Abstract
Background
Corticotropin-releasing hormone (CRH) plays an important role in the pathophysiology of irritable bowel syndrome (IBS) and regulates the stress response through two CRH receptors (R1 and R2). Previously, we reported that a CRHR1 gene polymorphism (rs110402, rs242924, and rs7209436) and haplotypes were associated with IBS. However, the association between the CRHR2 gene and IBS was not investigated. We tested the hypothesis that genetic polymorphisms and haplotypes of CRHR2 are associated with IBS pathophysiology and negative emotion in IBS patients.
Methods
A total of 142 IBS patients and 142 healthy controls participated in this study. Seven single nucleotide polymorphisms (SNPs) of the CRHR2 gene (rs4722999, rs3779250, rs2240403, rs2267710, rs2190242, rs2284217, and rs2284220) were genotyped. Subjects' psychological states were evaluated using the Perceived-Stress Scale, the State-Trait Anxiety Inventory, and the Self-Rating Depression Scale.
Results
We found that rs4722999 and rs3779250, located in intronic region, were associated with IBS in terms of genotype frequency (rs4722999: P = 0.037; rs3779250: P = 0.017) and that the distribution of the major allele was significantly different between patients and controls. There was a significant group effect (controls vs. IBS), and a CRHR2 genotype effect was observed for three psychological scores, but the interaction was not significant. We found a haplotype of four SNPs (rs4722999, rs3779250, rs2240403, and rs2267710) and two SNPs (rs2284217 and rs2284220) in strong linkage disequilibrium (D′ > 0.90). We also found that haplotypes of the CRHR2 gene were significantly different between IBS patients and controls and that they were associated with negative emotion.
Conclusion
Our findings support the hypothesis that genetic polymorphisms and haplotypes of CRHR2 are related to IBS. In addition, we found associations between CRHR2 genotypes and haplotypes and negative emotion in IBS patients and controls. Further studies on IBS and the CRH system are warranted.
Introduction
The stress response is an important mechanism that is indispensable for the maintenance of life. Corticotropin-releasing hormone (CRH) is released from the paraventricular nucleus of the hypothalamus under stress and it stimulates the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland. ACTH acts on the adrenal cortex and promotes the synthesis of glucocorticoid. This system is called the hypothalamic-pituitary-adrenal (HPA) axis [1]. In the hippocampus, hypothalamus, and pituitary gland, there are two receptors that interact with cortisol: the glucocorticoid receptor and mineralocorticoid receptor. When cortisol secretion is increased, the composition and secretion of CRH and ACTH is controlled through these receptors, which results in the suppression of cortisol secretion (negative feedback) [2]. Dysfunction of the HPA axis causes hippocampal atrophy [3] and it is also related to stress-related diseases such as depression, panic disorder, and post-traumatic stress disorder (PTSD) [4–7].
CRH is a polypeptide consisting of 41 amino acid residues [8]. The CRH family consists of several neuropeptides: CRH, urocortin 1, urocortin 2, and urocortin 3. CRH is primarily responsible for regulating and/or initiating stress responses via activation of the HPA axis [9]. Conversely, urocortins play an indispensable role in the recovery response to stress [9]. CRH released in the brain activates the sympathetic system and stimulates the cardiovascular system [10]. In addition, it stimulates sacral parasympathetic outflow and colonic motility [11]. The dysregulation of CRH increases anxiety and depression [12].
There are two G protein-coupled receptors for CRH: CRH receptor 1 (CRHR1, CRF1 in IUPHAR nomenclature) and CRH receptor 2 (CRHR2, CRF2 in IUPHAR nomenclature). CRHR1 is found at a high density in the brain and pituitary gland, while it is found at a low density in the adrenal gland, ovary, and testis [13–16]. Other than its expression in the brain, CRHR2 is distributed widely throughout peripheral organs such as the heart, skeletal muscle, intestines, and airway epithelium [17–20]. CRH activates both receptors but is more potent at CRHR1. Urocortin 1 can bind both receptors with equal affinity that is 10-fold greater than CRH binding affinity for CRHR1. urocortin and urocortin don’t bind to CRHR1, but CRH, urocortin 2 and urocortin 3 all bind to CRHR2 with equal affinity [21]. The distribution of these receptors and their binding affinities to endogenous ligands in individuals may reflect individual differences in physiological function.
Irritable bowel syndrome (IBS) is a prototypic functional gastrointestinal disorder [22] accompanied by visceral hypersensitivity [23], increased gut reactivity [24], and altered central processing [25] in response to various stressors [26]. A previous study demonstrated that mutual and reciprocal interactions between the brain and gut play a major role in the pathophysiology of IBS [27]. In IBS patients, the exogenous administration of CRH induced robust colonic motility [24]. Electrical stimulation of the rectum induced an increase in motility indices in IBS patients and this response was inhibited by the administration of a CRH antagonist [28]. Therefore, dysfunction of CRH signaling is considered to be related to the pathophysiology of IBS.
CRH plays a stimulatory role in the stress response through the activation of CRHR1, while the specific actions of urocortin 2 and urocortin 3 on CRHR2 may be important for dampening the stress response [4]. In the brain, CRHR1 stimulation causes anxiety, but CRHR2 stimulation induces anxiolysis. In regard to gut motility, CRHR1 stimulation evokes colonic motility, but CRHR2 stimulation inhibits gastric emptying [12]. From these studies, one of the important determinants of the brain-gut interaction in the stress response of IBS patients is signaling via CRHR1 and CRHR2.
The gene encoding CRHR1 is located on chromosome 17q21.31 and has a length of 51.55 Kb and contains 14 exons. The gene encoding CRHR2 is located on chromosome 7p14.3 and has a length 48.19Kb and contains 16 exons. There are many reports on the association between gene variants of CRH receptors and stress-related diseases. In CRHR1, variation of the gene has been found to be a risk factor for depression after childhood maltreatment [29–33]. A polymorphism of CRHR1 was reported to be related to recurrent major depressive disorder (MDD) [34]. In regard to CRHR2, gene variation may affect the risk of PTSD in women by attenuating the stress response and reducing symptoms of the disorder [35]. A polymorphism in the CRHR2 gene was also associated with MDD [36]. In a previous study from our laboratory, we showed that genetic polymorphisms and haplotypes of CRHR1 (rs110402, rs242924, and rs7209436) mediate IBS and related bowel patterns [37]. However, the association between CRHR2 genotypes and IBS has not been investigated. Therefore, we hypothesized that gene polymorphisms and/or haplotypes of CRHR2 may be associated with IBS pathophysiology and negative emotion in IBS patients.
