Abstract
Background
Marine-derived n-3 polyunsaturated fatty acids (PUFA) may have a beneficial effect on inflammation via lowering pro-inflammatory eicosanoid concentrations. We aimed to assess the effect of marine-derived n-3 PUFA on prostaglandin E2 (PGE2), thromboxane B2 (TXB2), and leukotriene B4 (LTB4) through systematic review and meta-analysis of randomized controlled trials.
Method and Findings
A structured search strategy on PubMed, Web of Science and Cochrane up to November 2015 was undertaken in this meta-analysis. Standard mean difference was used to calculate the effect size of marine-derived n-3 PUFA on PGE2, TXB2 and LTB4 in a random-effect model. A total of 18 RCTs with 826 subjects were included in this systematic review and meta-analysis. Supplementation of marine-derived n-3 PUFA significantly decreased concentrations of TXB2 in serum/plasma in subjects with high risk of cardiovascular diseases (SMD:-1.26; 95% CI: -1.65, -0.86) and LTB4 in neutrophils in unhealthy subjects (subjects with non-autoimmune chronic diseases or auto-immune diseases) (SMD:-0.59: 95% CI: -1.02, -0.16). Subgroup analyses showed a significant reduction of LTB4 in subjects with rheumatoid arthritis (SMD: -0.83; 95% CI: -1.37, -0.29), but not in non-autoimmune chronic disease patients (SMD: -0.33; 95% CI: -0.97, 0.31). No significant publication bias was shown in the meta-analysis.
Conclusions
Marine-derived n-3 PUFA had a beneficial effect on reducing the concentration of TXB2 in blood of subjects with high risk of CVD as well as LTB4 in neutrophils in unhealthy subjects, and that subjects with RA showed lower LTB4 content with supplementation of marine-derived n-3 PUFA.
Introduction
Previous studies have shown that inflammation plays a significant role in a number of widespread and destructive chronic diseases, including autoimmune diseases such as rheumatoid arthritis (RA) [1] and non-autoimmune chronic diseases including obesity and insulin resistance [2], cardiovascular disease (CVD) [3] and several neurodegenerative diseases such as Alzheimer’s disease [4]. Several arachidonic acid (AA) -derived eicosanoids exert their significant influence on the inflammatory response. Prostaglandin E2 (PGE2) is involved in the classic signs of inflammation and possesses both pro-inflammatory and anti-inflammatory actions [5]; thromboxane A2 (TXA2) (precursor of TXB2), formed by platelets, macrophages and poly-morphonuclear leukocytes (PMNs), can induce vasoconstriction and promotes aggregation of platelets as well as adhesiveness of PMNs [6]; leukotriene B4 (LTB4) can not only increase vascular permeability and enhance local blood flow by stimulating neutrophil secretion [7], but also stimulate other inflammatory substances.
Previous studies have shown that increased intake of n-3 polyunsaturated fatty acids (PUFA), especially marine-derived n-3 PUFAs (eicosapentaenoic acid [EPA] docosapentaenoic acid [DPA], and docosahexaenoic acid [DHA]), are beneficial for coronary heart disease [8], metabolic syndrome [9] and Alzheimer’s disease [10]. Previous evidence has suggested that the positive protective impact of n-3 PUFA on these diseases may be attributed to the anti-inflammatory function, including lowering of blood eicosanoids [11, 12]. However, the effects of n-3 PUFA on AA-derived major eicosanoids still remains controversial: several studies suggested that marine-derived n-3 PUFA resulted in decreased PGE2 [13], TXB2 [14] and LTB4 [15], while increased PGE2 [16] and TXB2 [17] in response to n-3 PUFA were also found in some other studies. There has been no systematic review and meta-analysis conducted to summarize the available evidence of the effects of n-3 PUFA on major eicosanoids. Therefore, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to assess the effect of marine-derived n-3 PUFA on AA-derived major eicosanoids (PGE2, TXA2/TXB2 and LTB4).
Materials and Methods
Search strategy and study selection
To identify randomized controlled studies involving the effects of marine-derived n-3 polyunsaturated fatty acids on major AA-derived eicosanoids (PGE2, TXA2/TXB2, and LTB4), we searched three electronic databases, PubMed, Web of Science and Cochrane Library, up to November 2015 for studies of humans published in all languages. We used the following key words for the literature search: (“fish oil” OR “seafood” OR “eicosapentaenoic acid” OR “docosahexaenoic acid” OR “docosapentaenoic acid” OR “n-3 PUFA”) AND (“eicosanoids” OR “prostaglandin” OR “thromboxane” OR “leukotriene”). References of included studies were also reviewed to identify potential publications. We contacted authors for the detailed information of primary studies when possible.
