPN116 | 1995 | VL | HIV | MON-1 | 34 | B | 1 | ||
| AM | Patient 53 | MHOM/FR/96/LPN141 | 1996 | VL | MON-1 | 34 | B | 1 | |||
| AM | Patient 54 | MHOM/FR/97/LPN150 | 1997 | IVL | MON-1 | 34 | B | 1 | |||
| AM | Patient 55 | MHOM/FR/2001/LPN181 | 2001 | IVL | MON-1 | 34 | B | 1 | |||
| AM | Patient 56 | MHOM/FR/2007/LPN313 | 2007 | CL | MON-1 | 34 | B | 1 | |||
| AM | Patient 57 | MHOM/FR/2011/LPN358 | 2011 | VL | MON-1 | 34 | B | 1 | |||
| AM | MCAN/FR/89/LPN57 | 1989 | CanL | MON-1 | 35 | A/B | |||||
| AM | Patient 58 | MHOM/FR/95/LPN120 | 1995 | VL | HIV | MON-1 | 35 | A/B | |||
| AM | Patient 60 | MHOM/FR/92/LPN84 | 1992 | IVL | MON-1 | 36 | A | 3 | 1 | ||
| AM | Patient 61 | MHOM/FR/94/LPN103 | 1994 | CL | MON-1 | 36 | A | 3 | 1 | ||
| AM | Patient 62 | MHOM/FR/95/LPN115 | 1995 | IVL | MON-1 | 36 | A | 3 | 1 | ||
| AM | Patient 63 | MHOM/FR/95/LPN119 | 1995 | VL | HIV | MON-1 | 36 | A | 3 | 1 | |
| CO | Patient 59 | MHOM/FR/96/LPM157 | 1996 | VL | MON-1 | 36 | A | 3 | 1 | ||
| AM | Patient 64 | MHOM/FR/2001/LPN187 | 2001 | VL | MON-1 | 36 | A | 3 | 1 | ||
| AM | Patient 64 | MHOM/FR/2002/LPN198 | 2002 | RVL | MON-1 | 36 | A | 3 | 1 | ||
| P | Patient 65 | MHOM/FR/94/LPM112 | 1994 | VL | HIV | MON-1 | 37 | A | 4/5/1 | 1/2/4 | |
| P | Patient 66 | MHOM/FR/2006/LPM250 | 2006 | VL | MON-1 | 37 | A | 4/5/1 | 1/2/4 | ||
| AM | Patient 67 | MHOM/FR/93/LPN92 | 1993 | VL | HIV | MON-1 | 38 | A | 2/1/3 | 3/4/1 | |
| P | Patient 68 | MHOM/FR/97/LPM173 | 1997 | VL | MON-1 | 39 | B | 1 | |||
| AM | Patient 69 | MHOM/FR/86/LPN29 | 1986 | VL | HIV | MON-1 | 40 | A/B | |||
| AM | Patient 71 | MHOM/FR/87/LPN33 | 1987 | IVL | MON-1 | 41 | A/B | ||||
| AM | MCAN/FR/87/LPN34 | 1987 | CanL | MON-1 | 41 | A/B | |||||
| AM | Patient 72 | MHOM/FR/89/LPN51 | 1989 | VL | HIV | MON-1 | 41 | A/B | |||
| AM | Patient 73 | MHOM/FR/98/LPN164 | 1998 | VL | HIV | 41 | A/B | ||||
| AM | Patient 70 | MHOM/FR/2005/LPM244 | 2005 | VL | HIV | MON-1 | 41 | A/B | |||
| AM | Patient 74 | MHOM/FR/96/LPN146 | 1996 | VL | HIV | MON-1 | 42 | B | 2 | ||
| AM | Patient 75 | MHOM/TR/94/LPN101 | 1994 | IVL | MON-1 | 43 | B | 1 | |||
| AM | Patient 76 | MHOM/FR/94/LPN104 | 1994 | IVL | MON-1 | 43 | B | 1 | |||
| AM | Patient 77 | MHOM/FR/95/LPN113 | 1995 | IVL | MON-1 | 43 | B | 1 | |||
| AM | Patient 78 | MHOM/FR/95/LPN121 | 1995 | VL | HIV | MON-1 | 43 | B | 1 | ||
| AM | Patient 86 | MHOM/FR/2002/LPN201 | 2002 | RVL | HIV | MON-1 | 43 | B | 1 | ||
| AM | Patient 87 | MHOM/FR/2004/LPN233 | 2004 | VL | HIV | MON-1 | 43 | B | 1 | ||
| AM | Patient 79 | MHOM/FR/96/LPN134* | 1996 | AC | MON-1 | 43 | B | 1 | |||
| AM | Patient 80 | MHOM/FR/96/LPN137* | 1996 | AC | MON-1 | 43 | B | 1 | |||
| AM | Patient 81 | MHOM/FR/96/LPN138* | 1996 | AC | MON-1 | 43 | B | 1 | |||
| AM | Patient 82 | MHOM/FR/96/LPN144* | 1996 | AC | MON-1 | 43 | B | 1 | |||
| AM | Patient 83 | MHOM/FR/96/LPN143* | 1996 | AC | MON-1 | 43 | B | 1 | |||
| AM | Patient 84 | MHOM/FR/96/LPN142* | 1996 | AC | MON-1 | 43 | B | 1 | |||
| AM | Patient 85 | MHOM/IT/96/LPN145* | 1996 | AC | MON-1 | 43 | B | 1 | |||
| AM | Patient 88 | MHOM/FR/80/LPN3 | 1980 | IVL | MON-1 | 44 | B | 1 | |||
| AM | Patient 89 | MHOM/00/94/LPN108 | 1994 | VL | HIV | MON-1 | 44 | B | 1 | ||
| AM | Patient 90 | MHOM/FR/2004/LPN236 | 2004 | VL | MON-1 | 45 | B | 1 | |||
| AM | MCAN/FR/82/LPN16 | 1983 | CanL | MON-1 | 46 | B | 1 | ||||
| AM | Patient 91 | MHOM/FR/83/LPN19 | 1983 | VL | MON-1 | 46 | B | 1 | |||
| AM | Patient 92 | MHOM/FR/84/LPN20 | 1984 | VL | MON-1 | 46 | B | 1 | |||
| AM | Patient 93 | MHOM/FR/85/LPN24 | 1985 | VL | MON-1 | 46 | B | 1 | |||
| AM | MCAN/FR/87/LPN37 | 1987 | CanL | MON-1 | 46 | B | 1 | ||||
| AM | Patient 94 | MHOM/FR/88/LPN46 | 1988 | VL | MON-1 | 46 | B | 1 | |||
| AM | Patient 95 | MHOM/FR/89/LPN59 | 1989 | VL | HIV | MON-1 | 46 | B | 1 | ||
| AM | Patient 96 | MHOM/FR/93/LPN91 | 1993 | IVL | MON-1 | 46 | B | 1 | |||
| AM | Patient 97 | MHOM/00/93/LPN94 | 1993 | VL | HIV | MON-1 | 46 | B | 1 | ||
| AM | Patient 98 | MHOM/FR/95/LPN107 | 1994 | IVL | MON-1 | 46 | B | 1 | |||
| AM | Patient 99 | MHOM/FR/95/LPN114 | 1994 | IVL | MON-1 | 46 | B | 1 | |||
| AM | Patient 100 | MHOM/FR/95/LPN118 | 1995 | VL | HIV | MON-1 | 46 | B | 1 | ||
| AM | Patient 101 | MHOM/FR/96/LPN125 | 1996 | VL | MON-1 | 46 | B | 1 | |||
| AM | Patient 102 | MHOM/FR/97/LPN148 | 1997 | VL | HIV | MON-1 | 46 | B | 1 | ||
| P | Patient 105 | MHOM/FR/97/LPM139 | 1995 | VL | HIV | MON-1 | 46 | B | 1 | ||
| AM | Patient 103 | MHOM/FR/97/LPN152 | 1997 | VL | MON-1 | 46 | B | 1 | |||
| AM | Patient 104 | MHOM/FR/2001/LPN180 | 2001 | IVL | MON-1 | 46 | B | 1 | |||
| AM | Patient 106 | MHOM/FR/2005/LPN257 | 2005 | IVL | MON-1 | 47 | B | 1 | |||
| AM | Patient 107 | MHOM/FR/84/LPN21 | 1984 | VL | MON-1 | 48 | B | 1 | |||
| AM | Patient 108 | MHOM/FR/92/LPN85 | 1992 | IVL | MON-1 | 48 | B | 1 | |||
| AM | Patient 109 | MHOM/FR/2004/LPN240 | 2004 | VL | MON-1 | 48 | B | 1 | |||
| AM | Patient 110 | n. d. | 2011 | IVL | MON-1 | 49 | B | 1 | |||
| AM | Patient 111 | MHOM/FR/92/LPN86 | 1992 | IVL | MON-1 | 50 | B | 2 | |||
| AM | Patient 112 | MHOM/FR/2001/LPN191 | 2001 | VL | HIV | MON-1 | 50 | B | 2 | ||
| AM | Patient 113 | MHOM/FR/2008/LPN321 | 2008 | VL | MON-1 | 50 | B | 2 | |||
| AM | Patient 114 | MHOM/FR/99/LPN170 | 1999 | VL | MON-1 | 51 | B | 2 | |||
| AM | Patient 115 | MHOM/FR/2006/LPN281 | 2006 | VL | MON-1 | 52 | B | 2 | |||
| CE | MCAN/FR/2006/LPN285 | 2006 | CanL | MON-1 | 52 | B | 2 | ||||
| AM | Patient 116 | MHOM/FR/87/LPN36 | 1987 | VL | HIV | MON-1 | 53 | B | 2 | ||
| AM | Patient 117 | MHOM/FR/91/LPN71 | 1991 | VL | HIV | MON-1 | 53 | B | 2 | ||
| AM | Patient 118 | MHOM/FR/93/LPN96 | 1992 | VL | MON-1 | 53 | B | 2 | |||
| AM | Patient 119 | MHOM/FR/93/LPN99 | 1993 | VL | HIV | MON-1 | 53 | B | 2 | ||
| AM | MCAN/FR/94/LPN102 | 1994 | CanL | MON-1 | 53 | B | 2 | ||||
| AM | Patient 120 | MHOM/FR/95/LPN117 | 1995 | VL | MON-1 | 53 | B | 2 | |||
| AM | Patient 121 | MHOM/FR/2001/LPN189 | 2001 | VL | MON-1 | 53 | B | 2 | |||
| AM | Patient 122 | MHOM/FR/78/LPN1 | 1978 | VL | MON-1 | 54 | B | 2 | |||
| AM | MCAN/FR/82/LPN6 | 1982 | CanL | MON-1 | 55 | B | 2 | ||||
| AM | Patient 123 | MHOM/FR/84/LPN22 | 1983 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 124 | MHOM/FR/86/LPN31 | 1986 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 125 | MHOM/FR/88/LPN48 | 1988 | CL | MON-1 | 55 | B | 2 | |||
| AM | Patient 126 | MHOM/FR/89/LPN55 | 1989 | IVL | MON-1 | 55 | B | 2 | |||
| AM | Patient 127 | MHOM/FR/90/LPN68 | 1990 | IVL | MON-1 | 55 | B | 2 | |||