Methods
Subjects
In total, 142 patients (62 males and 80 females) with IBS who were diagnosed at the Department of Psychosomatic Medicine, Tohoku University Hospital, were enrolled in this study (mean age 22.0 ± 0.2 years; range 18–31). Patients with organic diseases were excluded. In addition, 142 healthy volunteers (74 males and 68 females) were recruited at Tohoku University as controls (mean age 22.0 ± 0.2 years; range 19–31). Subjects without any symptoms or signs during a medical interview and physical examination were identified as healthy controls. There was no significant difference in age, sex ratio among the groups.
IBS patients were diagnosed according to the Rome III criteria [38]. IBS was defined as recurrent abdominal pain or discomfort for at least 3 days per month in the last 3 months associated with two or more of the following symptoms: improvement with defecation, onset associated with a change in the frequency of defecation, and/or onset associated with a change in the form (appearance) of stools. These criteria were fulfilled for the previous 3 months with symptom onset at least 6 months prior to diagnosis. According to the Rome III criteria, IBS was classified as IBS with diarrhea (D), constipation (C), or mixed symptoms of diarrhea and constipation (M). Unclassified IBS patients were classified as IBS-M. All subjects provided written informed consent and this study was approved by the Tohoku University Ethics Committee. Consecutive patients who agreed to participate in this study were enrolled.
Two hundred and twenty-two of 284 participants’ DNA samples were previously reported on polymorphisms of the CRHR1 polymorphic region [37]. There are apparently different from the targeted genes in this study.
SNP selection
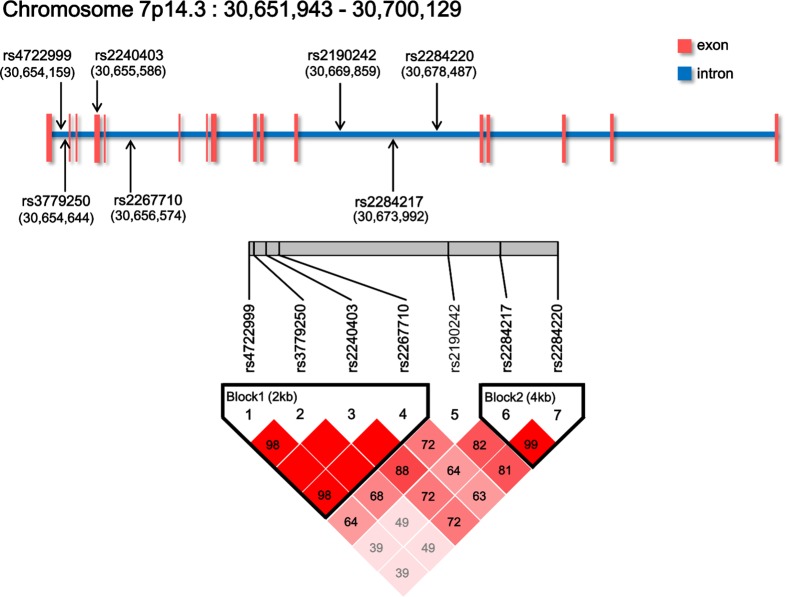
We used Generic Genome Browser in International HapMap Project (http://hapmap.ncbi.nlm.nih.gov/index.html.en) to search the allele frequency (minor allele frequency > 0.15 in Japanese population). Furthermore, we restricted seven candidate genes, which were selected from single nucleotide polymorphisms (SNPs) for genotyping based on previous literature [35,36,39]. The loci of these SNPs were searched from the dbSNP in the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/), and are shown in Fig 1.
Fig 1. SNPs of the CRHR2 gene examined in this study.
CRHR2 is located on chromosome 7p14.3. The arrows and the numbers in parentheses indicate the locus of each SNP. rs2240403 exists in the fourth exon, but six other SNPs exist in intronic regions. These SNPs had a minor allele frequency of >15% in the Japanese population. A linkage disequilibrium (LD) plot was generated using D′. D′ = 1 indicates complete LD. D′ values < 1.0 are shown in the respective squares for the SNP pairs, with darker shades of red representing higher levels of LD.
Genotyping
Peripheral blood was sampled from the forearm vein of each subject with a heparinized syringe. DNA was then extracted from the lymphocytes using a standard protocol [40]. The concentration of each DNA was diluted to 0.2 μg/μL. Seven SNPs (rs4722999, rs3779250, rs2240403, rs2267710, rs2190242, rs2284217, and rs2284220) in the regulatory region of the CRHR2 gene were genotyped using real-time polymerase chain reaction (PCR).
We use the CFX-96 TouchTM Real-time PCR Detection System (Bio-Rad Laboratories, Inc., Tokyo, Japan). PCR amplification was carried out using probe/primer sets for SNP genotyping that were purchased from Applied Biosystems, Tokyo, Japan (set of C1617682310 for rs2240403, C1587286110 for rs2190242, C1596058610 for rs2284217, C1596058010 for rs2284220, C34646010 for rs4722999, C2580980110 for rs3779250, and C1587287110 for rs2267710). PCR amplification was performed using a total volume of 10 μL consisting of 5 μL TaqMan Genotyping Master Mix, 4.4 μL ddH2O, 0.5 μL TaqMan SNP Genotyping Assays, and 0.1 μL DNA. After initial denaturation at 95°C for 10 min, amplification was performed for 40 cycles at 95°C for 15 s and 60°C for 1 min. To determine the positive control, sequence analysis of CRHR2 genes was performed using an ABI 3130 Genetic Analyzer (PE Applied Biosystems, Foster City, CA). All procedures were performed according to the manufacturer's instructions.
Evaluation of psychological state
Participants' psychological states were rated using the Perceived-Stress Scale (PSS; [41], [42]), the State-Trait Anxiety Inventory (STAI; [43], [44]), and the Self-Rating Depression Scale (SDS; [40], [45]). The Japanese versions of STAI, SDS, and PSS have been validated and their reliability has been confirmed [42].
