Abstract
The growth and survival of intracellular parasites depends on the availability of extracellular nutrients. Deprivation of nutrients viz glucose or amino acid alters redox balance in mammalian cells as well as some lower organisms. To further understand the relationship, the mechanistic role of L-arginine in regulation of redox mediated survival of Leishmania donovani promastigotes was investigated. L-arginine deprivation from the culture medium was found to inhibit cell growth, reduce proliferation and increase L-arginine uptake. Relative expression of enzymes, involved in L-arginine metabolism, which leads to polyamine and trypanothione biosynthesis, were downregulated causing decreased production of polyamines in L-arginine deprived parasites and cell death. The resultant increase in reactive oxygen species (ROS), due to L-arginine deprivation, correlated with increased NADP+/NADPH ratio, decreased superoxide dismutase (SOD) level, increased lipid peroxidation and reduced thiol content. A deficiency of L-arginine triggered phosphatidyl serine externalization, a change in mitochondrial membrane potential, release of intracellular calcium and cytochrome-c. This finally led to DNA damage in Leishmania promastigotes. In summary, the growth and survival of Leishmania depends on the availability of extracellular L-arginine. In its absence the parasite undergoes ROS mediated, caspase-independent apoptosis-like cell death. Therefore, L-arginine metabolism pathway could be a probable target for controlling the growth of Leishmania parasites and disease pathogenesis.
Author Summary
Leishmania donovani, the causative agent of Indian Visceral Leishmaniasis, resides in the gut of the insect vector and the macrophages of their mammalian host and avail nutrients for survival. Nutrient deprivation such as glucose or amino acid alters redox balance in mammalian cells as well as some lower organisms. However, the role of L-arginine, in regulation of redox balance and L. donovani survival yet not properly elucidated. In the present study, we found that L-arginine deprivation from the culture medium hinders growth and proliferation of Leishmania promastigotes. Starvation of L-arginine downregulates the expression of polyamine biosynthetic and thiol metabolic pathway enzymes leading to decreased production of polyamines in Leishmania parasites. Moreover, deprivation of L-arginine alters redox balance in Leishmania promastigotes characterized by the concomitant increase in ROS and decreased antioxidant level. Furthermore, L-arginine deprivation triggered phosphatidyl serine externalization, alteration in mitochondrial membrane potential, release of intraellular calcium and cytochrome-c followed by DNA damage. In summary, the growth and survival of Leishmania depends on the availability of extracellular L-arginine, in absence of which the parasite undergoes ROS mediated, caspase-independent apoptosis-like cell death. Therefore, targeting L-arginine metabolism pathway could be an alternative approach for controlling Leishmania growth and hence disease outcome.
Introduction
Leishmaniasis, one of the most neglected tropical diseases, is considered as a major global threat spread over 98 countries throughout 5 continents. Among different forms of leishmaniasis, Visceral Leishmaniasis (VL), the most severe one, has a disease burden of 0.2 to 0.4 million cases with a mortality rate of 20,000 to 40,000 reported per year [1]. Leishmania donovani, the etiological agent of Indian VL, is an obligatory parasite that harbors inside the sand fly mid-gut and the macrophages of their mammalian host [2, 3]. Maintenance of their niches is crucial for survival and replication of these parasites. Cell death in form of apoptosis carefully controls the population of these parasites which in turn helps in disease progression [4, 5].
Apoptosis or Programmed cell death (PCD) plays a critical role in development and defense response of multicellular organisms [6]. In unicellular trypanosomatids including Leishmania sp., apoptosis is a triggered response to different stimuli ranging from heat shock [7–9], reactive oxygen species (ROS) [10–13], antiparasitic drugs [14, 15], starvation [16–18] to antimicrobial peptides [19–20]. Sequentials events encompassing accumulation of reactive oxygen species (ROS), lipid peroxidation, release of intracellular calcium (Ca2+) into cytosol, mitochondrial membrane depolarization, externalization of phosphatidyl serine on the outer leaflet, leakage of cytochrome-c into cytosol and induction of caspases resulting in DNA cleavage characterize the onset of apoptosis [21, 22]. Though pathogenic protozoa lacks genes encoding caspases, the involvement of caspase like protease activity has been reported in the regulation of death process of some unicellular organisms [23]. Studies of Lee and co-workers showed that Leishmania promastigote undergoes an apoptosis like cell death independent of caspase activities after exposure with antimony [24].
When a cell fails to maintain cellular homeostasis utilizing the total available antioxidant capacity oxidative stress is generated that expedites the process of apoptosis [25]. To protect cells from ROS mediated apoptosis, the parasite must carefully control the level of ROS by upregulating antioxidant defense. Polyamines are one of the crucial molecules that have been shown to exert antioxidant activity [26, 27].
Amino acids in eukaryotes, serve as building blocks in protein biosynthesis and regulates osmotic balance by functioning as osmolytes. In some eukaryotes L-arginine, the precursor for the production of polyamines is not synthesized denovo and is imported to support cellular growth and to protect the cells under diseased conditions [28]. Apoptotic stimuli affect both cellular processes cell proliferation and apoptosis [29]. The role of L-arginine in the regulation of cell survival and apoptosis of some higher eukaryotes have been reported [30, 31]. Piacenza et al. showed the role of L-arginine in modulation of apoptotic death of T. cruzi epimastigotes [32].
Despite these advances, the precise role of L-arginine in the regulation of redox balance and ROS mediated apoptosis is still unclear in protozoan parasites particularly in Leishmania sp. In the present study, we have shown that L-arginine starvation hinders cell growth and proliferation of Leishmania parasite. As Leishmania parasite lacks the biosynthetic pathway of L-arginine, it upregulates the transport of L-arginine in starved conditions. While investigating the possible reason behind this reduced cell viability, we found that L-arginine deprivation downregulates the production of polyamines that are necessary for the Leishmania parasite and alters redox balance characterized by increased ROS level. This results in increased lipid peroxidation and NADP+/NADPH ratio followed by decreased Superoxide dismutase (SOD) activity and thiol levels. Simultaneously, it was observed that arginine starvation induces phosphatidyl serine externalization, mitochondrial membrane depolarization, release of intracellular calcium and cytochrome-c that ultimately damages DNA and promotes apoptosis. Collectively our study reveals, for the first time, the role of L-arginine in redox homeostasis and apoptosis-like cell death in Leishmania parasites.
Materials and Methods
Culture of parasite
Leishmania donovani strain AG83 (MHOM/IN/1983/AG83) originally obtained from an Indian Kala-azar patient was maintained routinely in golden hamsters, as described earlier [33]. In this study, Leishmania parasites were grown for 2 generations at 22°C separately in amino acids free and L-arginine free RPMI media (Invitrogen) and supplemented with 10% heat-inactivated dialyzed fetal bovine serum (FBS) (Gibco-BRL), 25 mM HEPES, pH-7.4, 4 mM NaHCO3, 100 U/ml of penicillin G-sodium (Sigma-Aldrich) and 100 mg/ml of streptomycin (Sigma-Aldrich) and these parasites were used for our experimental purpose. As per our experimental set up, the exponential phase promastigotes were further grown either in single amino acid (For eg. L-arginine, lysine, glutamine, proline) containing or single amino acid depleted RPMI medium (Invitrogen).
Cell viability assay
The effect of L-arginine on the viability of L. donovani promastigotes were analyzed by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenylterazolium bromide (MTT) assay as described previously [34, 35]. Leishmania promastigotes of the exponential phase were grown in complete RPMI media (Invitrogen), RPMI media with different concentrations (0–200 mg/L) of L-arginine (Sigma-Aldrich), single amino acid free or supplemented RPMI media as well as complete amino acid free RPMI media for 0–120 hrs. The OD was recorded on an ELISA reader (Multiskan EX; Thermo Fisher Scientific, Waltham, MA) at 570 nm and percent cell viability was determined. The growth of the parasite in absence of L-arginine but presence of L-lysine, glutamine, proline (all from Sigma-Aldrich) or L-ornithine, putrescine, spermidine, spermine (all from Merck-Millipore) as well as in presence of L-arginine was assessed by trypan blue dye exclusion method. Viable cells were quantified by counting the number of non-stained cells. Results were expressed as mean±SD for three independent experiments performed in triplicate.
BrdU cell proliferation assay
Rate of proliferation of the parasite was analyzed using Cell Proliferation ELISA BrdU colorimetric kit (Roche) as per manufacturer’s instruction as described previously [36]. Briefly ~1×105 parasites/100 μl media were cultured in a 96 well microplate. At different time points, the cells were incubated with 1 μl of 5-Bromo-2-DeoxyUridine (BrdU) labelling agent (final conc. 10 μM) for 5 hr and its uptake was measured at 450 nm on an ELISA reader (Multiskan EX; Thermo Fisher Scientific, Waltham, MA). Results were expressed as mean±SD for three independent experiments performed in triplicate.
Experimental infection of macrophages with L. donovani parasites
THP-1cells were treated with 20 nM phorbol 12-myristate 7-acetate (PMA) for 12 hr to become adherent, matured macrophage-like phenotype. Non-adherent cells were removed by washing with RPMI without FBS. The cells were infected with Leishmania parasite for 6 hrs at parasite/macrophage multiplicities of 10:1. The unbound parasites were removed by washing with RPMI without FBS followed by additional incubation of upto 24 and 48 hr. The cells were then fixed and stained with May-Gruenwald giemsa and observed under bright field microscope at 100X with oil immersion. Percent infected macrophage was calculated by counting the number of infected cells and parasite load was determined by counting the number of amastigotes per 100 macrophages. Each measurement was performed in triplicate and the data was expressed as mean±SD of three independent experiments.
Measurement of L-arginine, lysine, glutamine and proline transport
Uptake of L-arginine, lysine, glutamine and proline in Leishmania parasites was measured using a protocol as described elsewhere [37] with minor modification. Briefly, after individual incubations, promastigotes (1×107 parasites/ml) were pelleted down, washed twice with cold Earle’s Based Salt Solution (EBSS) containing (in mM) 117 NaCl, 26 NaHCO3, 5 KCl, 1.8 CaCl2, 1 NaH2PO4, 0.8 MgSO4, 5.5 glucose and resuspended in 100 μl of [3H]-L-arginine (0.5–5 μCi/ml) using stock from 1 mCi/ml (specific activity-51.5 Ci/mmole) or [3H]-lysine, [3H]-glutamine and [3H]-proline (1 μCi/ml) (Perkin-Elmer, Singapore) at 25°C. Uptake was stopped at different times by adding 200 μl of 50 mM ice-cold L-arginine (non-radioactive). Parasites were then washed twice with EBSS (200 μl), lysed in 200 μl lysing solution (0.1% SDS, 100 mM NaOH) and radioactivity was measured by liquid scintillation counter (Tri-Carb 2810 TR, Perkin Elmer, USA). Uptake was expressed as nmol/107 cells/min. Each measurement was performed in triplicate and data was expressed as mean±SD of three independent experiments.