Search strategy and study selection were undertaken independently by two investigators, with discrepancies resolved through group discussion. Inclusion criteria were: randomized controlled trial design including parallel and crossover design; the exposure of interest was marine-derived n-3 PUFA such as EPA and/or DHA on PGE2 or TXB2 or LTB4; LTB4 was determined in neutrophils by HPLC and PGE2 and TXB2 were measured by Radioimmunoassay or ELISA (enzyme-linked-immunosorbent assay). Studies were excluded if: subjects were diagnosed with acute disease or the duration was less than two weeks; there was dietary invention such as marine fish or others; there were other additional interventions apart from n-3 PUFA such as exercise and anti-inflammatory drugs compared with control group; data indispensable for a meta-analysis were not reported and still unavailable after contacting authors; studies with a crossover design which did not report a wash-out period; and studies which did not have a placebo group or proper control group.
Data extraction and quality assessment
Extracted data from each article included the first author’s name; year of publication; study population; study design and duration of intervention; number, age, gender or sex ratio and health status in subjects; method and dose of intervention; source of samples; data of mean changes and corresponding standard deviation for PGE2, TXB2 or LTB4. Risk of bias table based on Cochrane Criteria was used to assess the quality of studies in the present meta-analysis, including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), outcome data (attrition bias) and outcome reporting (reporting bias) [18].
Statistical analysis
We conducted three independent meta-analysis for PGE2, TXB2 and LTB4 and subgroup analysis was conducted to identify the source of heterogeneity. For parallel trials, mean changes and corresponding SDs from baseline to endpoint were collected for data analysis, data was estimated according to the previous method [19] if SDs of changes were not reported. For studies with a crossover design, mean changes between the levels of PGE2, TXB2 and LTB4 and corresponding SDs at the end of two intervention periods were used in data analysis, as suggested by Cochrane handbook for Systematic Reviews of Intervention [18]. The meta-analysis was performed with Stata/SE 11.0 software (Stata Corporation, College Station, TX) by using the random effect model. Standard mean differences and 95% CIs were calculated for net changes in values with PGE2, TXB2 and LTB4. Heterogeneity of treatment effects between studies was tested using the Chi-square method and I2 > 50% was considered to be significant. To explore the possible influence of covariates on net changes, subgroup analyses and meta-regression were conducted to evaluate the effects of dose of total marine-derived n-3 PUFA, EPA and DHA respectively, ratio of EPA/DHA, duration and design of study, body mass index (BMI) and age of subjects and type of intervention and placebo on the overall effect size. We classified studies according to duration as short term (< 12 weeks) or long term (> = 14 weeks). Region of subjects were mainly divided into four areas- Europe, America, Australia and Asia. For the following variables—age and BMI at baseline, dose of total marine-derived n-3 PUFA, EPA and DHA, ratio of EPA to DHA, we stratified studies according to their median. Meta-regression with restricted maximum likelihood estimation was performed to evaluate the potentially important covariates exerting substantial impact on heterogeneity between the trials. According to the Cochrane Handbook for Systemic Review, publication bias was examined by using funnel plots, Begg’s test and Egger’s regression test [20].
Results
Selection of relevant studies
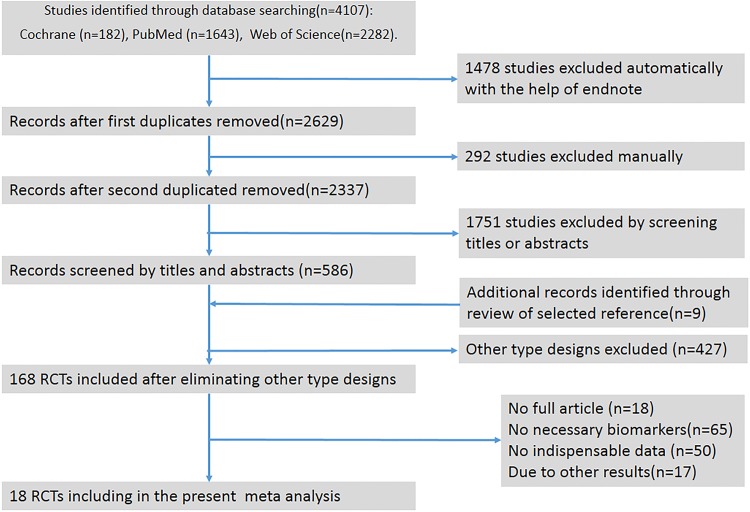
The initial electronic search yielded 4107 records (1643 from PubMed, 2282 from Web of Science and 182 from Cochrane with 1770 duplicate records). Titles and abstracts were screened for 1751 citations, and 168 RCTs were obtained after eliminating other type designs. Of these, 50 RCTs were excluded because data indispensable for meta-analysis was still not available. Four studies were excluded because they only provided figures without definite information, and two studies had insufficient information (no data at baseline or endpoint). We contacteded 15 authors for data and only received replies from 4 of them, but the required data was not available. Finally, 18 studies of RCTs, including 27 study arms with 826 subjects were included in this systematic review and meta-analysis. Detailed processes of the study selection are shown in Fig 1. PRISMA Flow Diagram PRISMA and checklist required for meta-analysis are shown in S1 Fig and S1 PRISMA Checklist.