Statistical analysis
The frequencies of genotypes, alleles, and haplotypes of the CRHR2 SNPs were compared between the IBS patients and controls, or between patients with different bowel patterns (normal, constipation, mixed, and diarrhea) using the χ2-test. The effects of variation in CRHR2 SNPs and haplotypes on psychological states between the IBS patients and controls were examined with two-way analysis of variance (ANOVA). The effects of variation in CRHR2 haplotypes on psychological states were examined with one-way ANOVA. Statistical analyses were performed using SPSS Statistics version 21.0 software (IBM, Inc., New York, NY). We used Haploview [46] to determine the LD of the SNPs within the CRHR2 gene and test for Hardy–Weinberg equilibrium. Results are expressed as the mean ± standard error, and a P value < 0.05 was considered to be significant.
Results
In the 284 samples, genotype distributions were in Hardy–Weinberg equilibrium for all seven SNPs. The genotype frequencies of the SNPs in the IBS group and healthy controls are listed in Table 1. The allele frequencies of the SNPs in the IBS group, healthy controls, and the subtypes of IBS patients are listed in Table 2.
Table 1. Genotypes frequencies for seven CRHR2 SNPs in the IBS patients and controls.
| Controls n (%) n = 142 | IBS Patients n (%) n = 142 | P value(Control vs. IBS) | ||||
|---|---|---|---|---|---|---|
| Male (n = 74) | Female (n = 68) | Male (n = 62) | Female (n = 80) | |||
| rs4722999 | CC | 25 (17.6) | 18 (12.7) | 15 (10.6) | 23 (16.2) | 0.037 |
| CT | 33 (23.2) | 34 (23.9) | 39 (27.5) | 47 (33.1) | ||
| TT | 16 (11.3) | 16 (11.3) | 8 (5.6) | 10 (7.0) | ||
| rs3779250 | CC | 27 (19.0) | 21 (14.8) | 16 (11.3) | 28 (19.7) | 0.017 |
| CT | 32 (22.5) | 31 (21.8) | 38 (26.8) | 44 (31.0) | ||
| TT | 15 (10.6) | 16 (11.3) | 8 (5.6) | 8 (5.6) | ||
| rs2240403 | CC | 54 (38.0) | 37 (26.1) | 38 (26.8) | 51 (35.9) | 0.855 |
| CT | 18 (12.7) | 30 (21.1) | 24 (16.9) | 27 (19.0) | ||
| TT | 2 (1.4) | 1 (0.7) | 0 (0.0) | 2 (1.4) | ||
| rs2267710 | CC | 28 (19.7) | 23 (16.2) | 20 (14.1) | 32 (22.5) | 0.107 |
| CT | 33 (23.2) | 32 (22.5) | 35 (24.6) | 41 (28.9) | ||
| TT | 13 (9.2) | 13 (9.2) | 7 (4.9) | 7 (4.9) | ||
| rs2190242 | CC | 25 (17.6) | 23 (16.2) | 21 (14.8) | 31 (21.8) | 0.518 |
| AC | 38 (26.8) | 30 (21.1) | 30 (21.1) | 41 (28.9) | ||
| AA | 11 (7.7) | 15 (10.6) | 11 (7.7) | 8 (5.6) | ||
| rs2284217 | GG | 22 (15.5) | 22 (15.5) | 22 (15.5) | 19 (13.4) | 0.171 |
| GA | 37 (26.1) | 42 (29.6) | 24 (16.9) | 46 (32.4) | ||
| AA | 15 (10.6) | 4 (2.8) | 16 (11.3) | 15 (10.6) | ||
| rs2284220 | AA | 21 (14.8) | 22 (15.5) | 23 (16.2) | 19 (13.4) | 0.289 |
| AG | 37 (26.1) | 41 (28.9) | 23 (16.2) | 46 (32.4) | ||
| GG | 16 (11.3) | 5 (3.5) | 16 (11.3) | 15 10.6 | ||
P value < 0.05 were indicated in bold.
Table 2. Allele expression in the controls, IBS patients, and IBS subtypes for seven SNPs of the CRHR2 gene.
| Controls | IBS Patients | Total | |||||
|---|---|---|---|---|---|---|---|
| All | C | M | D | ||||
| n = 142 | n = 142 | n = 41 | n = 36 | n = 65 | n = 284 | ||
| rs4722999 | C allele - | 32 | 18 | 9 | 1 | 8 | 50 |
| C allele + | 110 | 124 | 32 | 35 | 57 | 234 | |
| T allele - | 43 | 38 | 7 | 11 | 20 | 81 | |
| T allele + | 99 | 104 | 34 | 25 | 45 | 203 | |
| rs3779250 | C allele - | 31 | 16 | 6 | 1 | 9 | 47 |
| C allele + | 111 | 126 | 35 | 35 | 56 | 237 | |
| T allele - | 48 | 44 | 10 | 14 | 20 | 92 | |
| T allele + | 94 | 98 | 31 | 22 | 45 | 192 | |
| rs2240403 | C allele - | 3 | 2 | 0 | 2 | 0 | 5 |
| C allele + | 139 | 140 | 41 | 34 | 65 | 279 | |
| T allele - | 91 | 89 | 26 | 23 | 40 | 180 | |
| T allele + | 51 | 53 | 15 | 13 | 25 | 104 | |
| rs2267710 | C allele - | 26 | 14 | 5 | 1 | 8 | 40 |
| C allele + | 116 | 128 | 36 | 35 | 57 | 244 | |
| T allele - | 52 | 52 | 12 | 16 | 24 | 104 | |
| T allele + | 90 | 90 | 29 | 20 | 41 | 180 | |
| rs2190242 | C allele - | 26 | 19 | 4 | 1 | 14 | 45 |
| C allele + | 116 | 123 | 37 | 35 | 51 | 239 | |
| A allele - | 48 | 52 | 11 | 18 | 23 | 100 | |
| A allele + | 94 | 90 | 30 | 18 | 42 | 184 | |
| rs2284217 | G allele - | 19 | 31 | 8 | 9 | 14 | 50 |
| G allele + | 123 | 111 | 33 | 27 | 51 | 234 | |
| A allele - | 44 | 41 | 7 | 10 | 24 | 85 | |
| A allele + | 98 | 101 | 34 | 26 | 41 | 199 | |
| rs2284220 | A allele - | 21 | 31 | 8 | 9 | 14 | 52 |
| A allele + | 121 | 111 | 33 | 27 | 51 | 132 | |
| G allele - | 43 | 42 | 7 | 11 | 24 | 85 | |
| G allele + | 99 | 100 | 34 | 25 | 41 | 199 | |
IBS subtype: C, constipation; M, mixed; D, diarrhea.