Semi-quantitative RT-PCR
AD-Ld and AS-Ld parasites were harvested after 96 hr of incubation. RNA was extracted and c-DNA was prepared from 20μg of total RNA using c-DNA synthesis kit (Invitrogen). The c-DNAs were then amplified by PCR for polyamine biosynthetic and thiol metabolic pathway genes, such as ornithine decarboxylase (ODC), spermidine synthase (SPS), gamma-glutamyl cysteine synthetase (γ-GCS), trypanothione synthetase (TryS), trypanothione reductase (TR), cytoplasmic tryparedoxin (cTXN) and cytoplasmic tryparedoxin peroxidase (CTP) using gene specific primers (Supplementary S1 Table). PCR was performed and analysis was done according to the conditions as described previously [35]. Each measurement was performed in triplicate and data was expressed as mean±SD of three independent experiments.
Measurement of arginase activity
Arginase activity in L-arginine depleted Leishmania donovani (AD-Ld) and L-arginine supplemented Leishmania donovani (AS-Ld) parasite lysates was measured using a micromethod as described previously [38, 39]. Briefly, after individual incubations, cells were lysed in 0.1% Triton X-100 and added with 25 mM Tris-HCl. The cell lysate was then mixed with 10 mM MnCl2 and heated for 10 min at 56°C for enzyme activation. Following addition of 0.5 M L-arginine (pH 9.7) to the cell lysate and incubation at 37°C for 15–20 min, the reaction was stopped with H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v) mixture. α-isonitrosopropiophenone was added to the mixture and heated at 95°C for 30 min. The urea concentration was then measured spectrophotometrically at 540 nm and total protein concentration was estimated through Bradford method. Each measurement was performed in triplicate and data was expressed as mean±SD of three independent experiments.
Measurement of polyamines
The level of intracellular polyamines such as putrescine and spermidine in the leishmania parasites grown in arginine depleted and arginine supplemented media (AD-Ld and AS-Ld) for 24–120 hr were identified and quantified by high performance liquid chromatography (HPLC) using a prederivatization method as described previously [39, 40]. Briefly, after individual incubations, the parasites were harvested, washed with PBS (pH 7.4) and disintegrated with 5% (w/v) trichloroacetic acid for overnight. After centrifugation the supernatants were collected and neutralized with saturated NaHCO3 solution. Dansylation of the mixture was done using dansyl chloride (20 mg/ml) (Merck) in acetone (Merck, HPLC grade) at 50°C for overnight. The polyamines were extracted with toluene (Merck, HPLC grade), and evaporated under nitrogen stream. The residue was dissolved in acetonitrile (Merck, HPLC grade) and analyzed by HPLC on a C18 reverse-phase column (Shimadzu, Japan). Dansylated polyamines were detected and quantified by spectrofluorometric measurement (excitation wavelength 330 nm, emission wavelength 510 nm). The peak areas, retention times were recorded and calculated by a PC Integration Pack Programme. Polyamines were expressed as nmol per 107 promastigotes. There were three replicates in each test and the data were the mean±SD of three independent observations.
Measurement of reactive oxygen species
Leishmania parasites were grown in different invitro culture conditions (For eg. arginine depletion, AD-Ld; arginine depletion but L-ornithine supplementation, AD-Ld/Orn+; arginine depletion but putrescine supplementation, AD-Ld/Put+; arginine depletion but NAC treatment, AD-Ld/NAC+ and arginine supplementation, AS-Ld) for 0–120 hr. After individual incubations were over, the cells were harvested and the level of intracellular ROS was monitored by staining the cells with the oxidative fluorescent dye 2', 7'-dichlorodihydrofluorescein diacetate (H2DCFDA) (0.4 μM) (Sigma) for 15 min at 37°C and then analyzed using LS-55 spectrofluorometer (Perkin Elmer, USA) as described previously [41]. Absorbance and emission wavelength were 504 and 529 nm respectively. There were three replicates in each test, and the data were the mean±SD of three independent observations.
Measurement of total fluorescent lipid peroxidation product
Leishmania parasites were grown in different invitro culture media for 0–120 hr as mentioned earlier. After individual incubations, AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+ and AS-Ld parasites were harvested, washed with 1X PBS and dissolved in 15% SDS-PBS solution. The total fluorescent lipid peroxidation products were estimated spectrofluorometrically with excitation at 360 nm and emission at 430 nm. The results were expressed as relative fluorescence units with respect to quinine sulphate (1 mg/ml in 0.5 M H2SO4) [42, 43]. The data were mean±SD for three independent experiments performed in triplicate.
Determination of reduced thiol content
The total intracellular reduced thiol level was measured in deproteinized cell extracts from Leishmania parasites grown for 72–120 hr in different invitro culture conditions (AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+ and AS-Ld) by the method as described elsewhere [44]. Briefly, the cells were harvested, washed with a buffer [0.14 M Na3PO4, 0.14 M K3PO4, 0.14 M NaCl, and 3 mM KCl, pH-7.4] and suspended in 25% trichloroacetic acid. The denatured protein and cell debris were removed by centrifugation. Thiol content in the supernatant was determined with 0.6 mM 5, 5’-dithio-bis(2-nitrobenzoic acid) (DTNB, Ellman’s reagent) in 0.2 M Na3PO4 buffer, pH 8.0. DTNB derivatives of thiols were estimated spectrophotometrically at 412 nm. There were three replicates in each test, and the data were expressed as mean±SD of three independent observations.
Determination of SOD activity
SOD activity was measured by using SOD assay kit (SOD assay kit, Calbiochem) as per manufacturer’s instruction. Briefly, after individual incubations, the parasites, as mentioned earlier, grown in different invitro culture conditions (AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+ and AS-Ld) for 72–120 hr, were pelletted down, homogenized in cold 20 mM HEPES buffer (pH-7.2, containing 1 mM EGTA, 210 mM mannitol and 70 mM sucrose) and the supernatants were collected for assay. To 10 μl of supernatant, 200 μl of diluted radical detector was added followed by addition of 20 μl of diluted xanthine oxidase and incubation for 20 minutes. The absorbance was measured at 450 nm. One unit of SOD was defined as the amount of enzyme required to exhibit 50% dismutation of the superoxide radical. Data were expressed as mean±SD for three independent experiments performed in triplicate.
Measurement of NADP+/NADPH ratio
NADP+/NADPH ratio for the AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+ and AS-Ld parasites were assayed spectrophotometrically using NADP/NADPH Quantification Kit (Sigma) as per manufacturer’s instruction as described [45]. The reduced coenzyme was measured spectrophotometrically at 450 nm. Results were mean±SD for three independent experiments performed in triplicate.
Annexin-V binding assay
Annexin-V-FITC staining was performed by the method as described previously with Annexin-V-FLUOS staining kit (Roche) as per manufacturer’s instructions [46]. Briefly, after individual incubations, the Leishmania parasites grown in different invitro culture conditions (AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+ and AS-Ld) for 72–120 hr were harvested, washed twice in PBS (pH 7.2), resuspended in HEPES buffer followed by addition of Annexin V-FITC and PI. The cells were then incubated for 15 min in the dark at 25°C and acquired on BD FACS Aria II flow cytometer followed by analysis with BD FACS Diva software. Results were expressed as mean±SD for three independent experiments performed in triplicate.
Measurement of mitochondrial membrane potential (Δψm)
Change in mitochondrial membrane potential (Δψm) in Leishmania promastigotes was estimated using JC-1 dye as described previously [46]. Briefly, after 72–120 hr of incubation, Leishmania parasites grown in different invitro conditions (For eg. arginine depletion, AD-Ld; arginine depletion but L-ornithine supplementation, AD-Ld/Orn+; arginine depletion but putrescine supplementation, AD-Ld/Put+; arginine depletion but NAC treatment, AD-Ld/NAC+; L-arginine supplementation, AS-Ld and L-arginine supplementation but FCCP treatment, AS-Ld/FCCP+) were harvested and incubated with 10 μM JC-1 for 10 min at 37°C. The cells were washed, resuspended in media followed by fluorescence measurement using LS-55 spectrofluorometer (Perkin Elmer, USA). Relative Δψm value was expressed as ratio of the reading at 590 nm to the reading at 530 nm. Results were expressed as mean±SD from three independent experiments performed in triplicate.
Measurement of intracellular calcium (Ca2+) level
Intracellular Calcium concentration in AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+, AD-Ld/EGTA+ and AS-Ld parasites was measured following the protocol and using the fluorescent probe Fura-2-AM as described previously [43]. Each measurement was performed in triplicate and the data were expressed as mean±SD for three independent experiments.
TUNEL assay
The occurrence of DNA nicking generated during apoptosis was detected by the Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay as described previously using In situ Cell Death Detection kit, Fluorescein (Roche) as per manufacturer’s instruction [47]. Briefly, AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+ and AS-Ld parasites were harvested as mentioned earlier and dissolved in 100 μl of 1X PBS followed by addition of 50 μl of Fixation and Permeabilizing solution and incubation at 4°C for 15 min. The cells were pelleted down, washed with 1X PBS, resuspended in 50 μl of TUNEL mixture and incubated at 22°C BOD incubator for 1 hr. Finally, the cells were washed and resuspended in PBS and analyzed by flow cytometry (BD FACS Aria II). Each measurement was performed in triplicate and the data were expressed as mean±SD for three independent experiments.
Cell death detection ELISA
DNA fragmentation was assessed using Cell Death Detection ELISA plus kit (Roche) as per manufacturer’s instruction. Briefly, after individual incubations, AD-Ld, AD-Ld/Orn+, AD-Ld/Put+, AD-Ld/NAC+ and AS-Ld parasites were harvested, lysed and reacted with biotin-coupled mouse monoclonal anti-histone antibody. The complex was detected by peroxidase-conjugated mouse monoclonal anti-DNA antibody and ABTS [2, 2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)] as a developing reagent to quantify the cytoplasmic nucleosomes generated due to DNA fragmentation. Absorbance was measured in a Thermo Multiskan EX plate reader at 405 nm and the results were expressed as relative percentages. Each measurement was performed in triplicate and the data were expressed as mean±SD for three independent experiments.
Immunoblot analysis
Leishmania parasites grown in different invitro conditions (For eg. arginine depletion, AD-Ld; arginine depletion but L-ornithine supplementation, AD-Ld/Orn+; arginine depletion but putrescine supplementation, AD-Ld/Put+; arginine depletion but NAC treatment, AD-Ld/NAC+; L-arginine supplementation, AS-Ld and L-arginine supplementation but Camptothecin-B treatment, AS-Ld/CPT+) were harvested after 96 hrs of incubation and washed twice with 1X PBS, suspended in cell fractionation buffer (ApoAlert cell fractionation kit, BD), homogenized and cytosolic & mitochondrial fractions were separated by following the manufacturer’s instructions (Clontech, Palo Alto, CA, USA) [47]. For confirmation of the semi-quantitative RT-PCR data, 50μg cytosolic fractions collected from AD-Ld and AS-Ld parasites were run on 12% SDS-polyacrylamide gel, immunoblotted and probed with rabbit polyclonal anti-TryS (1:5000), anti-TR (1:2000) and anti-CTP (1:5000) antibodies. For cytochrome-c detection, the cytosolic fraction from all the treated parasites were immunoblotted and probed with the rabbit polyclonal anti-cytochrome-c antibody (1:1000). Horseradish peroxidase-conjugated secondary antibody (1:10000) was used for all the cases and alpha-tubulin (α-Tub) was used as an endogenous control. The protein band was visualized by enhanced chemiluminescence reaction and expressed as fold change.