| AM | Patient 128 | MHOM/FR/92/LPN76 | 1992 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 129 | MHOM/FR/92/LPN77 | 1992 | VL | HIV | MON-1 | 55 | B | 2 | ||
| AM | Patient 130 | MHOM/FR/92/LPN80 | 1992 | VL | HIV | MON-1 | 55 | B | 2 | ||
| AM | Patient 131 | MHOM/FR/92/LPN82 | 1992 | VL | HIV | MON-1 | 55 | B | 2 | ||
| CO | Patient 146 | MHOM/FR/93/LPN95 | 1993 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 132 | MHOM/FR/94/LPN106 | 1994 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 133 | MHOM/FR/94/LPN109 | 1994 | VL | HIV | MON-1 | 55 | B | 2 | ||
| AM | Patient 135 | MHOM/FR/96/LPN133 | 1996 | IVL | MON-1 | 55 | B | 2 | |||
| AM | Patient 134 | MHOM/FR/96/LPN130 | 1996 | VL | MON-1 | 55 | B | 2 | |||
| AM | MFEL/FR/96/LPN139 | 1996 | CatL | MON-1 | 55 | B | 2 | ||||
| AM | Patient 136 | MHOM/FR/97/LPN155 | 1997 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 137 | MHOM/FR/97/LPN158 | 1997 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 139 | MHOM/FR/2000/LPN176 | 2000 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 138 | MHOM/FR/2000/LPN175 | 2000 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 140 | MHOM/FR/2003/LPN209 | 2003 | IVL | MON-1 | 55 | B | 2 | |||
| AM | Patient 141 | MHOM/FR/2003/LPN217 | 2003 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 142 | MHOM/FR/2006/LPN259 | 2006 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 143 | MHOM/FR/2006/LPN282 | 2006 | IVL | MON-1 | 55 | B | 2 | |||
| AM | Patient 144 | MHOM/FR/2007/LPN316 | 2007 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 145 | MHOM/FR/2011/LPN357 | 2011 | VL | MON-1 | 55 | B | 2 | |||
| AM | Patient 147 | MHOM/FR/96/LPN127 | 1996 | VL | HIV | MON-1 | 56 | B | 2 | ||
| AM | MCAN/FR/82/LPN10 | 1982 | CanL | MON-1 | 57 | B | 2 | ||||
| AM | Patient 148 | MHOM/FR/89/LPN53 | 1989 | VL | MON-1 | 57 | B | 2 | |||
| AM | Patient 149 | MHOM/FR/90/LPN66 | 1990 | VL | MON-1 | 57 | B | 2 | |||
| AM | Patient 150 | MHOM/FR/93/LPN90 | 1993 | VL | MON-1 | 57 | B | 2 | |||
| AM | Patient 151 | MHOM/FR/93/LPN93 | 1993 | IVL | MON-1 | 57 | B | 2 | |||
| AM | Patient 152 | MHOM/FR/93/LPN98 | 1993 | VL | HIV | MON-1 | 57 | B | 2 | ||
| AM | Patient 153 | MHOM/FR/94/LPN110 | 1994 | VL | HIV | MON-1 | 57 | B | 2 | ||
| AM | Patient 154 | MHOM/FR/96/LPN140 | 1996 | IVL | MON-1 | 57 | B | 2 | |||
| AM | Patient 155 | MHOM/FR/97/LPN156 | 1997 | VL | HIV | MON-1 | 57 | B | 2 | ||
| AM | Patient 156 | MHOM/FR/2000/LPN178 | 2000 | IVL | MON-1 | 57 | B | 2 | |||
| AM | Patient 157 | MHOM/FR/2001/LPN190 | 2001 | IVL | MON-1 | 57 | B | 2 | |||
| AM | Patient 158 | MHOM/FR/2006/LPN277 | 2006 | VL | MON-1 | 57 | B | 2 | |||
| AM | Patient 159 | MHOM/FR/2011/LPN351 | 2011 | IVL | MON-1 | 57 | B | 2 | |||
| AM | Patient 160 | MHOM/FR/2011/LPN356 | 2011 | IVL | MON-1 | 57 | B | 2 | |||
| AM | Patient 161 | MHOM/FR/2011/LPN366 | 2011 | IVL | MON-1 | 57 | B | 2 | |||
| AM | Patient 162 | MHOM/FR/2004/LPN237 | 2004 | VL | MON-1 | 58 | B | 2 | |||
| CO | Patient 163 | MHOM/FR/94/LPN105 | 1994 | VL | HIV | MON-1 | 59 | B | 1 | ||
| P | Patient 164 | MHOM/FR/96/LPM154 | 1996 | IVL | MON-1 | 60 | A | 3/4/5/2 | 1 | ||
| P | Patient 165 | MHOM/FR/97/LPM174 | 1997 | VL | HIV | MON-1 | 61 | A | 4/3/2 | 1 | |
| AM | Patient 166 | MHOM/FR/99/LPN173 | 1999 | IVL | MON-1 | 62 | A/B | ||||
| P | Patient 167 | MHOM/FR/97/LPM170 | 1997 | IVL | MON-1 | 63 | A | 1 | 4 | ||
| P | Patient 168 | MHOM/FR/97/LPM177 | 1997 | VL | HIV | MON-1 | 63 | A | 1 | 4 | |
| P | Patient 169 | MHOM/FR/2000/LPM201 | 2000 | VL | HIV | MON-1 | 63 | A | 1 | 4 | |
| P | Patient 170 | MHOM/FR/2001/LPM209 | 2001 | VL | HIV | MON-1 | 64 | A | 1/3/4/2 | 4/1/2 | |
| P | Patient 171 | MHOM/FR/97/LPM166 | 1997 | VL | HIV | MON-1 | 65 | A | 1 | 4 | |
| P | Patient 172 | MHOM/FR/2004/LPM236 | 2004 | IVL | MON-1 | 66 | A | 1 | 4 | ||
| P | Patient 173 | MHOM/FR/2006/LPM252 | 2006 | VL | MON-1 | 67 | A | 1 | 4 | ||
| P | Patient 174 | MHOM/FR/94/LPM116 | 1994 | VL | HIV | MON-1 | 68 | A | 1 | 4 | |
| P | Patient 175 | MHOM/FR/96/LPM138 | 1996 | VL | HIV | MON-1 | 68 | A | 1 | 4 | |
| P | Patient 176 | MHOM/FR/99/LPM189 | 1999 | IVL | MON-1 | 68 | A | 1 | 4 | ||
| P | Patient 177 | MHOM/FR/2000/LPM204 | 2000 | IVL | MON-1 | 68 | A | 1 | 4 | ||
| P | Patient 178 | MHOM/FR/98/LPM183 | 1998 | VL | HIV | MON-1 | 69 | A | 1/3/2 | 4 | |
| P | Patient 179 | MHOM/FR/97/LPM178 | 1997 | UK | MON-1 | 69 | A | 1/3/2 | 4 | ||
| P | Patient 180 | MHOM/FR/2003/LPM232 | 2003 | IVL | MON-1 | 70 | A | 1 | 4 | ||
| P | Patient 181 | MHOM/FR/96/LPM152 | 1996 | VL | MON-1 | 71 | A | 1/3/2 | 4 | ||
| P | Patient 182 | MHOM/FR/97/LPM168 | 1997 | VL | HIV | MON-1 | 72 | A | 1 | 4 | |
| P | Patient 183 | MHOM/FR/98/LPM181 | 1998 | MCL | MON-1 | 73 | A | 5 | 2 | ||
| P | Patient 184 | MHOM/FR/96/LPM156 | 1996 | IVL | MON-1 | 74 | A | 5/1/4/2 | 2/4/1 | ||
| CE | IPER/FR/84/LEM576* | 1984 | PHLE | MON-1 | 75 | A | 4/1/2 | 1/4 | |||
| CE | IARI/FR/84/LEM595* | 1984 | PHLE | MON-1 | 75 | A | 4/1/2 | 1/4 | |||
| AM | Patient 185 | MHOM/FR/96/LPN129 | 1996 | VL | HIV | MON-1 | 76 | A | 1 | 4 | |
| AM | Patient 186 | MHOM/FR/88/LPN45 | 1988 | VL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 187 | MHOM/FR/89/LPN58 | 1989 | IVL | MON-1 | 77 | A | 1 | 4 | ||
| AM | Patient 188 | MHOM/FR/91/LPN69 | 1991 | VL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 189 | MHOM/FR/96/LPN128 | 1996 | VL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 190 | MHOM/FR/97/LPN151 | 1996 | VL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 86 | MHOM/FR/97/LPN153 | 1997 | VL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | MCAN/FR/98/LPN168 | 1998 | CanL | 77 | A | 1 | 4 | ||||
| AM | Patient 86 | MHOM/FR/2000/LPN177 | 2000 | RVL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 86 | MHOM/FR/2001/LPN185 | 2001 | RVL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 191 | MHOM/FR/2001/LPN186 | 2001 | VL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 86 | MHOM/FR/2003/LPN215 | 2003 | RVL | HIV | MON-1 | 77 | A | 1 | 4 | |
| AM | Patient 192 | MHOM/FR/2007/LPN314* | 2007 | VL | HIV | MON-1 | 78 | A | 1/6 | 4/3/1 | |
| AM | MCAN/FR/2006/LPN267 | 2006 | CanL | MON-1 | 79 | A | 1/6/3/4 | 4/2/3 | |||
| P | Patient 193 | MHOM/FR/2002/LPM222 | 2002 | VL | HIV | MON-1 | 80 | A | 1/5/6 | 4/2 | |
| P | Patient 194 | MHOM/FR/2001/LPM214 | 2001 | VL | MON-1 | 81 | A | 2/4 | 1 | ||
| P | Patient 195 | MHOM/FR/2002/LPM226 | 2002 | VL | HIV | MON-1 | 82 | A | 2/4 | 1 | |
| P | Patient 196 | MHOM/FR/2002/LPM228 | 2002 | VL | MON-1 | 83 | A | 2/4 | 1 | ||
| P | Patient 197 | MHOM/FR/96/LPM155 | 1996 | VL | MON-1 | 84 | A | 2/3/1 | 1 | ||