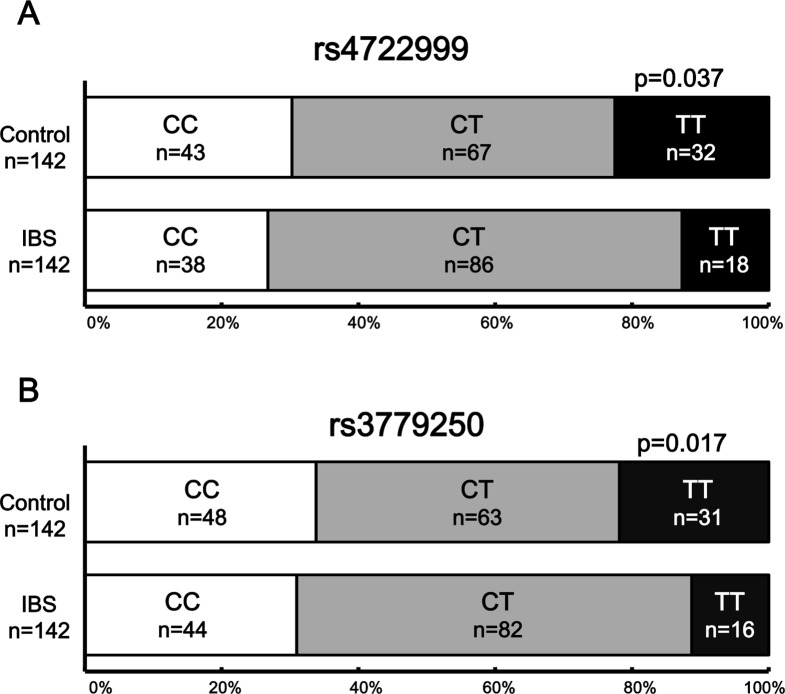
We found that rs4722999 (A) and rs3779250 (B) were significantly associated with IBS in terms of genotype distribution (rs4722999: P = 0.037; rs3779250: P = 0.017) (Fig 2). For these two SNPs, there was no significant difference in the frequency of the minor allele, but the frequency of the major allele was significantly different.
Fig 2. Difference in genotype of CRHR2 SNPs between the controls and IBS patients.
The SNPs rs4722999 (A) and rs3779250 (B) are shown. The SNPs rs4722999 (P = 0.037) and rs3779250 (P = 0.017, χ2-test) were significantly different in the IBS patients in comparison with the controls.
Table 3shows the scores of the psychological scales in the controls and IBS patients. There was no significant difference in the test scores for the three psychological scales between the IBS patients and controls.
Table 3. Perceived stress, depression, and anxiety in the controls and IBS patients.
| Controls | IBS Patients | P value | |||
|---|---|---|---|---|---|
| n = 142 | n = 142 | ||||
| Mean | SE | Mean | SE | ||
| PSS | 26.7 | 0.8 | 28 | 0.7 | 0.203 |
| STAI (state) | 44.1 | 0.8 | 45.2 | 0.8 | 0.361 |
| STAI (trait) | 47.9 | 0.9 | 47.5 | 0.9 | 0.739 |
| SDS | 39.4 | 0.7 | 40.6 | 0.6 | 0.23 |
PSS: Perceived Stress Scale, STAI: State Trait anxiety Inventry, SDS: Self-rating Depression Scale.
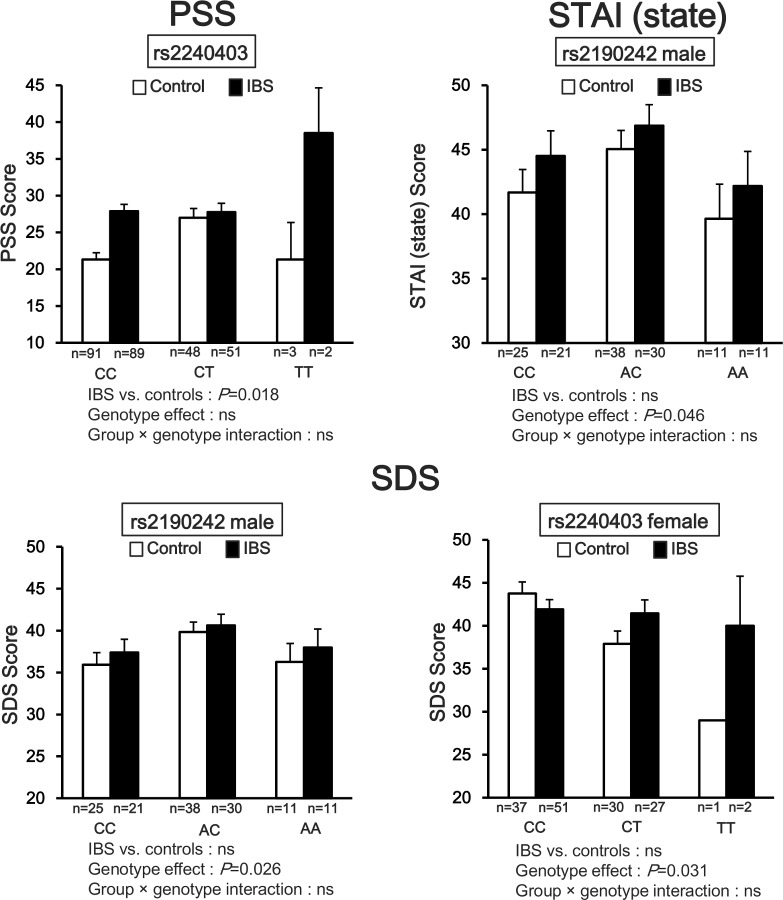
Fig 3shows that the three psychological scores were significantly different by CRHR2 genotype or group (IBS patients vs. controls). According to PSS in rs2240403, the IBS patients were significantly higher than the controls (P = 0.018), but there was no significant interaction. According to STAI (state) in rs2190242 in males, there was a significant genotype effect (P = 0.046), but there was no significant interaction. According to SDS in rs2190242 in males and rs2240403 in females, there was a significant genotype effect (rs2190242: P = 0.026; rs2240403: P = 0.031), but there was no significant interaction.
Fig 3. Psychological scales and CRHR2 SNPs in IBS patients and controls.