Assay of caspase activity
To determine the caspase activity (caspase—1, 3, 5, 8 and 9) of Leishmania parasites, a fluorogenic homogeneous caspase assay was performed using the Caspase Family Fluorometric Substrate Set plus kit (Bio-Vision) according to manufacturer’s instructions. The fluorometric measurement was recorded with a LS-55 spectrofluorometer (Perkin Elmer, USA) with excitation at 400 nm and emission at 505 nm. Each measurement was performed in triplicate and data was expressed as mean±SD of three independent experiments.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Program (Version 6.0, GraphPad Software, USA). All results were shown as mean±SD. Statistically significant differences were determined using students t-test. P values equal or below 0.05 were considered significant.
Results
L-arginine is crucial for growth of L. donovani promastigotes
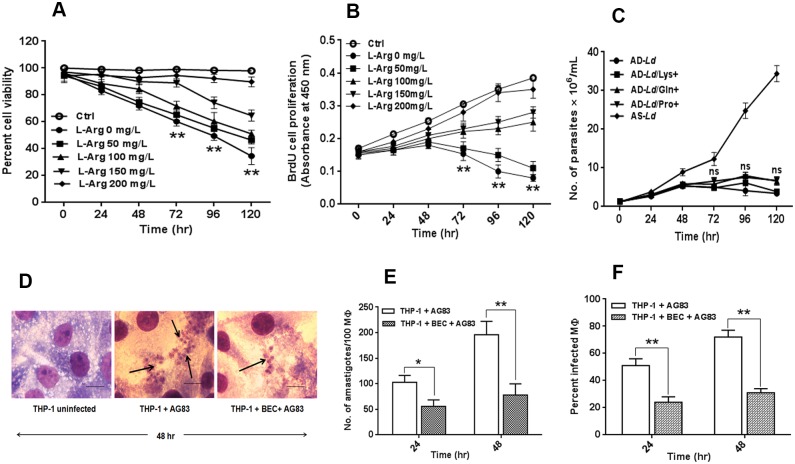
In this study, we used RPMI-1640 without L-arginine media and supplemented with upto 200 mg/L (1.149 mM) of L-arginine to characterize the role of L-arginine in the growth and survival of L. donovani promastigotes [48, 49]. Results showed that the percent cell viability decreased with increasing time interval. A significant difference (~1.65 fold, ~2.0 fold and ~2.8 fold) in reduction in cell viability was observed at 72 hr, 96 hr and 120 hr respectively for L-arginine deprived parasites compared to control parasites (Fig 1A).
Fig 1. L-arginine deprivation reduces cell viability, controls growth and proliferation of Leishmania parasite.
(A-C) L. donovani promastigotes were grown in normal RPMI (Ctrl or AS-Ld), L-arginine depleted RPMI media (L-Arg 0 mg/L or AD-Ld), supplemented with 50–200 mg/L of L-arginine or 200 mg/L lysine (AD-Ld/Lys+), glutamine (AD-Ld/Gln+) and proline (AD-Ld/Pro+) as described in “Materials and methods”. The percent cell viability of the parasite was determined by MTT assay (A). The rate of parasite proliferation was determined by BrdU colorimetric cell proliferation ELISA (B). The growth rate of the parasite was determined by counting the number of viable cells using trypan blue dye exclusion method (C). The data represents mean±SD of triplicate determinations and are representative of three independent experiments. *, P<0.05 (Student’s t test), **, P<0.001 compared to control (or AD-Ld as applicable). ns, non-significant. (D-F) THP-1 macrophages were either uninfected or infected with Leishmania parasites in absence or presence of BEC (200μM), an inhibitor of L-arginine utilizing enzyme, arginase for 24 hr and 48 hr. Intracellular parasites were visualized by staining the cells with giemsa followed by optical microscopy at 100X oil immersion (D). The horizontal bar indicates 5μm size. The parasite load was measured by counting the number of intracellular amastigotes per 100 macrophages (MΦ) (E). The rate of infection was analyzed by counting the percent infected macrophages (F). The data represent mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to control (or infected macrophage as applicable). ns, non-significant
The viability of the Leishmania parasite in single amino acid free (for eg. L-arginine, lysine, glutamine and proline) as well as complete amino acid free (AA-) RPMI media were also analyzed. It was found that the viability of the Leishmania parasite is significantly reduced in amino acid deprived media and L-arginine deprived media compared to normal RPMI media (ctrl). However, the reduction in cell viability observed in lysine, glutamine or proline free media was insignificant (S1A Fig). Simultaneously, cell viability in amino acid free but single amino acid supplemented (200 mg/L) media was also investigated. The result indicates that single amino acid L-arginine supplementation restored the cell viability upto ~80% whereas other amino acids failed to restore the growth (S1B Fig).
BrdU cell proliferation assay was conducted and found in all cases, that the rate of cell proliferation increased with increasing time intervals up to 48 hr. However, the rate of cell proliferation decreased from the 48 hr time point onwards. Despite L-arginine supplemented parasites (L-Arg 100–200 mg/L) showing considerably increased proliferation up to 120 hr, the rate of cell proliferation decreased gradually in L-arginine deprived parasites (L-Arg 50 mg/L and L-Arg 0 mg/L) up to 120 hr (Fig 1B). The minimum Leishmania parasites proliferation rate was observed when grown in L-arginine depleted media. The decrease in rate of cell proliferation was found to be ~2.0 fold, ~3.5 fold and ~4.8 fold at 72 hr, 96 hr and 120 hr respectively for L-arginine deprived parasites compared to parasites grown in normal RPMI media.
Experiments, to further understand the inhibition of growth, induced by L-arginine deprivation, were conducted to investigate whether supplementing equal amount of other amino acids such as lysine, glutamine and proline (200 mg/L) to the culture medium could reverse the effect. The addition of these amino acids did not produce any noticeable significant changes in the growth patterns of arginine depleted Leishmania donovani (AD-Ld) (Fig 1C).
To confirm the importance of L-arginine in the growth of the Leishmania parasite inside macrophages, we infected THP-1, a human monocytic macrophage like cell line with Leishmania parasite in the presence or absence of S-(2-Boronoethyl)-L-cysteine (BEC), an inhibitor of L-arginine-utilizing enzyme, arginase for 24 hr and 48 hrs. The sole objective behind the use of BEC was to prevent the macrophages from utilizing the available L-arginine in the extracellular milieu. This was to create an L-arginine deprived condition for the macrophages and to examine the resulting effect on parasite survival by determining the parasitic load and percent infectivity. It was found that the parasite load (Number of amastigotes per 100 macrophages) decreased by ~1.8 fold and ~2.5 fold at 24 hr and 48 hr respectively in presence of BEC (Fig 1D and 1E) when compared to control. Simultaneously, percent infectivity also decreased by ~2.1 fold and ~2.3 fold respectively at 24 hr and 48 hr in presence of BEC (Fig 1F). Therefore, our results indicate that inhibition of arginase or limited L-arginine availability led to reduced level of infection.
L-arginine starvation in Leishmania parasite up-regulates its transport in time and concentration dependent manner
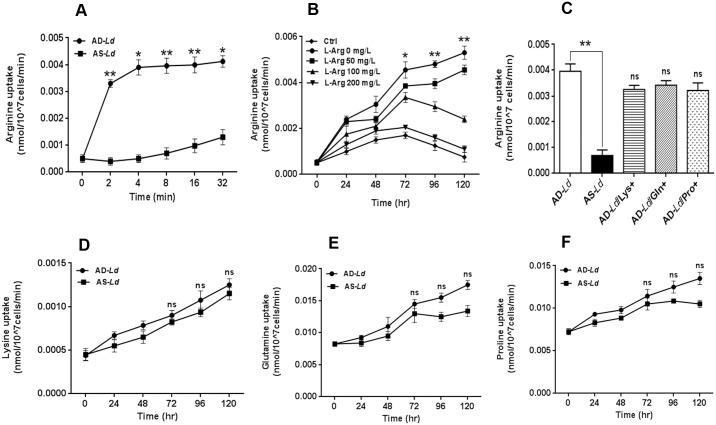
The importance of L-arginine was further characterized by measuring the radioactive L-arginine transport in Leishmania parasite. Firstly, experiments were performed to determine a concentration kinetics of radioactive L-arginine (0–5 μCi/ml) using stock from 1 mCi/ml (specific activity-51.5 Ci/mmole) and measure its transport in Leishmania parasites grown in L-arginine deprived or supplemented media. Results indicated that the uptake of L-arginine increases with increasing extracellular radioactive L-arginine concentration (S2 Fig). The rate of increase in transport was found to be comparatively higher in arginine depleted Leishmania donovani (AD-Ld) compared to arginine supplemented Leishmania donovani (AS-Ld). In both cases, measurable amounts of L-arginine uptake was observed when we used 1 μCi/ml of extracellular 3H L-arginine (S2 Fig). To understand how quickly L-arginine is taken up by the Leishmania parasite, we performed a time kinetics of L-arginine transport according to previously described methods [37]. We treated AD-Ld and AS-Ld parasites with 3H L-arginine for 0–32 minutes and found that within 4 minutes of incubation the uptake increases at a very high rate and afterwards reaches a plateau in case of AD-Ld parasites. In comparison, AS-Ld parasites uptake still occurred but with very slow rate (Fig 2A). This result indicated that the rate of L-arginine uptake depended on the time of incubation and most of the L-arginine was uptaken by the Leishmania parasites within 8 minutes. Therefore, based on the results obtained we selected 1 μCi/ml dose of L-arginine for 8 minutes incubation in all the subsequent experiments.
Fig 2. L-arginine deprivation in Leishmania parasite upregulates its transport in time and concentration dependent manner.
(A) Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media for 96 hr, incubated with 1μCi/ml of [3H] L-arginine uptake solution for 0–32 minutes and rate of uptake was measured. (B-C) Leishmania promastigotes were grown in normal RPMI (Ctrl or AS-Ld), L-arginine depleted RPMI media (L-Arg 0 mg/L or AD-Ld) as well as supplemented with 50–200 mg/L of L-arginine or 200 mg/L lysine (AD-Ld/Lys+), glutamine (AD-Ld/Gln+) and proline (AD-Ld/Pro+) as described in “Materials and methods” and incubated with 1μCi/ml of [3H] L-arginine uptake solution for 8 minutes. The rate of L-arginine transport was measured by counting the incorporation of [3H] L-arginine in liquid scintillation counter (A-C). The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.005 compared to AS-Ld or control (or AD-Ld as applicable) parasite as applicable. ns, non-significant. (D-F) Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media for 96 hr and incubated with 1μCi/ml of [3H] lysine, [3H] glutamine and [3H] proline uptake solution for 8 minutes. The rate of transport of lysine (D), glutamine (E) and proline (F) was measured by counting the radioactivity incorporation in liquid scintillation counter (D-F). The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AS-Ld parasite as applicable. ns, non-significant.