| P | Patient 198 | MHOM/FR/96/LPM159 | 1996 | VL | MON-1 | 84 | A | 2/3/1 | 1 | ||
| P | Patient 199 | MHOM/FR/94/LPM133 | 1994 | VL | HIV | MON-1 | 85 | A | 2 | 1 | |
| P | Patient 200 | MHOM/FR/96/LPM161 | 1996 | IVL | HIV | MON-1 | 85 | A | 2 | 1 | |
| P | Patient 201 | MHOM/FR/2009/LPM264 | 2009 | MCL | MON-1 | 85 | A | 2 | 1 | ||
| P | Patient 202 | MHOM/FR/94/LPM122-2 | 1994 | VL | HIV | MON-1 | 86 | A | 1/3/4 | 4/1 | |
| P | Patient 203 | MHOM/FR/2001/LPM212 | 2001 | CL | MON-1 | 87 | A | 4/3/2 | 1 | ||
| P | Patient 204 | MHOM/FR/2008/LPM260 | 2008 | VL | HIV | MON-1 | 88 | A | 2 | 1/3 | |
| P | Patient 205 | MHOM/FR/99/LPM191 | 1999 | CL | HIV | MON-1 | 89 | A | 1/3 | 1/4 | |
| P | Patient 206 | MHOM/FR/95/LPM137 | 1995 | VL | HIV | MON-1 | 90 | A | 3/6 | 1/3 | |
| P | Patient 207 | MHOM/FR/2000/LPM196 | 2000 | VL | HIV | MON-1 | 91 | A | 4 | 1 | |
| P | Patient 208 | MHOM/FR/94/LPM118 | 1994 | VL | HIV | MON-108 | 92 | A | 4/1/5 | 1/2/4 | |
| CE | Patient 212 | MHOM/FR/85/LEM716* | 1985 | VL | MON-1 | 93 | A | 4 | 1 | ||
| P | Patient 210 | MHOM/FR/97/LPM176 | 1997 | IVL | MON-1 | 93 | A | 4 | 1 | ||
| CO | Patient 209 | MHOM/FR/98/LPM186 | 1998 | VL | MON-1 | 93 | A | 4 | 1 | ||
| P | Patient 211 | MHOM/FR/2001/LPN183 | 2001 | VL | MON-1 | 93 | A | 4 | 1 | ||
| AM | Patient 213 | MHOM/FR/83/LPN18 | 1983 | VL | MON-1 | 94 | A | 4 | 1 | ||
| CE | Patient 214 | MHOM/FR/87/LEM1098* | 1987 | CL | MON-1 | 94 | A | 4 | 1 | ||
| CE | Patient 215 | MHOM/FR/78/LEM75* | 1978 | VL | MON-1 | 94 | A | 4 | 1 | ||
| CE | Patient 216 | MHOM/FR/85/LEM663* | 1985 | VL | MON-1 | 95 | A | 4 | 1 | ||
| AM | Patient 217 | MHOM/FR/88/LPN41 | 1988 | VL | HIV | MON-1 | 96 | A | 2/5 | 3/2 | |
| AM | Patient 218 | MHOM/FR/2003/LPN213 | 2003 | VL | MON-1 | 97 | A | 4 | 1 | ||
| AM | Patient 219 | MHOM/FR/96/LPM162 | 1996 | CL | MON-24 | 98 | A | 6 | 3 | ||
| AM | Patient 220 | MHOM/00/2004/LPN234 | 2004 | CL | MON-24 | 99 | A | 6 | 3 | ||
| AM | Patient 190 | MHOM/FR/2001/LPN182 | 2001 | RVL | HIV | MON-1 | 100 | A | 1 | 4 | |
| AM | Patient 190 | MHOM/FR/2001/LPN188 | 2001 | RVL | HIV | MON-1 | 100 | A | 1 | 4 | |
| P | Patient 221 | MHOM/FR/2000/LPM200 | 2000 | VL | HIV | MON-1 | 101 | A | 2/1 | 1/3/4 | |
| P | Patient 222 | MHOM/FR/94/LPM120 | 1994 | VL | HIV | MON-1 | 102 | A | 2/1/3 | 1/4 | |
| P | Patient 223 | MHOM/FR/99/LPM194 | 1999 | MCL | MON-1 | 103 | A | 4/3/2 | 1 | ||
| P | Patient 224 | MHOM/FR/96/LPM158 | 1996 | VL | MON-1 | 104 | A | 4/2/5 | 1/2 | ||
| AM | MCAN/FR/80/LPN4 | 1980 | CanL | MON-1 | 105 | A | 4/3/2 | 1 | |||
| AM | MCAN/FR/82/LPN8 | 1982 | CanL | MON-1 | 105 | A | 4/3/2 | 1 | |||
| AM | Patient 225 | MHOM/FR/99/LPN171 | 1999 | IVL | MON-1 | 106 | A | 3 | 1 | ||
| P | Patient 226 | MHOM/FR/2001/LPM211 | 2001 | VL | HIV | MON-1 | 107 | A | 3 | 1 | |
| P | Patient 227 | MHOM/00/00/LPM148 | 1995 | UK | MON-1 | 108 | A | 3 | 1 | ||
| AM | Patient 228 | n. d. | 2011 | VL | MON-1 | 109 | A | 3 | 1 | ||
| AM | MCAN/FR/86/LPN27 | 1986 | CanL | MON-1 | 110 | A | 3 | 1 | |||
| AM | Patient 229 | MHOM/FR/99/LPN174 | 1999 | CL | MON-24 | 111 | A | 6 | 3 | ||
| AM | Patient 230 | MHOM/FR/2005/LPN253 | 2001 | VL | HIV | MON-80 | 112 | A | 6 | 3 | |
| P | Patient 231 | MHOM/00/2003/LPM233 | 2003 | IVL | MON-1 | 113 | A | 2 | 3 | ||
| CO | Patient 232 | MHOM/FR/2000/LPM205 | 2000 | VL | MON-1 | 114 | A | 6 | 3 | ||
| CO | Patient 233 | MHOM/FR/92/LPN83 | 1991 | CL | HIV | MON-1 | 115 | A | 6 | 3 | |
| CO | Patient 234 | MHOM/FR/99/LPM190 | 1999 | VL | HIV | MON-1 | 116 | A | 6 | 3 | |
| AM | Patient 235 | MHOM/FR/89/LPN60 | 1989 | VL | HIV | MON-1 | 117 | A | 2 | 1/3 | |
| P | Patient 236 | MHOM/FR/2008/LPM259 | 2008 | VL | HIV | MON-1 | 118 | A | 2/1 | 1/4/3 | |
| P | Patient 237 | MHOM/FR/2007/LPM254 | 2007 | IVL | MON-1 | 119 | A | 6 | 3 | ||
| AM | Patient 238 | MHOM/FR/93/LPN97 | 1993 | CL | MON-24 | 120 | A | 6 | 3 | ||
| P | Patient 239 | MHOM/FR/95/LPM136 | 1995 | VL | HIV | MON-1 | 121 | A | 6 | 3 |
Legend of columns: Endemic area: AM: Alpes-Maritimes; P: Provence; CE: Cévennes; CO: Corsica. Patients: Anonymized name given to the patients. Sample WHO code: WHO code of the isolates; The MLMT profile of the samples indicated with * was already characterized by Hide et al [9]; n. d. = not defined. Y: Year of isolation. C F / H: Clinical form of the disease and/or host: VL–visceral leishmaniasis; IVL–Infant under 15 years with visceral leishmaniasis; CL–cutaneous leishmaniasis; MCL–muco-cutaneous leishmaniasis; RVL—New episode of leishmaniasis in VL patient; AC–asymptomatic carrier; CanL–canine leishmaniasis; CatL–leishmaniasis in cat; PHLE–sample isolated from phlebotomine sandfly; UK–unknown. Zymo: zymodeme of isolate. G: genotypes. P: population as defined by STRUCTURE.
Concerning the geographical distribution (Fig 1), the samples were collected from the endemic areas of AM (n = 178), P (n = 75), CO (n = 9) and CE (n = 8). The samples were isolated at the University Hospitals of Nice, Marseille and Montpellier (France) between 1978 and 2011. MLEE typing and cryoconservation were performed at the Centre National de Référence des Leishmania (Leishmania collection, BRC-Leish, Montpellier, France, BioBank N° BB-0033-00052). Overall, 259 isolates were characterized as zymodeme MON-1, six were MON-24, one was MON-11, one was MON-80 and one was MON-108. The data were unavailable for two isolates. For this study, the primary cultures from the patient stored at -80°C were thawed and the cells were cultured for six days before harvesting for DNA extraction.
Fig 1. Geographical distribution of isolates from Southeastern France.
Isolates clustered into four endemic areas. The geographic areas where samples were isolated from Cévennes (blue), Provence (green), Alpes-Maritimes (red) and Corsica (violet) are indicated. The Alpes-Maritimes and Provence endemic areas are located in the PACA region.
DNA extraction
The microsatellite data indicated with an asterisk in Table 1 were obtained from a previous study [9].
DNA of the remaining isolates was extracted from promastigotes grown in Schneider’s insect medium (Sigma Aldrich, France) supplemented with serum calf, urine, penicillin, streptomycin and L-glutamine (Sigma Aldrich). Promastigotes were harvested on the sixth day of culture, and DNA was extracted from a pellet of 2X108 parasites using a QIAamp DNA mini kit (Qiagen, France) according to the manufacturer’s instructions. DNA extracted from the strain MHOM/FR/85/LEM716 was used as a control to determine the size of amplified microsatellite fragments, as this microsatellite data have been published [9].