PSS: two-way ANOVA showed that the IBS patients with rs2240403 (P = 0.018) had significantly higher perceived stress scale scores than the controls. STAI (state): rs2190242 in males, genotype effect was significant (P = 0.046). SDS: rs2190242 in males and rs2240403 in females, genotype effect was significant (rs2190242: P = 0.026; rs2240403: P = 0.031). In all scale scores, there was no significant group × genotype interaction.
According to the selection criteria, four SNPs (rs4722999, rs3779250, rs2240403, and rs2267710) of CRHR2 with strong D′ (>0.90) were in one linkage block, and two SNPs (rs2284217 and rs2284220) with strong pairwise D′ (>0.90) were in another block (Fig 1). The selection criteria for haplotypes used in the haplotype analyses were adjacent SNPs with pairwise D′ > 0.90. Haplotype analysis of the IBS group and healthy controls is shown in Table 4. We found that three blocks were positively associated with IBS patients (T-C-C-C: P = 0.017; T-C-C-T: P = 0.017; C-T-C-C: P = 0.043). All haplotypes were found more frequently in the IBS patients than in the controls.
Table 4. Haplotype frequencies of the IBS patients and controls in the CRHR2 gene.
| Haplotype | Control—Freq | Case—Freq | P value |
|---|---|---|---|
| rs4722999—rs2267710 | |||
| TTCT | 0.641 | 0.620 | 0.806 |
| CCCC | 0.754 | 0.852 | 0.052 |
| CCTC | 0.359 | 0.373 | 0.902 |
| TTCC | 0.479 | 0.592 | 0.074 |
| TCCC | 0.479 | 0.627 | 0.017 |
| CTCT | 0.415 | 0.514 | 0.122 |
| TTTC | 0.155 | 0.211 | 0.283 |
| TTTT | 0.148 | 0.190 | 0.429 |
| CTTT | 0.148 | 0.190 | 0.429 |
| CCTT | 0.148 | 0.190 | 0.429 |
| TCCT | 0.479 | 0.627 | 0.017 |
| TCTT | 0.148 | 0.190 | 0.429 |
| CTTC | 0.155 | 0.211 | 0.283 |
| CTCC | 0.437 | 0.563 | 0.043 |
| TCTC | 0.176 | 0.225 | 0.374 |
| CCCT | 0.415 | 0.507 | 0.153 |
| rs2284217—rs2284220 | |||
| GA | 0.852 | 0.782 | 0.167 |
| AG | 0.690 | 0.704 | 0.897 |
| AA | 0.542 | 0.493 | 0.476 |
| GG | 0.563 | 0.486 | 0.235 |
P value < 0.05 were indicated in bold.
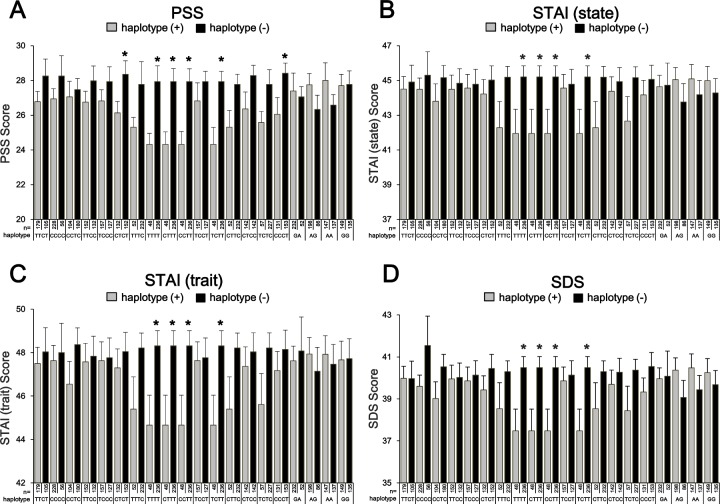
We tested whether the psychological scale scores were different by haplotypes using One-way ANOVA. The result was shown in Fig 4. The psychological scales PSS (A), STAI (state) (B), STAI (trait) (C) and SDS (D) were shown. The carriers with four haplotypes (T-T-T-T, C-T-T-T, C-C-T-T, and T-C-T-T) had significantly lower scores than the others in all psychological scales. The four haplotype carriers were all the same individuals. In addition, in PSS, two haplotypes (C-T-C-T and C-C-C-T) were similarly significant.
Fig 4. Psychological scale scores and CRHR2 haplotypes.
The psychological scales PSS (A), STAI (state) (B), STAI (trait) (C) and SDS (D) were shown. One-way ANOVA showed that the subjects with four haplotypes (T-T-T-T, C-T-T-T, C-C-T-T, and T-C-T-T) had significantly lower score than the others in all psychological scales. The four haplotype carriers were all the same individuals. In addition, in PSS, two haplotypes (C-T-C-T and C-C-C-T) were similarly significant.
We tested whether the psychological scale scores were different by haplotypes between the IBS patients and controls using two-way ANOVA. The result was shown in Table 5. In PSS, group effect (IBS vs. controls) was significant in six haplotypes. However, there was no group effect in other psychological scales. Haplotype effect was significant in all psychological scales. In STAI (trait), there was a significant group × haplotype interaction (P = 0.021). The four haplotype carriers (T-T-T-T, C-T-T-T, C-C-T-T, and T-C-T-T) were all the same individuals.
Table 5. Psychological state and CRHR2 SNPs in IBS patients and controls.
| Haplotype | IBS vs. controls (P value) | haplotype effect (P value) | group-haplotype interaction (P value) | |
|---|---|---|---|---|
| PSS | ||||
| rs4722999—rs2267710 | ||||
| TTTC | 0.047 | 0.035 | ns | |
| TTTT* | 0.041 | 0.005 | ns | |
| CTTT* | 0.041 | 0.005 | ns | |
| CCTT* | 0.041 | 0.005 | ns | |
| TCTT* | 0.041 | 0.005 | ns | |
| CTTC | 0.047 | 0.035 | ns | |
| CTCC | ns | 0.041 | ns | |
| CCCT | ns | 0.014 | ns | |
| STAI (state) | ||||
| rs4722999—rs2267710 | ||||
| TTTC | ns | 0.035 | ns | |
| TTTT* | ns | 0.020 | ns | |
| CTTT* | ns | 0.020 | ns | |
| CCTT* | ns | 0.020 | ns | |
| TCTT* | ns | 0.020 | ns | |
| CTTC | ns | 0.035 | ns | |
| STAI (trait) | ||||
| rs4722999—rs2267710 | ||||
| TTTT* | ns | 0.020 | ns | |
| CTTT* | ns | 0.020 | ns | |
| CCTT* | ns | 0.020 | ns | |
| TCTT* | ns | 0.020 | ns | |
| rs2284217—rs2284220 | ||||
| GG | ns | ns | 0.021 | |
| SDS | ||||
| rs4722999—rs2267710 | ||||
| TTTT* | ns | 0.012 | ns | |
| CTTT* | ns | 0.012 | ns | |
| CCTT* | ns | 0.012 | ns | |
| TCTT* | ns | 0.012 | ns | |
Data without significance were not shown.