To further understand the effect of L-arginine starvation on the transport of L-arginine in a broader time interval, we performed transport measurement experiments in Leishmania parasites grown in L-arginine depleted media supplemented with different conc. of L-arginine (0–200 mg/L) for different time intervals (0–120 hr). It was found that L-arginine transport increased gradually with increasing time of incubation. AD-Ld parasites were found to uptake L-arginine at a very high rate in comparison to AS-Ld parasites. Significant difference in L-arginine uptake was observed 72 hours onwards. ~2.7 fold, ~3.8 fold and ~7.1 fold increase in L-arginine uptake was observed for AD-Ld parasites compared with AS-Ld parasites at 72 hr, 96 hr and 120 hr respectively (Fig 2B). The transport of L-arginine in the presence of other amino acids such as lysine, glutamine and proline was also measured (Fig 2C). However, no difference in uptake was observed between AD-Ld and AD-Ld/Lys+ or AD-Ld/Gln+ or AD-Ld/Pro+ parasites. On the other hand, when we measured the transport of lysine (Fig 2D), glutamine (Fig 2E) and proline (Fig 2F) in AD-Ld and AS-Ld parasites, no difference in uptake was observed. Taken together, these results indicate that L-arginine deprivation in Leishmania parasite specifically up-regulates its transport in time and concentration dependent manner.
L-arginine deprivation reduces expression of polyamine biosynthetic and thiol metabolic pathway enzymes and polyamine production
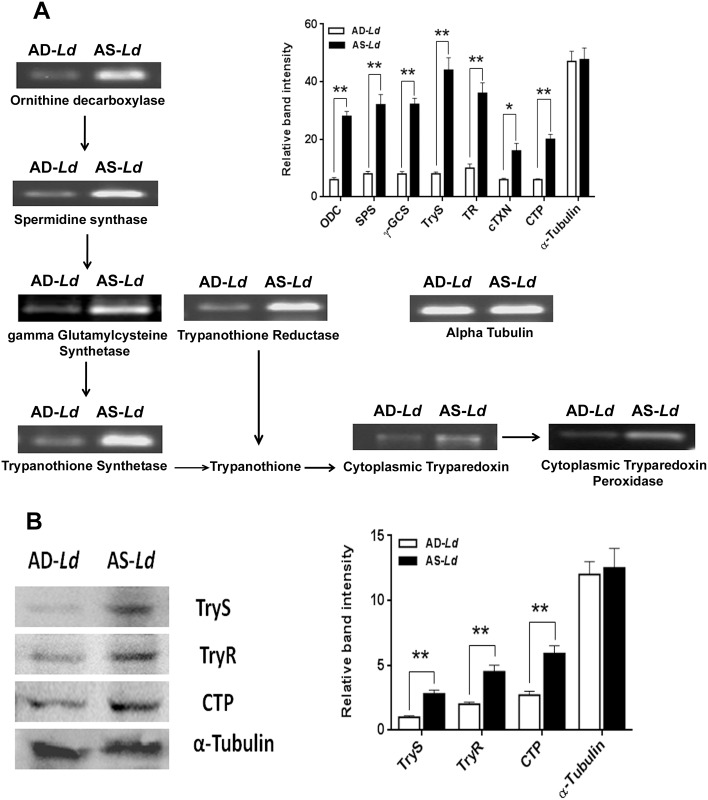
The polyamine biosynthetic and thiol metabolic pathway enzymes such as ODC, SPS, γ-GCS, TryS, TR, c-TXN and CTP are involved in the synthesis polyamines, trypanothione and tryparedoxin utilizing L-arginine as a primary substrate. Polyamines play a crucial role in the growth and survival of trypanosomatids including Leishmania parasites. The effect of L-arginine starvation on the transcripts of these enzymes were also determined. A significant decrease in transcript abundance of ODC (~4.7 fold), SPS (~4.0 fold), γ-GCS (~4.0 fold), TryS (~5.5 fold), TR (~3.6 fold), cTXN (~2.7 fold) and CTP (~3.3 fold) was observed for AD-Ld parasites as assessed by semi-quantitative RT PCR (Fig 3A).
Fig 3. L-arginine deprivation downregulates the expression of polyamine biosynthetic and thiol-metabolic pathway enzymes.
(A-B) Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media for 96 hr. (A) The cells were harvested, total RNA was extracted, c-DNA was prepared and expression of ornithine decarboxylase (ODC), spermidine synthase (SPS), γ-glutamyl cysteine synthetase (γ-GCS), trypanothione synthetase (TryS), trypanothione reductase (TR), cytoplasmic tryparedoxin (cTXN), cytoplasmic tryparedoxin peroxidase (CTP) and alpha-tubulin were analyzed by semi-QRT PCR using primers for the coding regions. The PCR products were run in ethidium bromide-stained 1.5% agarose gel and photographed in Gel documentation system. (B) The cells were harvested and cytoplasmic fractions were separated by the procedure as described in “Materials and method”. Cytosolic fractions collected from AD-Ld and AS-Ld parasites were run on SDS-polyacrylamide gel, immunoblotted and probed with rabbit polyclonal anti-leishmania TryS, TR, CTP and alpha-tubulin antibodies. The relative expression of the enzymes both at transcriptional and translational level were analyzed by densitometry (A-B). The data represent mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AS-Ld as applicable.
The expression of some of the enzymes of the polyamine and thiol metabolic pathway (For eg. TryS, TR and CTP) was also evaluated at the translational level by immunoblot analysis. A significant decrease in protein level of Trys (~2.8 fold), TR (~2.3 fold) and CTP (~2.2 fold) was observed for AD-Ld parasites compared to AS-Ld parasites (Fig 3B).
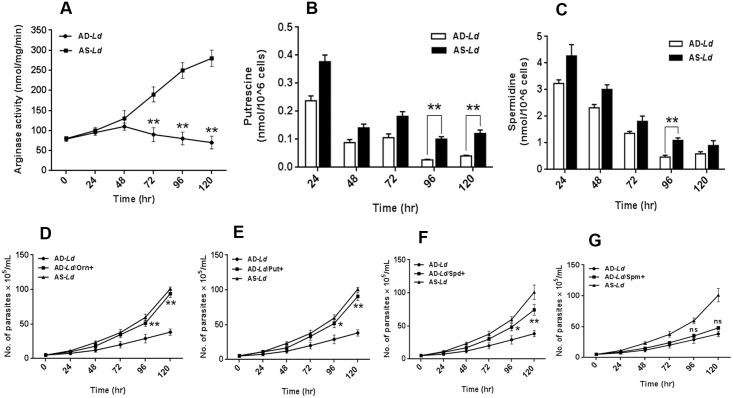
To study the effect of L-arginine availability in regulation of arginase, the first enzyme of arginine metabolism, the activity of the enzyme was also measured. Fig 4A shows the changes in arginase activity in arginine supplemented Leishmania donovani (AS-Ld) and arginine depleted Leishmania donovani (AD-Ld) parasites for 0–120 hr. Differences in arginase activity were apparent from 72 hours post incubation (Fig 4A). ~2.1 fold, ~3.1 fold and ~4.0 fold decrease in arginase activity was observed for AD-Ld parasites when compared with AS-Ld parasites at 72 hr, 96 hr and 120 hr respectively.
Fig 4. L-arginine deprivation reduces arginase activity and production of polyamines that supports the growth of Leishmania.
(A-C) Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media for 0–120 hr. Leishmanial arginase activity was measured spectrophotometrically following procedures as described in “Materials and methods”. The protein content was measured by Bradford method and the enzyme activity was expressed as nmol/mg/min (A). (B-C) The polyamines, putrescine and spermidine were extracted by TCA precipitation followed by dansyl chloride derivatization, separation by reverse phase HPLC as described in “Materials and methods”. Dansylated putrescine (B) and dansylated spermidine (C) were quantified by fluorescence spectrophotometry. The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable. ns, non-significant. (D-G) Leishmania parasites were grown in L-arginine depleted (AD-Ld), L-arginine supplemented (AS-Ld), L-arginine depleted but supplemented with 200 mg/L of L-ornithine (AD-Ld/Orn+) (D), putrescine (AD-Ld/Put+) (E), spermidine (AD-Ld/Spd+) (F) and spermine (AD-Ld/Spm+) (G) media for 0–120 hr. The growth rate of the parasites under different conditions was determined by microscopic counting of viable cells. The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable. ns, non-significant.
Subsequently, the level of intracellular polyamines (putrescine and spermidine) were quantified by HPLC. Interestingly, we found that at all the time intervals the level of polyamines were lower in arginine depleted Leishmania donovani (AD-Ld) parasites compared to arginine supplemented Leishmania donovani (AS-Ld) parasites. At 96 hr and 120 hour a marked difference in putrescine level (~3.7 and ~2.9 fold) was observed (Fig 4B), whereas the difference in spermidine content was found to be very less in AD-Ld and AS-Ld parasites (~1.7 and ~1.3 fold) (Fig 4C). However, the concentration of both polyamines decreased with increasing time in all cases.
L-ornithine and polyamine supplementation partially restores the growth of Leishmania parasite
We also determined the growth of the parasite in L-arginine depleted media and L-arginine depleted but supplemented with 200 mg/L of L-ornithine, putrescine, spermidine and spermine, in order to confirm that the inhibition of growth of Leishmania parasite in L-arginine deprived media is due to polyamine depletion. We have chosen this concentration of L-ornithine and polyamines because 200 mg/L of L-arginine was the optimum concentration required for Leishmania growth. L-ornithine and putrescine supplementation was found to almost completely restored the growth of the parasite (Fig 4D and 4E). In comparison, spermidine restored growth of the parasite to lesser extent (Fig 4F) and spermine had very negligible effects on restoration of growth (Fig 4G).
L-arginine deprivation alters redox balance in L. donovani promastigotes
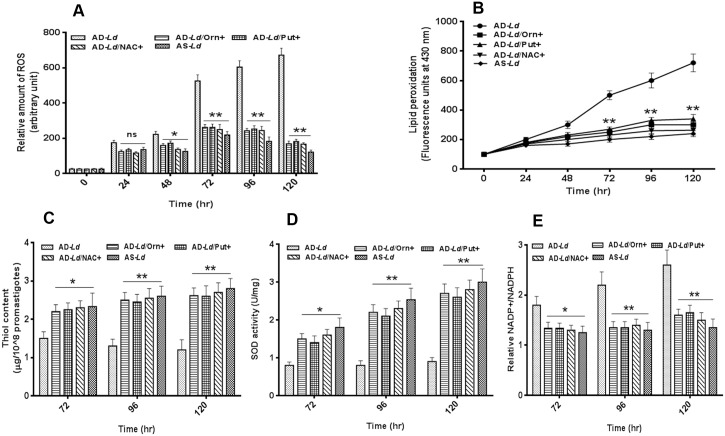
The involvement of different forms of ROS including superoxide and hydrogen peroxide (H2O2) has been reported to play a crucial role in regulation of cellular growth and cell death [50]. In this study, we targeted to explore whether L-arginine deprivation triggered intracellular ROS accumulation and induced apoptosis like cell death in L. donovani parasites. A gradual increase in ROS level was observed for arginine depleted Leishmania donovani (AD-Ld) promastigotes (Fig 5A) whereas no significant increase in ROS level was observed for arginine supplemented Leishmania donovani (AS-Ld) parasites. However, when AD-Ld parasites were pre-incubated with NAC, a ROS scavenger, the elevated ROS level fell to the control level. When, we compared the difference in ROS production between AD-Ld and AS-Ld parasites, it was found that ~2.4 fold, ~3.3 fold and ~3.5 fold induction in ROS level was observed after 72 hr, 96 hr and 120 hr of incubation respectively. Hence, this result indicated that induction in intracellular ROS production might be the possible reason for reduced cell viability for AD-Ld parasites.