Fig 1. Flow diagram of studies evaluated in the review.
RCT, randomized controlled trial.
Characteristics of the studies
Primary characteristics of the 18 studies including 27 study arms are shown in Table 1. A total of 826 subjects were randomly assigned to intervention or control groups with a completion rate of 57.23%. Of the 20 included randomized controlled trials, subjects in most studies were from Europe (n = 7), and others were from the United States (n = 4), Australia (n = 3), Brazil (n = 1), korea (n = 1), Iran (n = 1) and Canada (n = 1).
Table 1. Characteristics of included studies for systematic review and meta-analysis.
| Studies | Subjects | Treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Sample | Biomarkers | Duration | No | Country | Healthy | BMI | Age | n-3 | EPA | DHA | EPA | T |
| type | (weeks) | status | (kg/m2) | (yr) | (g) | (g) | (g) | /DHA | |||||
| Andrade et al.2007 | Plasma | PGE2 | 6 | 20 | Brazil | Health | 23.5 | 27.5 | 1.45 | 0.95 | 0.5 | 1.9 | Fish oil |
| Tempel et al.1990 | Neutrophils | LTB4 | 12 | 16 | Netherlands | RA | UN | 53 | 3.36 | 2.04 | 1.32 | 1.54 | Fish oil |
| Ve'ricel et al.1999 | Platelet | TXB2 | 6 | 20 | France | Healthy | UN | 76.5 | 0.18 | 0.03 | 0.15 | 0.2 | Fish oil |
| Park et al.2013 | Serum | PGE2 | 16 | 81 | Korea | RA | 22.4 | 48.4 | 3.255 | 2.09 | 1.165 | 1.79 | Fish oil |
| Peck et al.1996 (O) | Plasma | PGE2 | 8 | 13 | USA | Hemodialysis | 25.4 | 50.2 | UN | UN | UN | UN | Fish oil |
| Peck et al.1996 (S) | Plasma | PGE2 | 8 | 12 | USA | Hemodialysis | 24.4 | 52.2 | UN | UN | UN | UN | Fish oil |
| Pirich et al.1999 | Serum | TXB2 | 6 | 26 | Australia | Hyper cholesterolemia | UN | 57 | 0.356 | 0.216 | 0.14 | 1.54 | Fish oil |
| Sørensen et al.1992 | Serum | TXB2 | 7 | 34 | Denmark | Pregnant | UN | 29.6 | 2.7 | UN | UN | UN | Fish oil |
| Singer et al.2004 | Plasma | TXB2 | 24 | 65 | Germany | Cardiac arrhyamias | 25.9 | 43.5 | 0.9 | 0.54 | 0.36 | 1.5 | Fish oil |
| Turini et al.1994 | Neutrophils | LTB4 | 6 | 20 | Canada | Healthy | 25.3 | 26.5 | 4.5 | 3.3 | 1.2 | 2.75 | Fish oil |
| Tartibian et al.2011 | Plasma | PGE2 | 4 | 30 | Iran | Healthy | 24.9 | 30.2 | 0.54 | 0.324 | 0.216 | 1.5 | Fish oil |
| Thusgaard et al.2009 | Neutrophils | LTB4 | 12 | 48 | Denmark | HIV-infect | 24.7 | 45 | 3.36 | 1.84 | 1.52 | 1.21 | Fish oil |
| Maaloe et al.2011 | Neutrophils | LTB4 | 8 | 56 | Denmark | Chronic kidney diseases | UN | 59 | 1.44 | 0.864 | 0.576 | 1.5 | Fish oil |
| Knapp et al.1989 (L) | Serum | TXB2 | 4 | 16 | USA | Hypertension | UN | UN | 3 | 1.8 | 1.2 | 1.5 | Fish oil |
| Knapp et al.1989 (H) | Serum | TXB2 | 4 | 16 | USA | Hypertension | UN | UN | 15 | 9 | 6 | 1.5 | Fish oil |
| Mori et al.1992 | Neutrophils | LTB4 | 4 | 29 | Australia | Peripheral vascular disease | 25.4 | 61.9 | 4.6 | 2.8 | 1.8 | 1.56 | Fish oil |
| Phang et al.2013 | Plasma | TXB2 | 4 | 47 | Australia | Healthy | 24.6 | 39.6 | 1.2 | 1 | 0.2 | 5 | Fish oil |