*: The four haplotype carriers (T-T-T-T, C-T-T-T, C-C-T-T, and T-C-T-T) were all the same individuals.
Discussion
The present study is the first to show an association between IBS and SNPs/haplotypes of the CRHR2 gene in the Japanese population. These data suggest that CRHR2 gene polymorphisms and haplotypes are related to the pathophysiology of IBS.
Loss of CRHR2 leads to increased inflammation, delayed healing, and exacerbates the inflammatory insult [47–49]. Akiba et al. reported that peripheral CRHR2 activation induces colonic hyperemia through nitric oxide synthesis without involving prostaglandin synthesis or sensory nerve activation and may protect the colonic mucosa [50]. In a recent study, low grade inflammation was observed in the colonic mucosa of IBS patients [51]. We found that rs4722999 and rs3779250 were positively associated with IBS in terms of genotype frequency. Especially, for both SNPs, CT carriers were observed significantly more often in IBS patients. Ishitobi et al. investigated the association of six SNPs of CRHR2 with MDD and panic disorder [36]. They reported that rs3779250 was associated with MDD, but rs4722999 and the other SNPs were not [36]. In this study, major allele carriers were observed frequently in MDD patients. Similarly, in our study, the frequency of the major allele of rs3779250 was significantly different between the patients and controls. Like IBS, MDD is worsened by psychosocial stress with dysfunction of the HPA axis. From these results, the major allele of rs3779250 may be a risk allele for stress-related diseases. However, the C allele of rs3779250 is observed in approximately 77% of the normal population. Therefore, the increased frequency of the major allele of rs3779250 alone cannot explain the relationship between CRHR2 SNPs and IBS. Further study of other factors (e.g., other genetic polymorphisms) of IBS is required.
In this study, there was no significant difference in perceived stress, anxiety, and depression between the IBS patients and controls. Our previous report [52] and the results of others [53] showed that these factors are higher in IBS patients than in controls. By contrast, IBS patients were found to be neither depressive nor anxious in another study from our laboratory [54]. Therefore, IBS patients in this study were psychologically close to normal, but were physically stressed. This notion is supported by the fact that statistical analysis of stress, anxiety, and depression including CRHR2 genotypes with group (IBS patients vs. controls) showed significant differences. The relatively complex association between genotypes and IBS suggests a modifying role for CRHR2 in the pathophysiology of stress.
In seven SNP which we selected, six SNPs are in the intronic region. Only rs2240403 is in the exon region, but it is the synonymous SNP which does not cause amino acid substitution. In this study, there were significant differences in the genotype distribution of both groups in rs472299 and rs377950. In addition, there were significant interaction in association between genotype and group (IBS patients vs. controls) in psychological condition in rs2190242 and rs2284220. These SNPs are in intronic region, and the domain is spliced during transcription to mRNA. Therefore those SNPs are less likely to have an influence on the receptor expression directly. However, the SNPs influencing modulation of the psychological condition that is in vicinity of these SNP in the linkage disequilibrium in IBS may exist.
We found a haplotype of four SNPs (rs4722999, rs3779250, rs2240403, and rs2267710) and two SNPs (rs2284217 and rs2284220) in strong LD (D′ > 0.90). In this study, SNPs and haplotypes of the CRHR2 gene were significantly different between IBS patients and controls. Sato et al. reported that genetic polymorphisms and T-A-T haplotypes of CRHR1 mediate IBS and related bowel patterns [37]. Guillaume et al. reported that childhood sexual abuse and childhood emotional neglect interacted with CRHR1 and CRHR2 gene polymorphisms, respectively, to modulate adult decision-making in a cohort of suicide attempters [55]. Mahajan et al. reported that the balanced and coordinated expression of CRHR1 and CRHR2 is required for the proper regulation of urocortins in a rodent model of Crohn's colitis [56]. Therefore, haplotypes of both CRHR1 and CRHR2 may be related to the features of IBS.
We found that the psychological state varied from CRHR2 genotypes and haplotypes in IBS patients and controls. Especially, we discovered that the group with one of four haplotypes (T-T-T-T, C-T-T-T, C-C-T-T, and T-C-T-T) in CRHR2 showed a significantly lower score in perceived stress, anxiety, and depression in comparison with the group that did not have one of these haplotypes. In these four haplotypes, the common part is the T-T haplotype that consists of the T allele of rs2240403 and T allele of rs2267710. From this finding, it is suggested that having both these T alleles acts protectively against perceived stress and anxiety. To the best of our knowledge, this analysis of these four haplotypes of the CRHR2 gene has not been performed before [35, 36]. This is a novel finding and further studies investigating the association between the T-T haplotype and other stress-related disorders are warranted.
This study has several limitations. First, the sample size was relatively small. Particularly, there were small numbers of subjects with the minor allele of rs2240403 and the data had a wide standard error. However, our study has the strength of a higher precision of genome analyses than a genome-wide association study. Moreover, recently published genomic studies [57, 58] contained smaller number of participants than our study. Replication with a larger sample is necessary. Second, all subjects in this study were Japanese. Villafuerte et al. reported that the CRHR2 gene was unlikely to be involved in the genetic liability underlying HPA axis dysfunction and mood disorders [59]. Therefore, there may be ethnic differences in the function of CRHR2. Third, the molecular biologic differences in the CRHR2 gene polymorphisms examined in this study are not known. It is necessary to investigate any changes of function in vivo and behavior associated with the different CRHR2 gene polymorphisms in the future. Despite some limitations, the present study is the first to clarify the possible importance of CRHR2 in the pathophysiology of IBS.