Multi-Locus Microsatellite Typing (MLMT)
Twelve microsatellite loci were amplified using the PCR conditions as previously described: LiBTG, LiBTA, LIST7021, LIST7025, LIST7026, LIST7031, LIST7033, Li22-25, Li45-24, TubCA, Li71-5/2 and Rossi2 [9–12]. The amplification products were analyzed using an automated capillary ABI Prism 3130XL Genetic Analyzer (Applied Biosystems, France). The data were stored and analyzed using GeneMapper analysis software (version 4.0, Applied Biosystems). PCR fragment sizes were determined using the internal size standard GeneScan 500 LIZ (Applied Biosystems). All 270 Leishmania isolates were genotyped at each of the 12 loci. With the PCR fragment size of the control strain MHOM/FR/85/LEM716, we were able to include microsatellite data from the Hide et al. study (data indicated with an asterisk in Table 1). Four isolates from this previous study (MCAN/FR/95/LPN122, MCAN/FR/95/LPN123, MCAN/FR/95/LPN124, and MCAN/FR/95/LPN124) were re-extracted from culture and re-genotyped blindly. The same microsatellite results described by Hide et al. were obtained [8].
Genetic diversity and differentiation analysis
Descriptive statistics for the observed genetic populations were calculated using Genetix version 4.05.2 (2004) and FSTAT Version 2.9.3.2 [13]. Using these programs, we calculated allelic diversity (number of allelic variants per maker), expected (He) and observed (Ho) heterozygosity, genetic diversity within subsamples Hs, inbreeding coefficient (FIS) the migration rates (gene flow) (Nm) [14]. The Fst value, which indicates the degree of genetic differentiation and gene flow among populations was also calculated. Fst values above 0.25 with significant p- values (<0.05) indicated strong genetic differentiation [15].
Clustering and phenetic analyses
Phylogenetic analyses were performed based on the microsatellite profiles. A distance matrix was calculated using the Chord distance (Cavalli-Sforza and Edwards 1967) setting in the POPULATIONS 1.2.31 software with bootstrap values determined for 1,000 replicates (http://bioinformatics.org/~tryphon/populations/) [16]. The resulting distance matrix was processed using MEGA 4.0.2 to construct an unrooted Neighbor-Joining (NJ) tree [17].
The genetic characteristics of the Leishmania samples were also investigated using a model-based Bayesian clustering method implemented in STRUCTURE v 2.3.4 [18]. This algorithm simultaneously estimates the allele frequencies to assign individuals into genetically distinct populations (K) and each probability for the identification of the most likely number of populations. A series of ten independent runs was performed for each K value between one and ten. The following parameters were used: burn in period of 20,000 iterations, 200,000 Markov Chain Monte Carlo iterations and admixture model. The most probable number of clusters was identified via calculation of the Delta K (ΔK), which is based on the rate of change in the log probability of data between successive K values. The peak of the ΔK graph corresponds to the most probable number of populations in the data set [19].
Statistical analysis
A chi-square statistical test was performed to determine whether the observed data differed significantly from the expected ratios. The chi-square value was considered significant when p≤0.05. This test was used to compare the proportion of HIV+ patients in Populations A and B.
Results
Microsatellite analysis
In total, 270 isolates were typed at 12 microsatellite markers with one or two alleles at each locus. All markers were polymorphic. The number of alleles per locus (Na) ranged from 3 to 13. Li22-35, LIST7021, LiBTG, LiBTA and LIST7026 were the most polymorphic markers with 13, 12, 10, 9 and 8 alleles, respectively. LIST7031, Li71-5/2 and LIST7025 were the least polymorphic markers with three different alleles (Table 2).
Table 2. Descriptive statistics of the 270 isolates analyzed at the 12 microsatellite markers.
| Locus | Na | He | Ho | Hs | FIS |
|---|---|---|---|---|---|
| Li22-35 | 13 | 0.644 | 0.082 | 0.645 | 0.874 |
| LIST7021 | 12 | 0.547 | 0.037 | 0.548 | 0.933 |
| LiBTG | 10 | 0.620 | 0.041 | 0.621 | 0.935 |
| LiBTA | 9 | 0.809 | 0.174 | 0.811 | 0.786 |
| LIST7026 | 8 | 0.690 | 0.033 | 0.691 | 0.952 |
| TubCA | 7 | 0.541 | 0.041 | 0.542 | 0.925 |
| Li45-24 | 6 | 0.649 | 0.026 | 0.650 | 0.960 |
| Rossi2 | 6 | 0.553 | 0.019 | 0.554 | 0.967 |
| LIST7033 | 6 | 0.533 | 0.019 | 0.534 | 0.965 |
| LIST7031 | 3 | 0.230 | 0.026 | 0.231 | 0.888 |
| LIST7025 | 3 | 0.240 | 0.019 | 0.224 | 0.918 |
| Li71-5/2 | 3 | 0.502 | 0.011 | 0.503 | 0.978 |
| Overall | 7.17 | 0.545 | 0.044 | 0.546 | 0.920 |
Na: number of alleles per locus; He: expected heterozygosity; Ho: observed heterozygosity; Hs: genetic diversity; FIS: inbreeding coefficient.
Genetic variability was analyzed among the 12 microsatellite loci (Table 2). The Ho was weak and ranged from 0.011 to 0.174 for Li71-5/2 and LiBTA, respectively, with an overall Ho at 0.044. The mean intra-population Hs was 0.546 (0.224–0.811) for the entire sample set and 0.531 (0.208–0.804) for the MON-1 population (Table 2). The FIS for the entire population was 0.920, thereby indicating a considerable degree of inbreeding. A separate analysis was performed to investigate the genetic polymorphisms among the four geographically determined populations (Table 3). Extensive inbreeding in the four populations was observed, with the highest inbreeding coefficient found in the populations of the CO and AM endemic areas. The genetic differentiation among the four endemic areas was tested using FSTAT version 2.9.3.2 (Table 4). The Fst values ranged from 0.067 to 0.321. All Fst values between the four endemic areas were significant. We obtained lower values for P versus CE and P versus CO and higher values for AM versus CE and CO versus CE (which may be due to the low number of isolates collected from CE and CO). When comparing AM and P samples from 1993 to 2009 (corresponding to the time period of the isolation of samples from the P endemic area used in this study), the Fst value obtained was similar to the Fst value corresponding to the complete period of sample collection (1978 to 2011).
Table 3. Genetic diversity among the four endemic areas based on the MLMT profiles of the 12 analyzed markers.
| Na | He | Ho | FIS | |
|---|---|---|---|---|
| AM | 6.333 | 0.462 | 0.030 | 0.936 |
| P | 5.417 | 0.498 | 0.079 | 0.844 |
| CO | 3.000 | 0.568 | 0.028 | 0.956 |
| CE | 2.250 | 0.281 | 0.042 | 0.869 |
Na: Number of alleles per locus, He: expected heterozygosity, Ho: observed heterozygosity, FIS: inbreeding coefficient.
AM = Alpes-Maritimes, P = Provence, CO = Corsica and CE = Cévennes.
Table 4. Genetic differentiation between the isolates from the four endemic areas analyzed using F-statistics with the corresponding p-values.
| Endemic areas | Number of isolates | Fst | p-value |
|---|---|---|---|
| P vs. AM | 75 / 178 | 0.239 | 0.008 |
| P vs. CO | 75 / 9 | 0.126 | 0.008 |
| P vs. CE | 75 / 8 | 0.067 | 0.050 |
| CO vs. CE | 9 / 8 | 0.270 | 0.008 |
| AM vs. CO | 178 / 9 | 0.161 | 0.008 |
| AM vs. CE | 178 / 8 | 0.321 | 0.008 |
| P vs. AM isolates collected during same period of time (1993 to 2009) | 75 / 111 | 0.236 | 0.050 |
| Sub-population defined according to their position in relation to the Vars river | |||
| AM East Vars vs. AM West Vars | 122 / 50 | 0.062 | 0.050 |
| AM West Vars vs. P | 52 / 75 | 0.144 | 0.050 |
| P vs. AM East Vars | 75 / 122 | 0.308 | 0.050 |
| Sub-population within the P endemic area | |||
| Within P: Marseille vs. Toulon | 42 / 7 | 0.033 | 0.850 |
| Within P: Marseille vs. Other cities In P | 42 / 33 | 0.012 | 0.550 |
Sub-populations were also defined according to their position in relation to Vars River (S1 Table). This river is located in the southeast of France and flows in the Alpes-Maritimes Department. The Fst values highlighted a gradient of differentiation from the East Vars to the West Vars and up to the P endemic area. A high and significant genetic differentiation (Fst = 0.308) was obtained when comparing isolates from east of the Vars River and the P endemic area. The comparison of sub-populations isolated from the P endemic area failed to show any genetic differentiation (Marseille versus Toulon or Marseille versus other cities in the P endemic area), with the limitation that few samples were collected from Toulon (n = 7).