Fig 5. L-arginine availability regulates redox balance in L. donovani promastigotes.
(A-E) Leishmania parasites were grown in L-arginine depleted (AD-Ld), L-arginine depleted but supplemented with ornithine (AD-Ld/Orn+), putrescine (AD-Ld/Put+), NAC (AD-Ld/NAC+) and L-arginine supplemented (AS-Ld) RPMI media for 0–120 hr. Intracellular ROS production was measured using the fluorescent dye 2, 7-dicholorodihydroflourescein diacetate (H2DCFDA) and NAC was used as a ROS scavenger (A). The rate of lipid peroxidation was measured by spectrofluorometry and expressed as fluorescence units at 430 nm (B). The total intracellular reduced thiol levels were measured spectrophotometrically using DTNB (C). SOD activity was measured by using SOD assay kit as described in “Materials and methods (D). The relative NADP+/NADPH ratio was measured spectrophotometrically based on the measurement of the absorbance of the reduced coenzyme at 450 nm (E). The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable.
Further, total fluorescent lipid peroxidised products were spectrophotometrically measured in arginine depleted Leishmania donovani (AD-Ld) and arginine supplemented Leishmania donovani (AS-Ld) parasites at 0–120 hr. A gradual significant increase in fluorescence intensity was observed for AD-Ld parasites with increasing time of incubation whereas in case of AS-Ld parasites, the increase was not significant (Fig 5B).
Non protein thiols are important antioxidants which maintain cellular redox homeostasis through nullifying oxidative perturbations [51]. A gradual decrease in thiol levels was observed for arginine depleted Leishmania donovani (AD-Ld) parasites with increasing time interval, whereas in case of arginine supplemented Leishmania donovani (AS-Ld) parasites a slow increase in thiol level was observed. Significant difference (for eg. ~1.6 fold, ~2.0 fold and ~2.3 fold decrease) in thiol content between AD-Ld and AS-Ld parasites was observed at 72 hr, 96 hr and 120 hr respectively (Fig 5C). The results were consistent with the increased ROS production due to L-arginine deprivation in Leishmania parasites.
To determine whether this oxidative stress generated in L. donovani in case of L-arginine deprivation is associated with reduced antioxidant level, we measured the activity of an anti-oxidant enzyme, SOD, a metal containing enzyme that is present in parasite L. donovani [52]. ~2.2 fold, ~3.1 fold and ~3.3 fold decrease in SOD activity was observed for arginine depleted Leishmania donovani (AD-Ld) parasites compared to arginine supplemented Leishmania donovani (AS-Ld) parasites at 72 hr, 96 hr and 120 hr of incubation respectively (Fig 5D). Therefore, this decrease in SOD activity in AD-Ld parasites might be one of the reason for increased ROS production in Leishmania parasites and hence decreasing the cell viability.
NADP, an enzymatic cofactor shuttles between the reduced (NADPH) and oxidized (NADP) forms, profoundly supports the major antioxidants and redox regulatory enzymes, by providing reducing potential in the form of NADPH [53]. A gradual increase in relative NADP+/NADPH ratio was observed for arginine depleted Leishmania donovani (AD-Ld) parasites with increasing time of incubation, whereas no such change was observed for arginine supplemented Leishmania donovani (AS-Ld) parasites (Fig 5E). A significant difference in relative NADP+/NADPH ratio was observed for AD-Ld parasites for 72 hr onwards compared to AS-Ld parasites. For eg. ~1.7 fold and ~1.9 fold increase in NADP+/NADPH ratio was observed for AD-Ld parasites at 96 hr and 120 hr of incubation. This is correlated with increased ROS production in AD-Ld parasites compared to AS-Ld parasites.
Leishmania promastigote undergoes an apoptosis-like cell death in absence of L-arginine
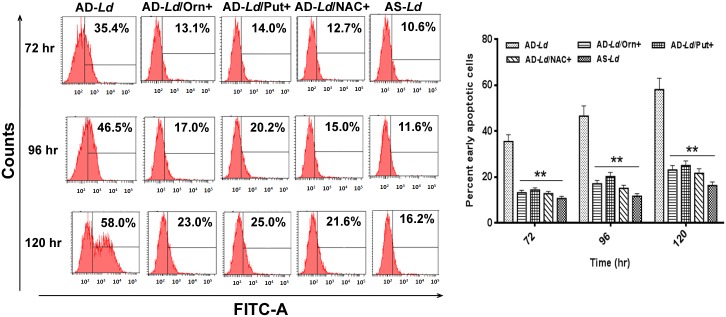
To elucidate the mechanism of cell death induced by L-arginine deprivation, both arginine depleted Leishmania donovani (AD-Ld) and arginine supplemented Leishmania donovani (AS-Ld) promastigotes were stained with Annexin-V-FITC and PI followed by analysis in flow cytometer. Although in both cases, gradual increase in apoptotic cells was observed, the percent of apoptotic cells was higher in case of L-arginine deprivation (Fig 6). Significant differences in percent apoptotic cells were observed at 72 hr, 96 hr and 120 hr (3.3 fold, 4.0 fold and 3.6 fold respectively). However, when AD-Ld parasites were supplemented with ornithine (200 mg/L) or putrescine (200 mg/L) or pre-incubated with NAC, a ROS scavenger, the percent apoptotic cells decreased to the control level.
Fig 6. L-arginine deprivation induces externalization of phosphatidyl serine in Leishmania parasite.
Leishmania parasites were grown in L-arginine depleted (AD-Ld), L-arginine depleted but supplemented with ornithine (AD-Ld/Orn+), putrescine (AD-Ld/Put+), NAC (AD-Ld/NAC+) and L-arginine supplemented (AS-Ld) RPMI media for 72–120 hr. The cells were co-stained with Annexin V-FITC and PI followed by analysis by flow cytometry as described in “Materials and methods”. Histogram of the Annexin-V assay in different conditions as mentioned were shown in figures. The graphs were plotted and percent early apoptotic Leishmanial cells were represented. The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test)**, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable.
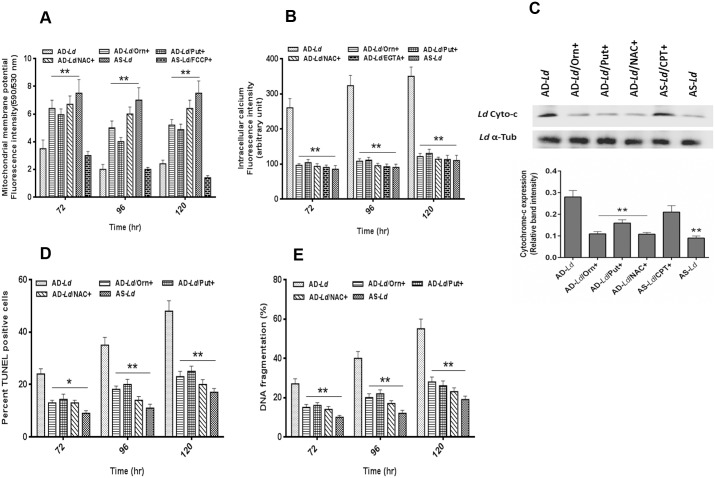
Apoptosis in protozoan parasites such as Leishmania is characterized by significant fall in mitochondrial membrane potential (Δψm) [10]. JC-1, a cationic lipophilic mito-sensor dye accumulates in the mitochondria of healthy cells. Our assessment of Δψm using JC-1 showed a gradual decrease in fluorescence intensity for arginine depleted Leishmania donovani (AD-Ld) parasites (Fig 7A). Simultaneously ~2.1 fold, ~3.5 fold and ~3.1 fold decrease in fluorescence intensity was observed for arginine depleted Leishmania donovani (AD-Ld) parasites when compared to arginine supplemented Leishmania donovani (AS-Ld) parasites at 72 hr, 96 hr and 120 hr incubation respectively. However, when AD-Ld parasites were supplemented with ornithine (200 mg/L) or putrescine (200 mg/L) or pre-incubated with NAC, the fluorescence intensity increased to the control level. Simultaneously, when AS-Ld parasites were treated with FCCP, a decrease in fluorescence intensity was observed. This result clearly demonstrated that overproduced ROS in L. donovani promastigotes led to alterations in mitochondrial membrane potential which validates previous reports [54].
Fig 7. L-arginine deprivation alters mitochondrial membrane potential (Δψm), regulates intracellular Ca2+ level, cytochrome-c release and damages DNA in Leishmania parasite.
(A-E) Leishmania parasites were grown in different invitro culture conditions (arginine depletion, AD-Ld; arginine depletion but L-ornithine supplementation, AD-Ld/Orn+; arginine depletion but putrescine supplementation, AD-Ld/Put+; arginine depletion but NAC treatment, AD-Ld/NAC+ and arginine supplementation, AS-Ld) for 0–120 hr. After individual incubations, the cells were harvested and stained with JC-1 probe and change in mitochondrial membrane potential (Δψm) was determined using spectrofluorometric measurement at 590 and 530 nm. FCCP was used as a positive control for mitochondrial membrane depolarization (A). Intracellular Ca2+ concentration was measured by using the fluorescent probe Fura-2-AM followed by spectrofluorometric measurement at 510 nm. EGTA was used as a calcium chelator in this experiment (B). The level of cytochrome-c in cytoplasmic fraction was measured by western blot followed by densitometric analysis and camptothecin-B (CPT) was used as positive control for release of cytochrome-c (C). The occurrence of DNA nicking was detected by the TUNEL assay using flow cytometer as described in “Materials and methods” and percent apoptotic (TUNEL positive) cells were counted (D). DNA fragmentation was measured by cell death detection ELISA (E). The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable. ns, non-significant.
Cytosolic increase in Ca2+ level has been found to be associated with mitochondrial membrane depolarization under oxidative stress in L. donovani [12]. A gradual increase in intracellular calcium concentration was observed for arginine depleted Leishmania donovani (AD-Ld) parasites whereas in case of arginine supplemented Leishmania donovani (AS-Ld) parasites no such increase was observed for 0–120 hr. Here, we found ~2.5 fold, ~3.1 fold, ~3.5 fold and ~3.2 fold increase in intracellular calcium level for AD-Ld parasites when compared to AS-Ld parasites at 48 hr, 72 hr, 96 hr and 120 hr respectively (Fig 7B). However, when AD-Ld parasites were supplemented with ornithine (200 mg/L) or putrescine (200 mg/L) or pre-incubated with NAC, the fluorescence intensity decreased to the control level. Furthermore, incubation with extracellular Ca2+ chelator, EGTA decreased the intracellular Ca2+ to normal level as depicted in the case of AS-Ld parasite. Therefore, the above findings mechanistically elucidate that ROS generation due to L-arginine deprivation causes irrepairable damage to calcium channels resulting in enhancement of intracellular calcium reserve followed by loss of Δψm leading to an apoptosis-like cell death.