In conclusion, our findings support the hypothesis that genetic polymorphisms and haplotypes of CRHR2 are associated with IBS. Further studies of IBS and the CRH system are warranted.
Acknowledgments
We thank Dr. Masaaki Kato, Naoko Shimakura, and Risa Ando, Department of Neurology, Tohoku University Graduate School of Medicine; members of the Department of Behavioral Medicine, Tohoku University Graduate School of Medicine, for their help with this study.
Data Availability
All data are fully available without restriction. All data and figure files are available from the Figshare database (accession number: http://dx.doi.org/10.6084/m9.figshare.1543516).
Funding Statement
This research was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan, No. 15H04799, 15H15267 (http://www.mext.go.jp/english/), and Grant-in-Aid for Scientific Research from the Ministry of Health, Welfare, and Labor of Japan (26-4). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. Epub 2007/07/07. 10.1152/physrev.00041.2006 . [DOI] [PubMed] [Google Scholar]
- 2.Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26(3–4):109–30. Epub 2005/11/18. 10.1016/j.yfrne.2005.09.001 . [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–77. Epub 2002/05/10. . [DOI] [PubMed] [Google Scholar]
- 4.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. Epub 2004/01/28. 10.1146/annurev.pharmtox.44.101802.121410 . [DOI] [PubMed] [Google Scholar]
- 5.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48(1):1–10. Epub 2005/05/28. 10.1016/j.yhbeh.2005.01.009 . [DOI] [PubMed] [Google Scholar]
- 6.Anisman H, Merali Z, Stead JD. Experiential and genetic contributions to depressive- and anxiety-like disorders: clinical and experimental studies. Neurosci Biobehav Rev. 2008;32(6):1185–206. Epub 2008/04/22. 10.1016/j.neubiorev.2008.03.001 . [DOI] [PubMed] [Google Scholar]
- 7.Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24(1):66–76. Epub 2006/07/18. 10.1002/da.20220 . [DOI] [PubMed] [Google Scholar]
- 8.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–7. Epub 1981/09/18. . [DOI] [PubMed] [Google Scholar]
- 9.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373(6513):427–32. Epub 1995/02/02. 10.1038/373427a0 . [DOI] [PubMed] [Google Scholar]
- 10.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15(2):71–100. Epub 1990/05/01. . [DOI] [PubMed] [Google Scholar]
- 11.Tache Y, Monnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann N Y Acad Sci. 1993;697:233–43. Epub 1993/10/29. . [DOI] [PubMed] [Google Scholar]
- 12.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42 Suppl 17:48–51. Epub 2007/01/24. 10.1007/s00535-006-1942-7 . [DOI] [PubMed] [Google Scholar]
- 13.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15(10):6340–50. Epub 1995/10/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, et al. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335(1):1–5. Epub 1993/11/29. . [DOI] [PubMed] [Google Scholar]
- 15.Nappi RE, Rivest S. Stress-induced genetic expression of a selective corticotropin-releasing factor-receptor subtype within the rat ovaries: an effect dependent on the ovulatory cycle. Biol Reprod. 1995;53(6):1417–28. Epub 1995/12/01. . [DOI] [PubMed] [Google Scholar]
- 16.Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83(8):2916–20. Epub 1998/08/26. 10.1210/jcem.83.8.5020 . [DOI] [PubMed] [Google Scholar]
- 17.Valdenaire O, Giller T, Breu V, Gottowik J, Kilpatrick G. A new functional isoform of the human CRF2 receptor for corticotropin-releasing factor. Biochim Biophys Acta. 1997;1352(2):129–32. Epub 1997/05/30. . [DOI] [PubMed] [Google Scholar]
- 18.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136(9):4139–42. Epub 1995/09/01. 10.1210/endo.136.9.7544278 . [DOI] [PubMed] [Google Scholar]
- 19.Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol. 1998;12(8):1077–85. Epub 1998/08/26. 10.1210/mend.12.8.0145 . [DOI] [PubMed] [Google Scholar]
- 20.Grammatopoulos D, Dai Y, Chen J, Karteris E, Papadopoulou N, Easton AJ, et al. Human corticotropin-releasing hormone receptor: differences in subtype expression between pregnant and nonpregnant myometria. J Clin Endocrinol Metab. 1998;83(7):2539–44. Epub 1998/07/14. 10.1210/jcem.83.7.4985 . [DOI] [PubMed] [Google Scholar]
- 21.Pal K, Swaminathan K, Xu HE, Pioszak AA. Structural basis for hormone recognition by the Human CRFR2{alpha} G protein-coupled receptor. The Journal of biological chemistry. 2010;285(51):40351–61. Epub 2010/10/23. 10.1074/jbc.M110.186072 ; PubMed Central PMCID: PMCPmc3001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–90. Epub 2006/05/09. 10.1053/j.gastro.2006.03.008 . [DOI] [PubMed] [Google Scholar]
- 23.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122(7):1771–7. Epub 2002/06/11. . [DOI] [PubMed] [Google Scholar]
- 24.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42(6):845–9. Epub 1998/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118(5):842–8. Epub 2000/04/28. . [DOI] [PubMed] [Google Scholar]
- 26.Kanazawa M, Palsson OS, Thiwan SI, Turner MJ, van Tilburg MA, Gangarosa LM, et al. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol. 2008;103(10):2550–61. Epub 2008/08/08. 10.1111/j.1572-0241.2008.02066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukudo S, Nomura T, Muranaka M, Taguchi F. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. A preliminary study. J Clin Gastroenterol. 1993;17(2):133–41. Epub 1993/09/01. . [DOI] [PubMed] [Google Scholar]