Geographical distribution of isolates from the two main endemic areas of AM and P
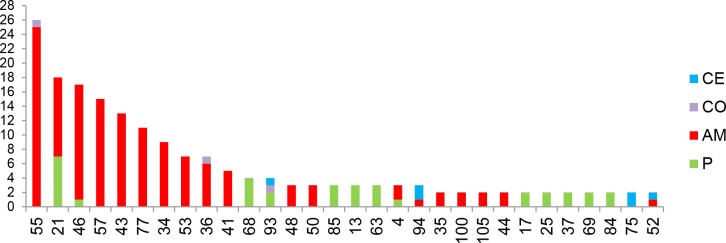
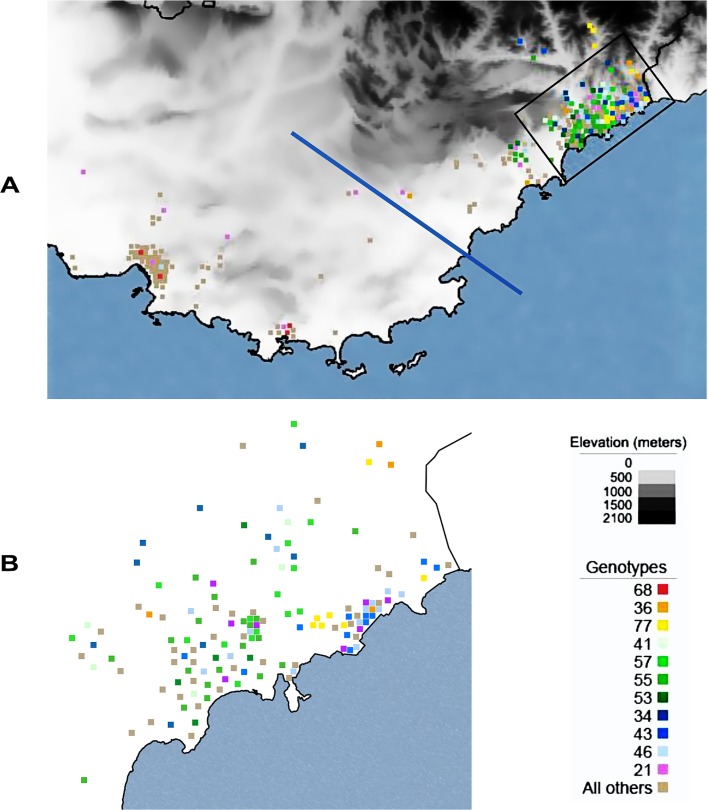
A total of 121 different genotypes were identified from the 270 isolates corresponding to a genotype frequency of 0.037. The entire sample set comprised 91 unique genotypes (75%). Among the 30 repeated genotypes, seven were common to two endemic areas, one was common to three endemic areas and 22 pertained to the same focus (Fig 2). The genotypes 21 (primarily) and 46 were found in both endemic areas of AM and P (Fig 2). Within these endemic areas, Faucher et al. have described high- and low-risk sub-areas of VL [5]. In our study, the repeated genotypes were almost exclusively found in high-risk areas, with the only exception of genotype 21 which was found in high and low risk sub-areas (Fig 3A and 3B). Among the 30 repeated genotypes, 11 were found in four or more samples. These 11 genotypes represented 132 isolates.
Fig 2. Repeated genotypes.
Arbitrary numbers assigned to the 30 repeated genotypes in (x-axis) and number of isolates belonging to each genotype (y-axis). The origin of each isolates is indicated by the different colors.
Fig 3. A. Localization of the genotypes.
Eleven repeated genotypes, which were observed in four or more isolates, are represented with colored squares on the map of the endemic areas of Alpes-Maritimes and Provence. The two endemic areas are separated by a blue line. The remaining genotypes are represented by light brown squares. The area in the black rectangle is shown in further detail in Fig 3B. B. Detailed localization of isolates in the Alpes-Maritimes endemic area and more precisely the surrounding region of Nice. Isolates were localized at the city level. The black line represents the delimitation of Nice.
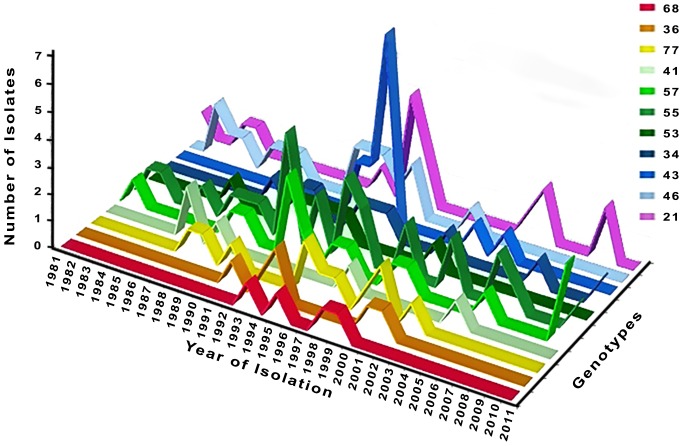
Temporal analysis of genotypes
The isolates were collected over the course of a 33-year period from 1978 to 2011 (Fig 4). The repeated genotypes, found in four or more samples, were isolated over a period of six (genotype 68) to 29 years (genotypes 57 and 55). All genotypes except 68, 36, 34 and 43 were isolated from both humans and dogs. Genotype 21 was found to be present in the AM and P endemic areas over the course of 28 years. This genotype was first isolated in 1981 in the AM endemic area and then in 1996, at which time it was first identified in both the AM and P endemic areas. After 1996, genotype 21 was only isolated in the P. Finally, in 2009, this genotype was found in both the AM and P endemic areas. Genotype 21 was isolated from a variety of patients including asymptomatic carriers (AC), adult VL, infant VL and HIV+ VL cases.
Fig 4. Temporal distribution of the 11 genotypes represented by more than four isolates.
Relapse and re-infection
Four patients (patients 4, 64, 86 and 190) experienced more than one episode of VL (Table 1). Three of these patients were HIV+ (patients 4, 86 and 190). The isolates derived from patient 4 and 64 (HIV+ and HIV-, respectively), which were collected during a first VL episode (2003 for patient 4 and 2001 for patient 64) and second VL episode (one year later), presented identical MLMT profiles. In both cases, relapse was suspected. Patient 190 (HIV+) presented three episodes of VL, for which the second and third (2001 for both episodes) isolates differed from the first isolate (1996) at only one marker (LIST7021). Re-infection was suspected for this patient; however, further studies are warranted to confirm whether only one allelic change can lead to the suspicion of a re-infection or reflects the evolution of the isolate over time. Patient 86 (HIV+) presented five different episodes of VL due to active chronic VL [20]. Isolates from the second (2000), third (2001) and last episode (2003) presented with the same MLMT profile compared with the isolate from the initial infection (1997). However, the MLMT profile of the fourth episode (2002) isolate varied at 11 loci; only Rossi2 remained the same. Re-infection and relapse were suspected for this patient. Each isolate of these multiple episodes was characterized zymodeme MON-1.
Association between genotype and clinical manifestation
Genetic differentiation among the various populations was tested using FSTAT Version 2.9.3.2.
In France, at the end of the 1990s and early 2000s, the repellent collar had been widely use to protect dogs from parasitic transmission [21,22]. To determine whether this had an impact on genetic differentiation, we compared the isolates before and after the introduction of repellent collar in the AM and P endemic areas. To minimize the temporal effect on genetic variability and determine whether the repellent collar led to a bottleneck effect, the samples collected between 1996 and 2004 were excluded (Table 5). No differentiation was found in both endemic areas, thereby suggesting no bottleneck effect due to repellent collar use.
Table 5. Differentiation measures (Fst) and testing (p-value) between different Leishmania infantum isolates according to the use of collar repellent and clinical manifestations.
| Subsamples | Number of isolates | Fst | p-value |
|---|---|---|---|
| Effect of collar repellent. Comparison of isolates from 1978 to 1995 and 2005 to 2011 (years 1996–2004 excluded) | |||
| P endemic area | 11 / 12 | 0.0412 | 0.15 |
| AM endemic area | 87 / 40 | ≈ 0 | 0.95 |
| Comparison of clinical manifestations. Other means VL, CL, MCL | |||
| AM AC vs other 1991–2001 | 9 / 172 | 0.0763 | 0.10000 |
| AM AC vs HIV isolated from 1994 to 1998 in the same endemic area | 11 / 9 | 0.0830 | 0.10000 |
| HIV + vs HIV - | 95 / 150 | 0.0683 | 0.05000 |
| HIV + vs VL adult | 95 / 72 | 0.0857 | 0.05000 |
| HIV + vs CanL CatL | 95 / 21 | 0.0167 | 0.50000 |
| P HIV + vs VL adult | 40 / 11 | 0.0020 | 0.80000 |
| AM HIV + vs VL adult | 52 / 50 | 0.1510 | 0.05000 |
| IVL vs CanL CatL | 58 / 21 | -0.0013 | 0.90000 |
| VL vs IVL | 70 / 57 | 0.0003 | 0.30000 |
Nine isolates from asymptomatic carriers were compared with 11 isolates from HIV patients collected between 1994 and 1998 in the same restricted endemic area. To avoid bias, we selected isolates collected two years before and after the date of the collection of the asymptomatic carrier isolates (1996). Unlike the findings reported by Hide et al., no genetic differentiation was observed between isolates from asymptomatic carriers and those derived from HIV+ patients (Table 5) [9]. However, additional isolates from asymptomatic carriers are required to strengthen these findings. A genetic differentiation was observed between isolates from HIV+ and VL adult patients in AM (Table 5).
Clustering analysis
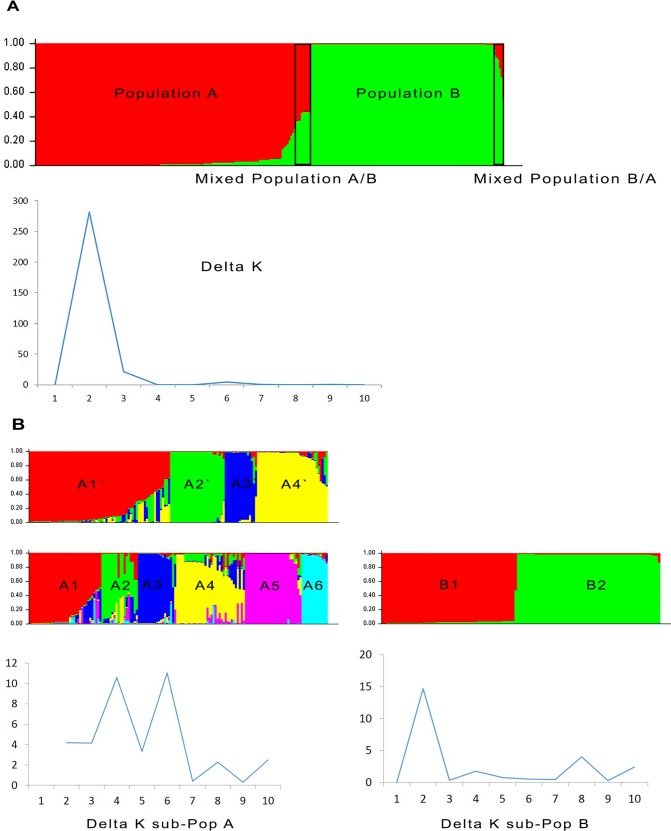
Bayesian model-based analysis of the 270 isolates using STRUCTURE (with calculation of ΔK) indicated two distinct genetic populations (Fig 5A). Population A consisted of 148 samples: 73 from P, 61 from AM, seven from CO and seven from CE. This population consisted of zymodemes MON-1 and nine isolates with zymodemes other than MON-1. Population B consisted of 109 isolates: 104 from AM, two from P, two from CO and one from CE. Isolates from Population B were all characterized zymodeme MON-1. Among the Populations A and B, 13 isolates had mixed genotypes (11 isolates corresponding to seven genotypes and two isolates corresponding to two genotypes in Populations A and B, respectively). The isolates with mixed genotypes shared allele characteristics of each population. All isolates with mixed genotypes were collected in the AM endemic area and were characterized zymodeme MON-1. Thus, Population A is a mixed population with almost an equivalent number of isolates from AM and P corresponding to MON-1 and all non-MON-1 isolates. The estimated gene flow between isolates from AM and P within Population A (Nm value) was 8.38. Population A also displayed a marked proportion of isolates from HIV+ patients (44.7%) compared with Population B (21.6%) (p<0.05). The two populations A and B defined by STRUCTURE were significantly different as shown by the Fst value 0.503 and p-value equal to 0.05. The estimated gene flow between the populations (Nm value) was 0.25, thereby indicating very few exchanges between those two populations.