Cytochrome-c, localized in the inner mitochondrial membrane space, comprises the electron transport chain and is released into the cytosol during the initial steps of apoptosis. We performed western blot hybridization (Fig 7C) to determine the localization of cytochrome-c in arginine depleted Leishmania donovani (AD-Ld) and arginine supplemented Leishmania donovani (AS-Ld) parasites. A small amount of cytochrome-c was found in the cytoplasmic fraction of the AS-Ld promastigotes whereas cytochrome-c was abundant in the cytoplasmic fraction of AD-Ld promastigotes. ~3.1 fold increase in cytochrome-c band intensity was observed for AD-Ld parasites compared to AS-Ld parasite at 96 hr. However, when AD-Ld parasites were supplemented with ornithine (200 mg/L) or putrescine (200 mg/L) or pre-incubated with NAC, significant decrease in release of cytochrome-c compared to AD-Ld parasites were observed. Significant increase in release of cytochrome-c was also observed for AS-Ld parasites treated with camptothecin (CPT).
Caspase-like protease activity responsive to different apoptotic stimuli has been reported to control the cellular death of unicellular kinetoplastid parasites [10, 24]. Therefore, in the present study, we investigated whether there is any change in caspase-like protease activity due to L-arginine deprivation in L. donovani parasites. However, we did not find any significant increase in caspase activity (Caspase-1, 3, 5, 8, 9) in arginine depleted Leishmania donovani (AD-Ld) parasites as compared to arginine supplemented Leishmania donovani (AS-Ld) parasites (S3A–S3E Fig).
The occurrence of DNA nicking and internucleosomal DNA digestion is a hallmark of apoptosis-like cell death [55]. TUNEL assay based analysis detected FITC labeled dUTP attached to the nick ends through TdT and revealed the presence of nicking of DNA in AD-Ld parasites. ~ 2.0 fold, ~2.5 fold and ~2.5 fold increase in percent TUNEL positive cells were observed for arginine depleted Leishmania donovani (AD-Ld) parasites compared to arginine supplemented Leishmania donovani (AS-Ld) parasites at 72 hr, 96 hr and 120 hr of incubation respectively (Fig 7D). However, when AD-Ld parasites were supplemented with ornithine (200 mg/L) or putrescine (200 mg/L) or pre-incubated with NAC, significant decrease in TUNEL positive cells were observed when compared to AD-Ld parasites.
To investigate whether L-arginine deprivation of L. donovani cells also resulted in occurrence of DNA fragmentation, we carried out genomic DNA fragmentation assay by determining cytoplasmic histone associated mono and oligonucleosomes as described in ‘‘Materials and methods”. ~ 2.7 fold, ~3.3 fold and ~2.9 fold increase in percent DNA fragmentation was observed for arginine depleted Leishmania donovani (AD-Ld) parasites compared to arginine supplemented Leishmania donovani (AS-Ld) parasites at 72 hr, 96 hr and 120 hr of incubation respectively (Fig 7E). Furthermore, when AD-Ld parasites were supplemented with ornithine (200 mg/L) or putrescine (200 mg/L) or pre-incubated with NAC, significant decrease in percent DNA fragmentation compared to AD-Ld parasites were observed.
Discussion
The importance of L-arginine for the growth of Leishmania parasite was demonstrated in 1970s by Krassner et al. [56] indicating that these parasites import this amino acid for the growth in invitro culture media [56, 57]. However, how L-arginine is utilized by the Leishmania parasite and promotes growth has not been clearly elucidated.
Our study revealed that the growth of the Leishmania parasite is hindered in absence of L-arginine. Simultaneously the rate of proliferation of Leishmania parasite also gets reduced in absence of L-arginine, whereas L-arginine supplementation again increases the rate of proliferation, indicating that the growth and proliferation of the Leishmania promastigote depends on the availability of L-arginine in the extracellular milieu. Therefore, the decrease in Leishmania growth and viability is associated with reduced proliferation. Interestingly supplementation of L-arginine deprived parasite with other amino acids such as lysine, glutamine or proline alone could not restore the growth of the parasite. The survival of the Leishmania parasite inside macrophage also gets reduced in presence of inhibitor of arginase. Therefore, it indicates that L-arginine is essential for the growth of the parasite and other amino acids could not act as replacement. This establishes the importance and uniqueness of this amino acid in the survival of the Leishmania parasite. Findings from our experiment is in agreement with the previous report [58, 59] which states that as there is no evidence for denovo synthesis of L-arginine in the Leishmania parasite, its metabolism depends on extracellular supplies, making L-arginine, an essential amino acid for the growth and survival. Similar type of result was also observed for T. gondii parasite, an obligate intracellular parasite [60].
L-arginine deprivation also resulted in its increased uptake. However, no difference in uptake of other amino acids such as lysine, glutamine or proline was observed between AD-Ld and AS-Ld parasites. Our observation is in agreement with the studies of Darlyuk I et al. [61] and indicates that upon sensing low concentration of L-arginine, L. donovani promastigotes specifically increases the L-arginine uptake probably by upregulating the expression of its transporter.
Polyamines (PAs) like putrescine and spermidine provide critical biosynthetic input in the synthetic machinery of trypanothione, thereby promoting growth and survival of trypanosomatid protozoa [62]. The metabolic process which generates L-ornithine following enzymatic hydrolysis of L-arginine involves arginase on one hand and ODC on the other, to generate putrescine via decarboxylation of L-ornithine. Our previous study suggested that the ROS generated due to autooxidation of amphotericin B was efficiently nullified by polyamine biosynthetic and thiol metabolic pathway enzymes in resistant parasite [35]. Therefore, it is evident that L-ornithine, putrescine along with spermidine feeds the cellular antioxidant machinery to cope up with different forms of oxidative stress. In this study, we found a very low m-RNA expression of polyamine biosynthetic and thiol metabolic pathway genes such as ODC, γ-GCS, SPS and in particular TryS, TR, c-TXN and CTP for L-arginine deprived parasites whereas in case of L-arginine supplemented parasites all these genes were upregulated. The activity of arginase, the first enzyme of arginine metabolism was also found to be downregulated for L-arginine deprived parasites. The activity of the enzyme depends on the availability of the substrate. Here, as the substrate, L-arginine is limited; the activity of the downstream enzyme gets decreased both at transcript and translational level as observed through semi-quantitative RT-PCR and immunoblot analysis respectively. It can therefore be concluded that due to non-availability of arginine, the expression of polyamine biosynthetic and thiol metabolic pathway enzymes get downregulated and that results in the decreased production of polyamines such as putrescine and spermidine.
To understand the mechanism of reduced growth rate and cell viability of L. donovani parasite in L-arginine depleted media, we assayed intracellular ROS generation. Interestingly, significant increase in intracellular ROS generation was observed for AD-Ld parasites compared to AS-Ld parasites. It was also observed that L-arginine supplementation decreases the ROS level. Induction of ROS due to nutrient deprivation (such as glucose and amino acid) has been reported previously [63]. To the best of our knowledge this is the first report describing ROS generation due to L-arginine deprivation in L. donovani. Therefore, it can be concluded that ROS generation might be one of the causes for reduced cell viability and growth of Leishmania parasite in L-arginine depleted media.
Studies of Das et al. [64] showed that curcumin treatment to Leishmania parasite leads to an increase in lipid peroxides levels. Increase in lipid peroxide with increasing time was also observed for L. donovani after spiningerin treatment by Sardar et al. [43]. In this study, we found a significant increase in lipid peroxide level for Leishmania promastigotes grown in L-arginine depleted media with increasing time interval, whereas L-arginine supplementation restores this increase in lipid peroxidation. Hence, it can be concluded that ROS generation due to L-arginine deprivation might be one of the causes for increasing lipid peroxidation that ultimately leads to reduced cell viability and growth of Leishmania parasite.
The free radicals elicited by the phagocytic macrophages is successfully evaded by the parasitic protozoan Leishmania via utilization of elaborate network of non-protein thiols like glutathione and trypanothione [51]. In this study, a significant difference in thiol level between AD-Ld and AS-Ld parasite was observed with increasing time of incubation. The decreased intracellular thiol level in AD-Ld parasites is correlated with increased intracellular ROS production leading to decreased parasite survival.
The cellular concentration of ROS is controlled by anti-oxidant enzymes. The balance between ROS generation and ROS elimination by antioxidant enzymes helps to maintain cellular function. Therefore, a decrease in anti-oxidant enzyme levels leads to an overall increase in intracellular ROS levels and causes cell death [65]. The involvement of SOD in detoxification of reactive superoxide radicals produced by activated macrophages and survival had been described in Leishmania promastigotes [66]. In this study, we tried to investigate the probable reason of reduced cell viability of Leishmania donovani in L-arginine deprived condition. As increased ROS level is associated with reduced cell survival and apoptosis and SOD is an anti-oxidant enzyme that helps to neutralize ROS, we measured the activity of SOD. In this study, a gradual decrease in SOD activity was observed for Leishmania parasites grown in L-arginine deprived media, whereas in case of parasites grown in L-arginine enriched media, SOD activity gradually increased. Therefore, it is obvious that this reduced SOD activity led to increased ROS generation in L-arginine deprived condition. Finally, we concluded that the reduction in cell viability of AD-Ld parasite was due to increased ROS generation and decreased thiol levels.
NADP+/NADPH ratio critically regulates the oxidative homeostasis inside cell. In this study an increased NADP+/NADPH ratio was observed for L-arginine deprived parasites with increasing time interval which further supports our hypothesis of ROS production due to L-arginine deprivation resulting in reduced viability of Leishmania parasite.
Apoptosis-like phenomenon was observed in L. donovani promastigotes upon treatment with hydrogen peroxide [10, 12] and nutrient deprivation [24] whereas staurosporine treatment [67] and serum withdrawal [18] demonstrated similar changes in case of L. major promastigotes. To the best of our knowledge, this is the first report where we showed significant increase in early apoptotic cells in L-arginine deprivation condition with increasing time of incubation.
When we measured the alteration in mitochondrial membrane potential (Δψm), we found that as expected with increasing time interval, a significant decrease in Δψm was observed for AD-Ld parasites compared to AS-Ld parasites. High ROS levels bring about cationic influx in cytosol which leads to mitochondrial dysfunction and depolarization [68]. With already established evidences of calcium influx modulating the activation of different proteases and regulating the exhibition of phosphatidyl serine on the outer leaflet of plasma membrane during apoptosis [69], we considered to measure the Ca2+ concentration in L-arginine deprived and L-arginine supplemented Leishmania parasites. Interestingly, a gradual increase in intracellular cytosolic Ca2+ level was observed for AD-Ld parasites whereas no such change was observed for AS-Ld parasites. Simultaneously, significant increase in cytosolic cytochrome-c was also observed for AD-Ld parasite compared to AS-Ld parasite. Therefore, the increase in cytosolic Ca2+ and cytochrome-c is correlated with increased ROS level resulting in decrased cell viability in L-arginine deprivation condition. Collectively, our results clearly suggest that the biochemical basis behind reduced L-arginine availability and increased ROS generation is due to downregulation of SOD activity, increased NADP+/NADPH ratio, reduced thiol level, release of intracellular calcium and decreased mitochondrial membrane potential (Fig 8).