- 28.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, et al. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53(7):958–64. Epub 2004/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. Epub 2008/02/06. 10.1001/archgenpsychiatry.2007.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeYoung CG, Cicchetti D, Rogosch FA. Moderation of the association between childhood maltreatment and neuroticism by the corticotropin-releasing hormone receptor 1 gene. J Child Psychol Psychiatry. 2011;52(8):898–906. Epub 2011/03/29. 10.1111/j.1469-7610.2011.02404.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3:41 Epub 2010/02/18. 10.3389/neuro.08.041.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66(9):978–85. Epub 2009/09/09. 10.1001/archgenpsychiatry.2009.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumner JA, McLaughlin KA, Walsh K, Sheridan MA, Koenen KC. CRHR1 genotype and history of maltreatment predict cortisol reactivity to stress in adolescents. Psychoneuroendocrinology. 2014;43:71–80. Epub 2014/04/08. 10.1016/j.psyneuen.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Liu W, Yao L, Yang C, Xiao L, Wan Q, et al. Negative life events and corticotropin-releasing-hormone receptor1 gene in recurrent major depressive disorder. Sci Rep. 2013;3:1548 Epub 2013/03/27. 10.1038/srep01548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf EJ, Mitchell KS, Logue MW, Baldwin CT, Reardon AF, Humphries DE, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30(12):1161–9. Epub 2013/10/15. 10.1002/da.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishitobi Y, Nakayama S, Yamaguchi K, Kanehisa M, Higuma H, Maruyama Y, et al. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(4):429–36. Epub 2012/04/03. 10.1002/ajmg.b.32046 . [DOI] [PubMed] [Google Scholar]
- 37.Sato N, Suzuki N, Sasaki A, Aizawa E, Obayashi T, Kanazawa M, et al. Corticotropin-releasing hormone receptor 1 gene variants in irritable bowel syndrome. PLoS One. 2012;7(9):e42450 Epub 2012/09/08. 10.1371/journal.pone.0042450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. Epub 2006/05/09. 10.1053/j.gastro.2005.11.061 . [DOI] [PubMed] [Google Scholar]
- 39.Poon AH, Tantisira KG, Litonjua AA, Lazarus R, Xu J, Lasky-Su J, et al. Association of corticotropin-releasing hormone receptor-2 genetic variants with acute bronchodilator response in asthma. Pharmacogenetics and genomics. 2008;18(5):373–82. Epub 2008/04/15. 10.1097/FPC.0b013e3282fa760a ; PubMed Central PMCID: PMCPmc3208318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno T, Aoki M, Shimada Y, Inoue M, Nakaya K, Takahashi T, et al. Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. J Psychosom Res. 2006;60(1):91–7. Epub 2005/12/29. 10.1016/j.jpsychores.2005.06.068 . [DOI] [PubMed] [Google Scholar]
- 41.Iwahashi S, Tanaka Y, Fukudo S, Hongo M. The development of the Japanese version of the Perceived Stress Scale. Jpn J Psychosom Med. 2002;42(7):459–66. [Google Scholar]
- 42.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 43.Nakazato K, Shimonaka Y. The Japanese State-Trait Anxiety Inventory: age and sex differences. Percept Mot Skills. 1989;69(2):611–7. Epub 1989/10/01. 10.2466/pms.1989.69.2.611 . [DOI] [PubMed] [Google Scholar]
- 44.Zung WW. A self-rating depression scale. Archives of general psychiatry. 1965;12(1):63–70. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda K, Kobayashi S. [A study on a self-rating depression scale (author's transl)]. Seishin Shinkeigaku Zasshi. 1973;75(10):673–9. Epub 1973/10/01. . [PubMed] [Google Scholar]
- 46.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. Epub 2004/08/07. 10.1093/bioinformatics/bth457 . [DOI] [PubMed] [Google Scholar]
- 47.Chang J, Adams MR, Clifton MS, Liao M, Brooks JH, Hasdemir B, et al. Urocortin 1 modulates immunosignaling in a rat model of colitis via corticotropin-releasing factor receptor 2. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G884–94. Epub 2011/02/19. 10.1152/ajpgi.00319.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92(2):537–76. Epub 2012/04/27. 10.1152/physrev.00027.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Im E, Rhee SH, Park YS, Fiocchi C, Tache Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010;138(7):2457–67, 67 e1-5. Epub 2010/03/09. 10.1053/j.gastro.2010.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiba Y, Kaunitz JD, Million M. Peripheral corticotropin-releasing factor receptor type 2 activation increases colonic blood flow through nitric oxide pathway in rats. Dig Dis Sci. 2015;60(4):858–67. Epub 2015/02/24. 10.1007/s10620-015-3579-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305(8):G529–41. Epub 2013/07/28. 10.1152/ajpgi.00207.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanazawa M, Endo Y, Whitehead WE, Kano M, Hongo M, Fukudo S. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig Dis Sci. 2004;49(6):1046–53. Epub 2004/08/18. . [DOI] [PubMed] [Google Scholar]
- 53.Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, et al. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130(5):1435–46. Epub 2006/05/09. 10.1053/j.gastro.2005.09.071 . [DOI] [PubMed] [Google Scholar]
- 54.Aizawa E, Sato Y, Kochiyama T, Saito N, Izumiyama M, Morishita J, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology. 2012;143(5):1188–98. Epub 2012/07/31. 10.1053/j.gastro.2012.07.104 . [DOI] [PubMed] [Google Scholar]
- 55.Guillaume S, Perroud N, Jollant F, Jaussent I, Olie E, Malafosse A, et al. HPA axis genes may modulate the effect of childhood adversities on decision-making in suicide attempters. J Psychiatr Res. 2013;47(2):259–65. Epub 2012/11/28. 10.1016/j.jpsychires.2012.10.014 . [DOI] [PubMed] [Google Scholar]
- 56.Mahajan S, Liao M, Barkan P, Takahashi K, Bhargava A. Urocortin 3 expression at baseline and during inflammation in the colon: corticotropin releasing factor receptors cross-talk. Peptides. 2014;54:58–66. Epub 2014/01/28. 10.1016/j.peptides.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu F, Huang S, Zhou F, Luo Q, Xie X, Zheng C. Correlation between DQB1 genetic polymorphism and genetic susceptibility in patients diagnosed with irritable bowel syndrome with diarrhea. Genet Mol Res. 2014;13(4):10285–93. Epub 2014/12/17. 10.4238/2014.December.4.23 . [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Wu Z, Qiao H, Zhang Y. A genetic association study of single nucleotide polymorphisms in GNbeta3 and COMT in elderly patients with irritable bowel syndrome. Med Sci Monit. 2014;20:1246–54. Epub 2014/07/20. 10.12659/msm.890315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villafuerte SM, Del-Favero J, Adolfsson R, Souery D, Massat I, Mendlewicz J, et al. Gene-based SNP genetic association study of the corticotropin-releasing hormone receptor-2 (CRHR2) in major depression. Am J Med Genet. 2002;114(2):222–6. Epub 2002/02/22. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are fully available without restriction. All data and figure files are available from the Figshare database (accession number: http://dx.doi.org/10.6084/m9.figshare.1543516).