Fig 5. Estimated population structure for L. infantum from Southeastern France assessed using STRUCTURE software based on the analysis of the 270 L. infantum DNA samples at 12 microsatellite markers.
A: The plots show the estimated membership coefficient (Q) of each isolates. Each isolate is represented by a single vertical line divided into K colors, in which K is the number of populations assumed. Each color represents one population, and the length of the colored segment shows the estimated proportion of isolates membership in that population. The derived graph for delta K shows K = 2, thereby indicating the presence of two populations in the investigated sample set. B: Isolates of the sub-populations A and B with delta K values for each sub-population. Two close values were observed for K in sub-population A: K = 4 and K = 6. For sub-population B, K = 2.
The STRUCTURE analysis of Population A separately from Population B, excluding admixed isolates (13 isolates), revealed a delta K graph with two peaks at K = 4 and K = 6 (Fig 5B). The main difference between K = 4 and K = 6 was that sub-population A1’ at K = 4 was split into three populations at K = 6: sub-population A2 (four isolates from sub-population A1’), A3 (16 isolates from sub-population A1’) and A4 (15 isolates from sub-population A1’). Whether at K = 4 or K = 6, the sub-population defined contained isolates from the AM and P endemic areas and from HIV+ patients. The non-MON-1 isolates were grouped into sub-population A1’ and A3’ with K = 4 and into sub-population A2, A4 and A6 with K = 6. No cluster based on clinical data, geographic area or zymodeme profile was found within the defined sub-populations. Some isolates displayed mixed genotypes within the A sub-populations. At K = 4 and K = 6, 26 and 44 isolates had mixed genotypes, respectively. At K = 6, isolates with mixed genotypes came from P (n = 33; 75%), AM (n = 8; 18.2%) and CE (n = 3; 6.8%).
When excluding mixed genotypes, the sub-populations A1, A2, A3, A4, A5 and A6 as well as A1’, A2’, A3’ and A4’ were significantly different, as shown by the significant Fst values ranging from 0.249 to 0.833 and from 0.322 to 0.813 for K = 4 and K = 6, respectively (Table 6). At K = 4, the highest Nm value was obtained between sub-population A1’ and sub-population A3’ (0.53), whereas at K = 6, the highest Nm value was 0.75 between sub-population A2 and sub-population A6, thereby indicating only limited genotype flow between these sub-populations (Table 6).
Table 6. Differentiation measures, migration rate and statistical significance between different Leishmania infantum isolates according to sub-populations defined using STRUCTURE.
In sub populations A, the isolates with mixed genotypes have been excluded (26 and 44 isolates for K = 4 and K = 6, respectively). Only the highest and lowest Fst values are represented in this table.
| Sub populations | Number of Isolates | Fst | p-value | Nm | |
|---|---|---|---|---|---|
| Sub Pop A | Pop A2’ vs A4’ | 26 / 30 | 0.813 | 0.008 | 0.06 |
| K = 4 | Pop A1’ vs A3’ | 52 / 14 | 0.322 | 0.008 | 0.53 |
| Sub Pop A | Pop A3 vs A5 | 16 / 26 | 0.833 | 0.003 | 0.05 |
| K = 6 | Pop A2 vs A6 | 7 / 13 | 0.249 | 0.003 | 0.75 |
| Sub Pop B | |||||
| K = 2 | Pop B1 vs B2 | 51 / 58 | 0.537 | 0.050 | 0.22 |
Fst: degree of genetic differentiation, Nm: migration rates, p-value: statistical significance.
Two main sub-populations were defined using STRUCTURE for Population B: sub-population B1 and sub-population B2. No mixed genotypes were present. Sub-population B1 consisted of 48 isolates from AM, two from P and one from CO. Sub-population B2 consisted of 56 isolates from AM, one from CO and one from CE. These two sub-populations were genetically different as evidenced by the Fst value (0.537 and p-value = 0.05). Few exchanges occurred between these two sub-populations (Nm = 0.22 was obtained between sub-population B1 and sub-population B2).
Sub-populations A3’ (sub-population A K = 4) and A6 (sub-population A K = 6) displayed the highest number of alleles per population (Table 7). The expected heterozygosity (He), a measure of genetic diversity, was higher in the A sub-populations with non-MON-1 isolates compared with the A sub-populations with only MON-1 isolates (Table 7). All A sub-populations displayed a high inbreeding coefficient (FIS) (> 0.7), whereas the sub-population B2 displayed a low inbreeding coefficient (0.343) (Table 7).
Table 7. Descriptive statistics per population.
| Name of the sub population | n | Na | He | Ho | FIS | |
|---|---|---|---|---|---|---|
| Sub Pop A | Pop A1’ | 52 | 3.500 | 0.380 | 0.066 | 0.830 |
| K = 4 | Pop A2’ | 26 | 1.500 | 0.052 | 0.013 | 0.763 |
| Pop A3’ | 14 | 5.417 | 0.695 | 0.048 | 0.936 | |
| Pop A4’ | 30 | 2.000 | 0.161 | 0.014 | 0.917 | |
| Sub Pop A | Pop A1 | 27 | 1.917 | 0.149 | 0.012 | 0.920 |
| K = 6 | Pop A2 | 7 | 2.417 | 0.395 | 0.071 | 0.843 |
| Pop A3 | 16 | 1.917 | 0.169 | 0.016 | 0.913 | |
| Pop A4 | 15 | 1.917 | 0.184 | 0.006 | 0.972 | |
| Pop A5 | 26 | 1.500 | 0.052 | 0.013 | 0.763 | |
| Pop A6 | 13 | 5.000 | 0.680 | 0.051 | 0.931 | |
| Sub Pop B | Pop B1 | 51 | 1.500 | 0.080 | 0.011 | 0.860 |
| K = 2 | Pop B2 | 58 | 1.917 | 0.067 | 0.0445 | 0.34337 |
In sub populations A, the isolates with mixed genotypes have been excluded (26 and 44 isolates for K = 4 and K = 6, respectively). n: number of isolates per population; Na: number of alleles; Ho: observed heterozygosity; He: expected heterozygosity; FIS: inbreeding coefficient.
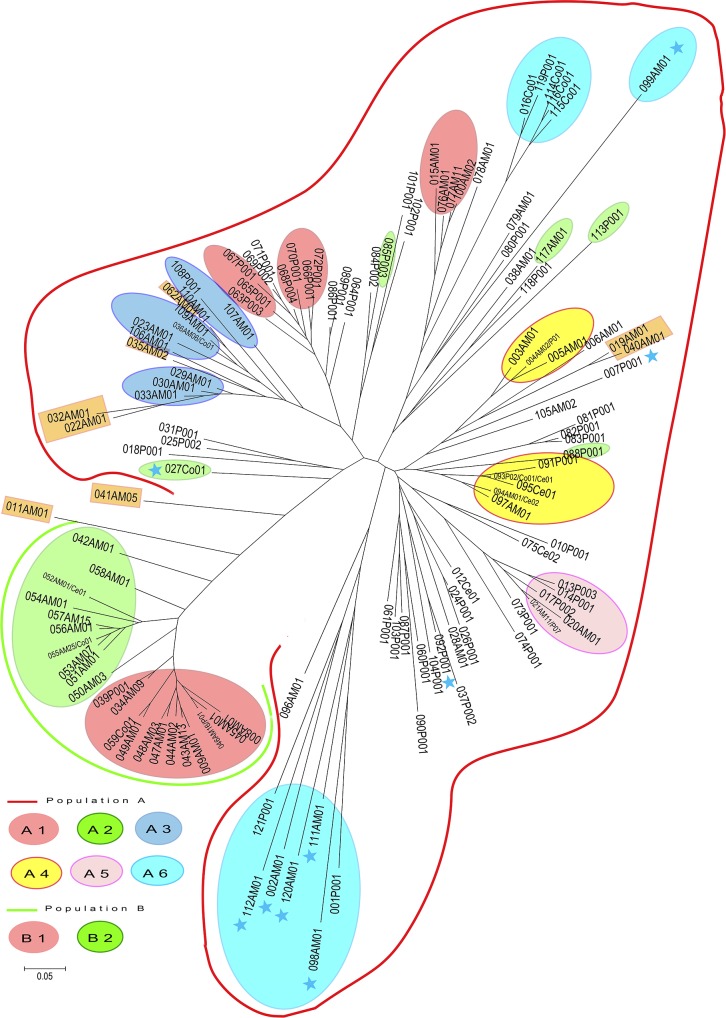
The NJ tree presented in Fig 6 provides a graphic representation of the data. The bootstrap values based on the re-sampling of loci were low and therefore not included in the NJ tree (Fig 6). This was due to the presence of admixed genotypes and the high number of shared alleles even if the allelic frequencies are different between the populations and sub-populations. Two main clusters were found to correspond to the two populations obtained using STRUCTURE at K = 2. Population B formed a separate cluster from Population A. The two sub-clusters defined using STRUCTURE for Populations B1 and B2 are shown on the NJ tree. However, for Population A, the clusters defined by the NJ tree did not perfectly correlate with the A sub-populations defined using STRUCTURE for neither K = 4 nor K = 6. The sub-populations A1, A2 and A3 (A1: four genotypes from AM and seven genotypes from P; A2: one genotype from AM, one from CO and three from P; and A3: eight genotypes from AM, two from P and one from CO) are dispersed throughout the NJ tree. Within Population A, the isolates from AM and P are dispersed throughout the cluster with no correlation with endemic area, clinical form or host background. The mixed genotypes were present between the two main clusters of Populations A and B as well as within the cluster of A sub-populations. Some non-MON-1 isolates grouped together as a paraphyletic group in the sub-population A6, while the others were dispersed among the MON-1 isolates and are present at the end of the branches. As previously described, the MON-108 isolate is closely related to the MON-1 isolates [23–25]. Regarding the nine isolates from AC, two (genotype 21) were present in sub-population A5, whereas seven (genotype 43) belonged to sub-population B1 and grouped with isolates from infant VL and HIV + VL patients. None of the isolates from AC has a mixed genotype. Samples isolated from CanL grouped together with human isolates, and no correlation was found between host and MLMT profile.