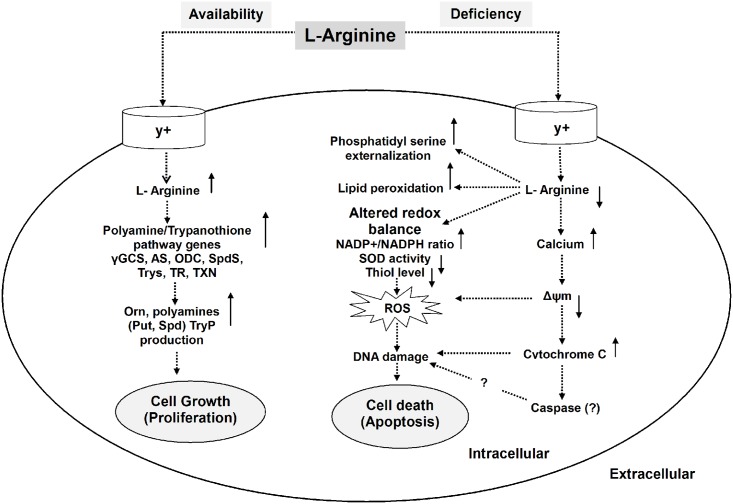
Fig 8. Schematic representation demonstrating the role of L-arginine in the modulation of cell proliferation or apoptosis-like cell death in Leishmania parasite.
The model illustrates that the fate of Leishmania parasites depend on the availability of extracellular L-arginine. If L-arginine is available, it is taken up from the external medium by cationic L-arginine transporters (y+) and metabolized by arginase (AS) and other polyamine biosynthetic and thiol metabolic pathway enzymes such as γ-glutamylcysteine synthetase (γ-GCS), ornithine decarboxylase (ODC), spermidine synthase (SpdS), trypanothione synthetase (TryS), trypanothione reductase (TR), tryparedoxin (TXN) to produce L-ornithine (Orn), putrescine (Put), spermidine (Spd) and thiols such as trypanothione (TryP) which promotes parasite proliferation and cell growth. Whereas, if extracellular L-arginine is unavailable or deficient, cellular redox balance is altered characterized by increased NADP+/NADPH ratio, decreased antioxidant levels such as SOD activity and thiol content that led to increased ROS production. L-arginine unavailability also triggered the phenomenons like phosphatidyl serine externalization, increased lipid peroxidation and release of intracellular calcium. Calcium influx in turn causes mitochondrial membrane depolarization leading to release of cytochrome-c from mitochondrion and intracellular ROS generation that ultimately damages DNA and promotes an apoptosis-like cell death.
As the apoptotic pathways are stringently regulated by caspases, the presence of caspase-like activity in the parasites under L-arginine deprivation condition was examined. However, recent apoptosis work challenges the accepted roles of caspase proteases [23]. Dolai et al. [70] also reported ROS dependent but caspase independent apoptosis-like cell death in L. major promastigotes. Interestingly in our study, no significant increase in caspase activity was observed for AD-Ld parasites compared to AS-Ld parasites with increasing time intervals.
To further confirm apoptosis-like cell death of Leishmania parasite, we analyzed DNA nicking by TUNEL assay using flow cytometer and DNA fragmentation by Cell Death Detection ELISA. As expected, a significant increase in TUNEL positive cells and DNA fragmentation was observed for AD-Ld parasites compared to AS-Ld parasites with increasing time intervals.
From this study, it can be concluded that L-arginine is a crucial molecule for survival and growth of Leishmania parasite, in absence of which the parasite undergoes an apoptosis-like cell death. Our study is the first report describing the mechanism of L-arginine dependent regulation of cell survival and apoptosis in L. donovani promastigotes (Fig 8). The molecular mechanism for L-arginine regulated caspase independent apoptosis-like cell death involves ROS mediated DNA degradation through intracellular calcium influx and subsequent depolarization of mitochondrial membrane potential along with cytosolic cytochrome-c release. Therefore, this important amino acid might be targeted for the efficient control of Leishmania growth and thereby occurrence of VL.
Supporting Information
(A) L. donovani promastigotes were grown in amino acid free (AA-), L-arginine free (Arg-), L-lysine free (Lys-), L-glutamine free (Gln-), L-proline free (Pro-) and normal (ctrl) RPMI media separately for 0–120 hr. The percent cell viability of the parasite was determined by MTT assay. (B) L. donovani promastigotes were grown in amino acid free (AA-) as well as amino acid free but supplemented with individual amino acids such as L-arginine (AA-/Arg+), L-lysine (AA-/Lys+), L-glutamine (AA-/Gln+), L-proline (AA-/Pro+) and normal (ctrl) RPMI separately for 0–120 hr. The percent cell viability of the parasite was determined by MTT assay. The data represents mean±SD of triplicate determinations and are representative of three independent experiments. *, P<0.05 (Student’s t test), **, P<0.001 compared to control (or AA- as applicable). ns, non-significant
(TIF)
Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media. After 96 hr of incubation the parasites were harvested, incubated with different conc. of [3H] L-arginine (0–5 μCi/ml) for 8 minutes and rate of uptake was measured. The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable. ns, non-significant.
(TIF)
(A-E) Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media for 0–120 hr. The activity of different caspases [caspase-1 (A), caspase-3 (B), caspase-5 (C), caspase-8 (D) and caspase-9 (E)] were measured using a fluorogenic homogeneous caspase assay kit as described in “Materials and methods”. The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable. ns, non-significant.
(TIF)
(DOCX)
Acknowledgments
We are grateful to Dr. Sujoy Dasgupta and Mr. Swaroop Biswas (Bose Institute, Kolkata, India) for providing and helping with the HPLC analysis. We are thankful to Mr. Subhash Kumar for providing technical assistance for this study. We are also thankful to Dr. Vahab Ali (RMRIMS, Patna, India) for kind gift of anti-leishmania TryS and TR antibodies and Dr. Chandrima Shaha (NII, New Delhi, India) for providing anti-leishmania CTP antibody.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by an intramural grant (INT-76-BAS) from the Indian Council of Medical Research (ICMR), Ministry of Health and Family Welfare, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012; 7: e35671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander J and Russell DG. The interaction of Leishmania species with macrophages. Advances in Parasitology. 1992; 31: 174–254 [DOI] [PubMed] [Google Scholar]
- 3.Desjardins M and Descoteaux A. Survival strategies of Leishmania donovani in mammalian host macrophages. Res. Immunol. 1998; 149: 689–692. [DOI] [PubMed] [Google Scholar]
- 4.Welburn SC, Barcinski MA and Williams GT. Programmed cell death in Trypansomatids. Parasitol. Today. 1997; 13: 22–26. [DOI] [PubMed] [Google Scholar]
- 5.Welburn SC and Maudlin I. Control of Trypanosoma brucei brucei infections in tsetse, Glossina morsitans. Med. Vet. Entomol. 1997; 11: 286–289. [DOI] [PubMed] [Google Scholar]
- 6.Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001; 41: 367–401. [DOI] [PubMed] [Google Scholar]
- 7.Alzate JF, Arias AA, Moreno-Mateos D, Alvarez-Barrientos A, Jimenez-Ruiz A. Mitochondrial superoxide mediates heat-induced apoptotic-like death in Leishmania infantum. Mol Biochem Parasitol. 2007; 152: 192–202. [DOI] [PubMed] [Google Scholar]
- 8.Moreira ME, Del Portillo HA, Milder RV, Balanco JM, Barcinski MA. Heat shock induction of apoptosis in promastigotes of the unicellular organism Leishmania (Leishmania) amazonensis. J Cell Physiol. 1996; 167: 305–313. [DOI] [PubMed] [Google Scholar]
- 9.Raina P, Kaur S. Chronic heat-shock treatment driven differentiation induces apoptosis in Leishmania donovani. Mol Cell Biochem. 2006; 289: 83–90. [DOI] [PubMed] [Google Scholar]
- 10.Das M, Mukherjee SB, Shaha C. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J Cell Sci. 2001; 114: 2461–2469. [DOI] [PubMed] [Google Scholar]
- 11.Irigoin F, Inada NM, Fernandes MP, Piacenza L, Gadelha FR, Vercesi AE et al. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem J. 2009; 418: 595–604. 10.1042/BJ20081981 [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee SB, Das M, Sudhandiran G, Shaha C. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J Biol Chem. 2002; 277: 24717–24727. [DOI] [PubMed] [Google Scholar]
- 13.Ridgley EL, Xiong ZH, Ruben L. Reactive oxygen species activate a Ca2+-dependent cell death pathway in the unicellular organism Trypanosoma brucei brucei. Biochem J. 1999; 340: 33–40. [PMC free article] [PubMed] [Google Scholar]
- 14.De Souza EM, Menna-Barreto R, Araujo-Jorge TC, Kumar A, Hu Q, Boykin DW, Soeiro MN. Antiparasitic activity of aromatic diamidines is related to apoptosis-like death in Trypanosoma cruzi. Parasitology. 2006; 133: 75–79. [DOI] [PubMed] [Google Scholar]
- 15.Paris C, Loiseau PM, Bories C, Breard J: Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2004; 48: 852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez VE, Kosec G, Sant’Anna C, Turk V, Cazzulo JJ, Turk B. Autophagy is involved in nutritional stress response and differentiation in Trypanosoma cruzi. J Biol Chem. 2008; 283: 3454–3464. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez V, Paredes R, Sosa MA, Galanti N. Natural programmed cell death in T. cruzi epimastigotes maintained in axenic cultures. J Cell Biochem. 2008; 105: 688–698. 10.1002/jcb.21864 [DOI] [PubMed] [Google Scholar]
- 18.Zangger H, Mottram JC, Fasel N. Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ. 2002; 9: 1126–1139. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni MM, McMaster WR, Kamysz W, McGwire BS. Antimicrobial peptide induced apoptotic death of Leishmania results from calcium-dependent, caspase-independent mitochondrial toxicity. J Biol Chem. 2009; 284: 15496–15504. 10.1074/jbc.M809079200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luque-Ortega JR, Cruz LJ, Albericio F, Rivas L. The Antitumoral Depsipeptide IB-01212 Kills Leishmania through an Apoptosis-like Process Involving Intracellular Targets. Mol Pharm. 2010; 5: 1608–1617. [DOI] [PubMed] [Google Scholar]
- 21.Deponte M: Programmed cell death in protists. Biochim Biophys Acta. 2008; 1783:1396–1405. 10.1016/j.bbamcr.2008.01.018 [DOI] [PubMed] [Google Scholar]
- 22.Duszenko M, Figarella K, Macleod ET, Welburn SC. Death of a trypanosome: a selfish altruism. Trends Parasitol. 2006; 22: 536–542. [DOI] [PubMed] [Google Scholar]
- 23.Wanderley Joaõ Luiz M., Barcinski Marcello A. Apoptosis and apoptotic mimicry: the Leishmania connection. Cell Mol Life Sci. 2010; 10: 1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, Nakhasi HL. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002; 9: 53–64. [DOI] [PubMed] [Google Scholar]
- 25.Bejarano J, Espino SD, Paredes Á, Ortiz G, Pariente Lozano JA, Apoptosis Rodríguez AB., ROS and Calcium Signaling in Human Spermatozoa: Relationship to Infertility, Male Infertility 2012, Bashamboo Anu Dr. (Ed.), ISBN: 978-953-51-0562-6: 51–76. [Google Scholar]