Fig 6. Unrooted neighbor-joining tree inferred from genetic distances derived from the proportion of alleles shared among the 270 isolates of L. infantum based on 12 microsatellite markers.
The two populations defined using STRUCTURE are highlighted. The blue stars correspond to the non-MON-1 strains. At the end of each branche of the network, the first three characters correspond to the arbitrarily assigned number for each genotype, the following two characters (AM, P, Ce, Co) correspond to the endemic area of sample collection, and then the last two characters correspond to the number of isolates with the given genotype. The orange rectangles represent the isolates with mixed genotypes. The sub populations are represented with colored ovals at K = 6 for A sub-populations and K = 2 for B sub-populations. The isolates with no color are those with mixed genotypes within A sub-populations at K = 6. MEGA 4 software was used to visualize the neighbor-joining tree.
Discussion
Leishmaniasis due to L. infantum is endemic in Southern France. In this study, we used MLMT, a molecular tool useful for population genetic studies, to analyze an extensive set of isolates from four endemic areas in Southern France (AM, P, CE and CO). To the best of our knowledge, this study is the first to investigate a large number of L. infantum isolates from different endemic areas in Southern France. We also focused on the AM and P endemic areas over an extended period of time. A greater number of samples came from AM than P (AM = 178 versus P = 75) because AM is the most active foci in France with the greatest number of leishmaniasis cases per year [2]. The study period was also longer for the AM area than for the P endemic area (AM: 1978–2011; P: 1993–2009). This aspect may generate a sampling bias, although no significant genetic differentiation was found when comparing isolates from AM and P during the same time period. Although MON-1 is the most prevalent zymodeme, other zymodemes also circulate in the south of France [7]. Microsatellite characterization of L. infantum isolates revealed a total of 121 different genotypes. Overall, 91 unique genotypes and 30 repeated genotypes were found. A greater number of repeated genotypes were observed in AM compared with P for the same period, thereby suggesting variations in the transmission cycle between the two areas such as outbreak, vector diversity or density, or host density.
In the AM endemic area, the isolates belonged to two main populations as defined by STRUCTURE: Population A and Population B. Within Population A, gene flow occurred between the AM and P endemic areas. The spread of isolates seems to be from AM to P, as indicated by the results of genotype 21, which was found 15 years later in P.
Substantial genetic diversity was found to be comparable to other endemic areas, even within zymodeme MON-1, thereby confirming previous analyses assessed by other markers [26]. As genetic differentiation depends on the area, our findings suggest strong epidemiological structuring. This is in agreement with the known mechanism of Leishmania transmission and spread in micro-foci and the entomologic data that have demonstrated limited sandfly dispersion [27–31]. Indeed, considering the behavior of the phlebotomine sandfly, it seems less likely that the spread of isolates from AM to P is due to sandfly movement [32]. Unfortunately, due to the small sample size of the phlebotomine sandfly isolates, we cannot investigate the transmission between sandflies, humans and canine hosts in further detail. We suspect that people traveling with their infected dogs between the endemic areas plays a possible role in the etiology of these exchanges. This has been already described for the emergence of L. infantum in South America probably via Conquistadores infected dogs from Portugal [33]. More recently, an intercontinental transportation from France to French Guiana was also reported due to the probable importation of L. infantum from an infected dog [34]. Notably, some repeated genotypes in the AM endemic area are well settled and continue to spread through the area over time. Indeed, some repeated genotypes were detected during a limited period, ranging from 6 to 15 years, and are no longer detected (genotypes 36, 43, 46, 53, 68 and 77). However, other genotypes were still detected in 2011 (genotypes 34, 55 and 57) and one genotype spread to P endemic area (genotype 21). The mixed genotypes between Population A and Population B were isolated in the AM endemic area, whereas in the A sub-populations, 75% of the mixed genotypes came from the P endemic area. We also observed a predominance of isolates from HIV+ patients in Population A (44.7%) compared with Population B (21.6%), which may indicate a variation in virulence. Indeed, these isolates from HIV+ patients may produce leishmaniasis in immunocompromised patients, whereas affected immunocompetent patients may develop only an asymptomatic infection [35]. This hypothesis is in agreement with the distribution of isolates from AC in both Population A and Population B. These isolates belonged to the genotypes 21 (2 AC) and 43 (7 AC), with samples from HIV+ VL, IVL, VL and CanL cases and samples from infant VL and HIV+ VL patients, respectively. The comparison of isolates from AC and those from HIV+ patients isolated during the same period and within the same restricted endemic area revealed no genetic differentiation between these populations. This finding contrasts with previous data reported by Hide et al. [9] and as suspected, does not reflect a difference in virulence. However, further isolates from asymptomatic carriers must be assessed to confirm our hypotheses. This is critical in the endemic areas of Southern France (as well as all endemic foci of leishmaniasis), where the isolates responsible for leishmaniasis represent only the tip of the iceberg [36]. Indeed, depending on the test used to detect asymptomatic carriage, prevalence varies from 30% to 46.8% in the AM endemic area [36]. Although our study provides important insight into leishmaniasis epidemiology in AM and P, our panel represents only a small proportion of the L. infantum population circulating in Southern France as samples from asymptomatic carriers, dogs and sandflies are underrepresented.
Microsatellite analyses may be useful to estimate relapse and re-infection rates, which is important to evaluate anti-Leishmania drug efficacy and transmission dynamics, respectively. This aspect is particularly important for the follow-up of patient treatment. MLMT may also be a useful tool to differentiate between relapses from re-infection cases [11,25,26,35,37,38]. Moreover, Bourgeois et al. have described “active chronic visceral leishmaniasis” in patients with several episodes of VL [20]. In this particular form of the disease, identifying the MLMT profile of each isolate responsible of each episode of VL could be useful to monitor and optimize treatment regimes. In our study, we detected probable treatment failure in HIV+ and non-HIV patients, as the MLMT profiles were indistinguishable from one episode to another. Certain patients likely experienced re-infection, as isolates from two different episodes of leishmaniasis in same patient displayed different MLMT profiles. However, we cannot exclude the possibility of a mixed infection with differential strain isolation depending on the time of sampling. Due to the small number of patients with isolates from several biological samples at different times, the rate of relapse and re-infection needs to be confirmed on a larger sample set. Thus, the results on relapse and re-infection should be interpreted with caution. Further investigations are required to assess these hypotheses in further detail.
The high ratio of repeated genotypes in HIV patients (81.6%) compared with the remaining population (41%) may be due to an outbreak amongst this fragile human population. Outbreaks have already been reported among intravenous drug users, a population also frequently affected by HIV infection [39,40]. Nevertheless, we have no information concerning this aspect of the case population in our study.
Faucher et al. have highlighted the heterogeneity of environments associated with VL transmission in Southeastern France [5]. The authors showed two distinct foci strongly associated with specific environments. One focus, corresponding to the AM endemic area, was characterized by scattered habitation and mixed forest in the foothills. In contrast, the other focus in the P endemic area was centered in urban areas of Marseille. These environmental differences correlate with the strong genetic differentiation we found between the Leishmania populations from AM and P. Indeed, the ecosystem influences the transmission cycle and thus the population dynamics of parasites. Moreover, in the P endemic area, Toscana virus, which is responsible for summer meningitis, and L. infantum share the same vector, Phlebotomus perniciosus. A recent study has described dogs co-infected by these two organisms [41]. Although cases of co-infection in humans or vectors have not been reported, we suspect that is also possible. This phenomenon of co-infection may have an impact on Leishmania transmission and should be addressed in future studies to understand whether this may also influence parasite evolution.
Other wild reservoirs of L. infantum have been demonstrated in Europe such as fox, rats and hare [42]. These wild reservoirs are able to transmit L. infantum to sandflies. However, the isolates from wild reservoirs have indistinguishable genotypes from those derived from domestic dogs and humans [26,43]. No isolates from wild animals were included in our study. The only uncommon host included in our study was a cat, and the isolate from this animal shared the genotype 55 with isolates from dog, IVL, HIV+ VL and VL samples.
In some studies, the isolates with a zymodeme other than MON-1 grouped together either via neighbor joining tree or STRUCTURE analysis [25,26]. We did not find such correlations with our data which is probably due to the high number of strains with mixed genotypes. Indeed the nine non-MON-1 isolates did not group into a separate population but rather clustered into Population A with a majority of the MON-1 isolates. In the NJ tree, some non-MON-1 isolates appeared as a paraphyletic group, while others were either isolated or dispersed among other zymodemes [23–25]. The zymodeme MON-108 (genotype 92) isolate appeared very close to MON-1 isolates with the genotype 37 [23–25].
In our study, no correlation was found between MLMT profile, clinical expression of the disease, immune status and host. Finally, MLMT is more discriminant and thus more appropriate than MLEE to evaluate epidemiological changes among parasite population in Southern France. MLMT data provided a better understanding of gene flow between L. infantum populations within the Southeastern France endemic area.
Supporting Information
AM: Alpes-Maritimes; P: Provence; CE: Cévennes; CO: Corsica. VL–Visceral leishmaniasis; IVL–Infant under 15 years visceral leishmaniasis; CL–Cutaneous leishmaniasis; MCL–Muco-cutaneous leishmaniasis; RVL—New episode of leishmaniasis in patients; AC–Asymptomatic carrier; CanL–Canine leishmaniasis; CatL–Leishmaniasis in cat; PHLE: isolate from phlebotomine sandfly; UK–Unknown. n. d. = not defined.