- 26.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casera RA Jr. The natural polyamine spermine functions directly as a free radical scavenge. Proc Natl Acad Sci. 1998; 95: 11140–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujisawa S, Kadoma Y. Kinetic evaluation of polyamines as radical scavengers. Anticancer Res. 2005; 25: 965–70. [PubMed] [Google Scholar]
- 28.Landfear SM. Nutrient Transport and Pathogenesis in Selected Parasitic Protozoa. Eukaryot Cell. 2011; 4: 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alenzi FQB. Links between apoptosis, proliferation and the cell cycle. British Journal of Biomedical Science. 2004; 61: 1–4. [DOI] [PubMed] [Google Scholar]
- 30.Gong H, Zo¨lzer F, von Recklinghausen G., Havers W and Schweigerer L. Arginine deiminase inhibits proliferation of human leukemia cells more potently than asparaginase by inducing cell cycle arrest and apoptosis. Leukemia. 2000; 14: 826–829. [DOI] [PubMed] [Google Scholar]
- 31.Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12: 7126–31. [DOI] [PubMed] [Google Scholar]
- 32.Piacenza L, Peluffo G and Rafael RR. L-Arginine-dependent suppression of apoptosis in Trypanosoma cruzi: Contribution of the nitric oxide and polyamine pathways. PNAS. 2001; 98: 7301–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das S, Pandey K, Kumar A, Sardar AH, Purkait B, Kumar M et al. TGF-β1 re-programs TLR4 signaling in L. donovani infection: enhancement of SHP-1 and ubiquitin-editing enzyme A20. Immunol Cell Biol. 2012; 90: 640–54. 10.1038/icb.2011.80 [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 35.Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother. 2012; 56: 1031–41. 10.1128/AAC.00030-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Browne CD, Hindmarsh EJ, Smith JW. Inhibition of endothelial cell proliferation and angiogenesis by orlistat, a fatty acid synthase inhibitor. FASEB J. 2006; 20: 2027–35. [DOI] [PubMed] [Google Scholar]
- 37.Castilho-Martins EA, Laranjeira da Silva MF, dos Santos MG, Muxel SM, Floeter-Winter LM. Axenic Leishmania amazonensis Promastigotes Sense both the External and Internal Arginine Pool Distinctly Regulating the Two Transporter-Coding Genes. PLoS ONE. 2011; 11: e27818 10.1371/journal.pone.0027818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994; 174: 231–5. [DOI] [PubMed] [Google Scholar]
- 39.Reguera RM, Balaña-Fouce R, Showalter M, Hickerson S, Beverley SM. Leishmania major lacking Arginase (ARG) are auxotrophic for polyamines but retain infectivity to susceptible BALB/c mice. Mol Biochem Parasitol. 2009; 165: 48–56. 10.1016/j.molbiopara.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaña-Fouce R, Escribano MI, Alunda JM. Leishmania infantum: polyamine biosynthesis and levels during the growth of promastigotes. Int J Biochem. 1991; 23: 1213–7. [DOI] [PubMed] [Google Scholar]
- 41.Das S, Pandey K, Rabidas VN, Mandal A, Das P. Effectiveness of miltefosine treatment in targeting anti-leishmanial HO-1/Nrf-2 mediated oxidative responses in visceral leishmaniasis patients. J Antimicrob Chemother. 2013; 68: 2059–65. 10.1093/jac/dkt162 [DOI] [PubMed] [Google Scholar]
- 42.Shimasaki H. Assay of fluorescent lipid peroxidation products. Methods Enzymol. 1994; 233: 338–46. [DOI] [PubMed] [Google Scholar]
- 43.Sardar AH, Das S, Agnihorti S, Kumar M, Ghosh AK, Abhishek K et al. Spinigerin induces apoptotic like cell death in a caspase independent manner in Leishmania donovani. Exp Parasitol. 2013; 135: 715–25. 10.1016/j.exppara.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay R, Dey S, Xu N, Gage D, Lightbody J, Ouellette M, and Rosen BP. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc. Natl. Acad. Sci. 1996; 93: 10383–10387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koulajian K, Desai T, Liu GC, Ivovic A, Patterson JN, Tang C et al. NADPH oxidase inhibition prevents beta cell dysfunction induced by prolonged elevation of oleate in rodents. Diabetologia. 2013; 56: 1078–87. 10.1007/s00125-013-2858-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das S, Ghosh AK, Singh S, Saha B, Ganguly A, Das P. Unmethylated CpG motifs in the L. donovani DNA regulate TLR9-dependent delay of programmed cell death in macrophages. J Leukoc Biol. 2015; 97: 363–78. 10.1189/jlb.4A0713-378RR [DOI] [PubMed] [Google Scholar]
- 47.Kumar A, Das S, Purkait B, Sardar AH, Ghosh AK, Dikhit MR et al. Ascorbate peroxidase, a key molecule regulating amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother. 2014; 58: 6172–84. 10.1128/AAC.02834-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozbilgin A, Ozbel Y, Alkan MZ, Atambay M, Ozcel MA. Cultivation of Leishmania sp. in nutrient broth. J Egypt Soc Parasitol. 1995; 25: 437–41. [PubMed] [Google Scholar]
- 49.Steiger RF, Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977; 24: 437–41. [DOI] [PubMed] [Google Scholar]
- 50.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010; 6: 749–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandal G, Wyllie S, Singh N, Sundar S, Fairlamb AH, Chatterjee M. Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology. 2007; 134: 1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee S, Bandyapadhyay R, Basu MK. Leishmania donovani: superoxide dismutase level in infected macrophages. Biosci Rep. 1988; 8: 131–7. [DOI] [PubMed] [Google Scholar]
- 53.Ying W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxidants & Redox Signaling. 2008; 2: 179–206. [DOI] [PubMed] [Google Scholar]
- 54.Mehta A, Shaha C. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J Biol Chem. 2004; 279: 11798–813. [DOI] [PubMed] [Google Scholar]
- 55.Compton MM. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992; 11: 105–19. [DOI] [PubMed] [Google Scholar]
- 56.Krassner SM, Flory B. Essential amino acids in the culture of Leishmania tarentolae. J Parasitol. 1971; 57: 917–20. [PubMed] [Google Scholar]
- 57.Steiger RF, Meshnick SR. Amino-acid and glucose utilization of Leishmania donovani and L. braziliensis. Trans R Soc Trop Med Hyg. 1977; 5: 441–3. [DOI] [PubMed] [Google Scholar]
- 58.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005; 309: 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaked-Mishan P, Suter-Grotemeyer M, Yoel-Almagor T, Holland N, Zilberstein D, Rentsch D. A novel high-affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol Microbiol. 2006; 60: 30–8. [DOI] [PubMed] [Google Scholar]
- 60.Fox BA, Gigley JP, Bzik DJ. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int J Parasitol. 2004; 34: 323–331. [DOI] [PubMed] [Google Scholar]
- 61.Darlyuk I, Goldman A, Roberts SC, Ullman B, Rentsch D, Zilberstein D. Arginine homeostasis and transport in the human pathogen Leishmania donovani. J Biol Chem. 2009; 284: 19800–7. 10.1074/jbc.M901066200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colotti G, Ilari A. Polyamine metabolism in Leishmania: from arginine to trypanothione. Amino Acids. 2011; 40: 269–285. 10.1007/s00726-010-0630-3 [DOI] [PubMed] [Google Scholar]
- 63.Filomeni G, De Zio D and Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death and Differentiation. 2015; 22: 377–388. 10.1038/cdd.2014.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das R, Roy A, Dutta N, Majumder HK. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis. 2008; 13: 867–882. 10.1007/s10495-008-0224-7 [DOI] [PubMed] [Google Scholar]
- 65.Garrel C, Ceballos-Picot I, Germain G, Al-Gubory KH. Oxidative stress-inducible antioxidant adaptive response during prostaglandin F2alpha-induced luteal cell death in vivo. Free Radic Res. 2007; 41: 251–259. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh S, Goswami S, Adhya S. Role of superoxide dismutase in survival of Leishmania within the macrophage; Biochem J. 2003; 369: 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnoult D, Akarid K, Grodet A, Petit PX, Estaquier J, Ameisen JC. On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ. 2002; 9: 65–81. [DOI] [PubMed] [Google Scholar]
- 68.Koya RC, Fujita H, Shimizu S, Ohtsu M, Takimoto M, Tsujimoto Y et al. Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. J Biol Chem. 2000; 275: 15343–9. [DOI] [PubMed] [Google Scholar]
- 69.Sen N, Das BB, Ganguly A, Mukherjee T, Bandyopadhyay S and Majumder HK. Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J. Biol. Chem. 2004; 279: 52366–52375. [DOI] [PubMed] [Google Scholar]
- 70.Dolai S, Pal S, Yadav RK, Adak S. Endoplasmic reticulum stress induced apoptosis in Leishmania through Ca2+-dependent and caspase-independent mechanism. J Biol Chem. 2011; 286: 13638–46. 10.1074/jbc.M110.201889 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) L. donovani promastigotes were grown in amino acid free (AA-), L-arginine free (Arg-), L-lysine free (Lys-), L-glutamine free (Gln-), L-proline free (Pro-) and normal (ctrl) RPMI media separately for 0–120 hr. The percent cell viability of the parasite was determined by MTT assay. (B) L. donovani promastigotes were grown in amino acid free (AA-) as well as amino acid free but supplemented with individual amino acids such as L-arginine (AA-/Arg+), L-lysine (AA-/Lys+), L-glutamine (AA-/Gln+), L-proline (AA-/Pro+) and normal (ctrl) RPMI separately for 0–120 hr. The percent cell viability of the parasite was determined by MTT assay. The data represents mean±SD of triplicate determinations and are representative of three independent experiments. *, P<0.05 (Student’s t test), **, P<0.001 compared to control (or AA- as applicable). ns, non-significant
(TIF)
Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media. After 96 hr of incubation the parasites were harvested, incubated with different conc. of [3H] L-arginine (0–5 μCi/ml) for 8 minutes and rate of uptake was measured. The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable. ns, non-significant.
(TIF)
(A-E) Leishmania parasites were grown in L-arginine depleted (AD-Ld) and L-arginine supplemented (AS-Ld) RPMI media for 0–120 hr. The activity of different caspases [caspase-1 (A), caspase-3 (B), caspase-5 (C), caspase-8 (D) and caspase-9 (E)] were measured using a fluorogenic homogeneous caspase assay kit as described in “Materials and methods”. The data represents mean±SD of triplicate determinations and are representative of three independent experiments.*, P<0.05 (Student’s t test), **, P<0.001 compared to AD-Ld or AS-Ld parasite as applicable. ns, non-significant.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.