(XLS)
Acknowledgments
We thank Mohammad Akhoundi for his precious assistance. The data used in this study were processed at the GenSeq capillary electrophoresis facilities of the labex « Centre Méditerranéen de l’Environnement et de la Biodiversité », Montpellier.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
SA received a grant from Infectiopôle Sud and Infectiopôle Sud provided some equipment used in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization, others. Control of the leishmaniases: report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22–26 March 2010. 2010; http://apps.who.int/iris/handle/10665/44412
- 2.Lachaud L, Dedet JP, Marty P, Faraut F, Buffet P, Gangneux JP, et al. Surveillance of leishmaniases in France, 1999 to 2012. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2013;18: 20534. [PubMed] [Google Scholar]
- 3.Pigott DM, Bhatt S, Golding N, Duda KA, Battle KE, Brady OJ, et al. Global distribution maps of the leishmaniases. eLife. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marty P, Izri A, Ozon C, Haas P, Rosenthal E, Del Giudice P, et al. A century of leishmaniasis in Alpes-Maritimes, France. Ann Trop Med Parasitol. 2007;101: 563–574. [DOI] [PubMed] [Google Scholar]
- 5.Faucher B, Gaudart J, Faraut F, Pomares C, Mary C, Marty P, et al. Heterogeneity of Environments Associated with Transmission of Visceral Leishmaniasis in South-Eastern France and Implication for Control Strategies. PLoS Negl Trop Dis. 2012;6: e1765 10.1371/journal.pntd.0001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyrefitte CN, Grandadam M, Bessaud M, Andry P-E, Fouque F, Caro V, et al. Diversity of Phlebotomus perniciosus in Provence, southeastern France: Detection of two putative new phlebovirus sequences. Vector Borne Zoonotic Dis Larchmt N. 2013;13: 630–636. [DOI] [PubMed] [Google Scholar]
- 7.Pratlong F, Rioux J-A, Marty P, Faraut-Gambarelli F, Dereure J, Lanotte G, et al. Isoenzymatic analysis of 712 strains of Leishmania infantum in the south of France and relationship of enzymatic polymorphism to clinical and epidemiological features. J Clin Microbiol. 2004;42: 4077–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schönian G, Kuhls K, Mauricio IL. Molecular approaches for a better understanding of the epidemiology and population genetics of Leishmania. Parasitology. 2011;138: 405–425. 10.1017/S0031182010001538 [DOI] [PubMed] [Google Scholar]
- 9.Hide M, Marion E, Pomares C, Fisa R, Marty P, Bañuls AL. Parasitic genotypes appear to differ in leishmaniasis patients compared with asymptomatic related carriers. Int J Parasitol. 2013;43: 389–397. 10.1016/j.ijpara.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 10.Jamjoom MB, Ashford RW, Bates PA, Kemp SJ, Noyes HA. Towards a standard battery of microsatellite markers for the analysis of the Leishmania donovani complex. Ann Trop Med Parasitol. 2002;96: 265–270. [DOI] [PubMed] [Google Scholar]
- 11.Ochsenreither S, Kuhls K, Schaar M, Presber W, Schönian G. Multilocus microsatellite typing as a new tool for discrimination of Leishmania infantum MON-1 strains. J Clin Microbiol. 2006;44: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rougeron V, De Meeûs T, Hide M, Le Falher G, Bucheton B, Dereure J, et al. Multifaceted population structure and reproductive strategy in Leishmania donovani complex in one Sudanese village. PLoS Negl Trop Dis. 2011;5: e1448 10.1371/journal.pntd.0001448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goudet J, Perrin N, Waser P. Tests for sex-biased dispersal using bi-parentally inherited genetic markers. Mol Ecol. 2002;11: 1103–1114. [DOI] [PubMed] [Google Scholar]
- 14.Nei M, Chesser RK. Estimation of fixation indices and gene diversities. Ann Hum Genet. 1983;47: 253–259. [DOI] [PubMed] [Google Scholar]
- 15.Wright S. Vol. 4: Variability within and among natural populations [Internet]. Chicago: [etc.]: University of Chicago Press; 1978. http://library.wur.nl/WebQuery/clc/1876572 [Google Scholar]
- 16.Cavalli-Sforza LL, Edwards AW. Phylogenetic analysis. Models and estimation procedures. Am J Hum Genet. 1967;19: 233–257. [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- 20.Bourgeois N, Bastien P, Reynes J, Makinson A, Rouanet I, Lachaud L. “Active chronic visceral leishmaniasis” in HIV-1-infected patients demonstrated by biological and clinical long-term follow-up of 10 patients. HIV Med. 2010;11: 670–673. 10.1111/j.1468-1293.2010.00846.x [DOI] [PubMed] [Google Scholar]
- 21.Brianti E, Gaglio G, Napoli E, Falsone L, Prudente C, Solari Basano F, et al. Efficacy of a slow-release imidacloprid (10%)/flumethrin (4.5%) collar for the prevention of canine leishmaniosis. Parasit Vectors. 2014;7: 327 10.1186/1756-3305-7-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maroli M, Mizzon V, Siragusa C, D’Oorazi A, Gradoni L. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med Vet Entomol. 2001;15: 358–363. [DOI] [PubMed] [Google Scholar]
- 23.Amro A, Hamdi S, Lemrani M, Mouna I, Mohammed H, Mostafa S, et al. Moroccan Leishmania infantum: genetic diversity and population structure as revealed by multi-locus microsatellite typing. PloS One. 2013;8: e77778 10.1371/journal.pone.0077778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amro A, Schönian G, Al-Sharabati MB, Azmi K, Nasereddin A, Abdeen Z, et al. Population genetics of Leishmania infantum in Israel and the Palestinian Authority through microsatellite analysis. Microbes Infect Inst Pasteur. 2009;11: 484–492. [DOI] [PubMed] [Google Scholar]
- 25.Kuhls K, Chicharro C, Cañavate C, Cortes S, Campino L, Haralambous C, et al. Differentiation and gene flow among European populations of Leishmania infantum MON-1. PLoS Negl Trop Dis. 2008;2: e261 10.1371/journal.pntd.0000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes S, Maurício IL, Kuhls K, Nunes M, Lopes C, Marcos M, et al. Genetic diversity evaluation on Portuguese Leishmania infantum strains by multilocus microsatellite typing. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2014;26: 20–31. [DOI] [PubMed] [Google Scholar]
- 27.Rioux JA, Killick-Kendrick R, Leaney AJ, Turner DP, Bailly M, Young CJ. [Ecology of leishmaniasis in the south of France. — 12. Horizontal dispersion of Phlebotomus ariasi Tonnoir, 1921. Preliminary experiments (author’s transl)]. Ann Parasitol Hum Comparée. 1979;54: 673–682. [PubMed] [Google Scholar]
- 28.Killick-Kendrick R. Recent advances and outstanding problems in the biology of phlebotomine sandflies. A review. Acta Trop. 1978;35: 297–313. [PubMed] [Google Scholar]
- 29.Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44: 535–551. [PMC free article] [PubMed] [Google Scholar]
- 30.Izri A, Depaquit J, Parola P. [Phlebotomine sandflies and transmission of disease agents around the Mediterranean basin]. Médecine Trop Rev Corps Santé Colon. 2006;66: 429–435. [PubMed] [Google Scholar]
- 31.Rougeron V, De Meeûs T, Hide M, Waleckx E, Dereure J, Arevalo J, et al. A battery of 12 microsatellite markers for genetic analysis of the Leishmania (Viannia) guyanensis complex. Parasitology. 2010;137: 1879–1884. 10.1017/S0031182010000776 [DOI] [PubMed] [Google Scholar]
- 32.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27: 123–147. 10.1111/j.1365-2915.2012.01034.x [DOI] [PubMed] [Google Scholar]
- 33.Leblois R, Kuhls K, François O, Schönian G, Wirth T. Guns, germs and dogs: On the origin of Leishmania chagasi. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2011;11: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 34.Rotureau B, Ravel C, Aznar C, Carme B, Dedet J- P. First Report of Leishmania infantum in French Guiana: Canine Visceral Leishmaniasis Imported from the Old World. J Clin Microbiol. 2006;44: 1120–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelanew T, Kuhls K, Hurissa Z, Weldegebreal T, Hailu W, Kassahun A, et al. Inference of Population Structure of Leishmania donovani Strains Isolated from Different Ethiopian Visceral Leishmaniasis Endemic Areas. Bates PA, editor. PLoS Negl Trop Dis. 2010;4: e889 10.1371/journal.pntd.0000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel G, Pomares C, Ferrua B, Marty P. Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Trop. 2011;119: 69–75. 10.1016/j.actatropica.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 37.Botilde Y, Laurent T, Quispe Tintaya W, Chicharro C, Cañavate C, Cruz I, et al. Comparison of molecular markers for strain typing of Leishmania infantum. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2006;6: 440–446. [DOI] [PubMed] [Google Scholar]
- 38.Seridi N, Amro A, Kuhls K, Belkaid M, Zidane C, Al-Jawabreh A, et al. Genetic polymorphism of Algerian Leishmania infantum strains revealed by multilocus microsatellite analysis. Microbes Infect Inst Pasteur. 2008;10: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 39.Cruz I, Morales MA, Noguer I, Rodríguez A, Alvar J. Leishmania in discarded syringes from intravenous drug users. Lancet. 2002;359: 1124–1125. [DOI] [PubMed] [Google Scholar]
- 40.Molina R, Gradoni L, Alvar J. HIV and the transmission of Leishmania. Ann Trop Med Parasitol. 2003;97 Suppl 1: 29–45. [DOI] [PubMed] [Google Scholar]
- 41.Dincer E, Gargari S, Ozkul A, Ergunay K. Potential Animal Reservoirs of Toscana Virus and Coinfections with Leishmania infantum in Turkey. Am J Trop Med Hyg. 2015;92: 690–697. 10.4269/ajtmh.14-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millán J, Ferroglio E, Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol Res. 2014;113: 2005–2014. 10.1007/s00436-014-3929-2 [DOI] [PubMed] [Google Scholar]
- 43.Chicharro C, Llanes-Acevedo IP, García E, Nieto J, Moreno J, Cruz I. Molecular typing of Leishmania infantum isolates from a leishmaniasis outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2013;18: 20545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AM: Alpes-Maritimes; P: Provence; CE: Cévennes; CO: Corsica. VL–Visceral leishmaniasis; IVL–Infant under 15 years visceral leishmaniasis; CL–Cutaneous leishmaniasis; MCL–Muco-cutaneous leishmaniasis; RVL—New episode of leishmaniasis in patients; AC–Asymptomatic carrier; CanL–Canine leishmaniasis; CatL–Leishmaniasis in cat; PHLE: isolate from phlebotomine sandfly; UK–Unknown. n. d. = not defined.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.