| Phang et al.2013 (H) | Plasma | TXB2 | 4 | 47 | Australia | Healthy | 24.6 | 39.6 | 1.2 | 0.2 | 1 | 0.2 | Fish oil |
| Rees et al.2006 (YL) | MNCs | PGE2 | 12 | 31 | UK | Healthy | 24.8 | 24.3 | 1.65 | 1.35 | 0.3 | 4.5 | EPA |
| Rees et al.2006 (OL) | MNCs | PGE2 | 12 | 22 | UK | Healthy | 27.6 | 61 | 1.65 | 1.35 | 0.3 | 4.5 | EPA |
| Rees et al.2006 (YM) | MNCs | PGE2 | 12 | 31 | UK | Healthy | 23.5 | 24.8 | 3.3 | 2.7 | 0.6 | 4.5 | EPA |
| Rees et al.2006 (OM) | MNCs | PGE2 | 12 | 20 | UK | Healthy | 28.1 | 60.9 | 3.3 | 2.7 | 0.6 | 4.5 | EPA |
| Rees et al.2006 (YH) | MNCs | PGE2 | 12 | 31 | UK | Healthy | 24 | 24 | 4.95 | 4.05 | 0.9 | 4.5 | EPA |
| Rees et al.2006 (OH) | MNCs | PGE2 | 12 | 20 | UK | Healthy | 27 | 60.1 | 4.95 | 4.05 | 0.9 | 4.5 | EPA |
| Kremer et al.1990 (L) | Neutrophils | LTB4 | 24 | 26 | USA | RA | UN | 58.8 | UN | UN | UN | UN | EPA |
| Kremer et al.1990 (H) | Neutrophils | LTB4 | 24 | 23 | USA | RA | UN | 58 | UN | UN | UN | UN | EPA |
| Kremer et al.1987 | Neutrophils | LTB4 | 14 | 26 | USA | RA | UN | 56.8 | 4.5 | 2.7 | 1.8 | 1.5 | EPA |
T, intervention in treatment group; UN, unclear; RA, rheumatoid arthritis; MNCs, mononuclear cells; PGE2, prostaglandin E2; TXB2, thromboxane B2; LTB4, leukotriene B4; L, low, H, high; YL, young and low, OL, old and low, YM, young and middle, OM, old and middle, YH, young and high, OH, old and high.
For studies that reported values of PGE2, TXB2 or LTB4 at more than 2 time points, only changes from baseline to endpoint were used to calculate the overall effect size [21]. For studies that included more than one intervention group, to avoid the duplication of subjects for analysis, ‘shared’ control group was split into two or more control groups with smaller sample size, which included two or more (reasonably independent) comparisons.
Knapp et al [22] compared a high dose and low dose of fish oil with safflower oil and mixed oils. The high dose arm was compared with mixed oil arm labelled Knapp L, whereas the low dose arm was compared with safflower oil arm labelled Knapp H. Kremer et al [23] included low dose, high dose and control, the control was isolated into two units and labeled Kremer L and Kremer H. Phang et al [24] compared EPA group and DHA group with Placebo group. The EPA arm was compared with Placebo arm labelled Phang E, wheras DHA arm was compared with Placebo arm labelled Phang D. Peck et al [16] compared fish oil with various control oils including olive oil and safflower oil, the fish oil arm was seperated into two small arms labelled Peck O and Peck S respectively. There were eight groups in the study reported by Rees et al [25], six study arms were labelled Rees (YL), Rees (OL), Rees (YM), Rees (OM), Rees (YH), Rees (OH) respectively according to age (Y-young, O-old) and dose (L-low, M-moderate, H-high).
Quality of studies
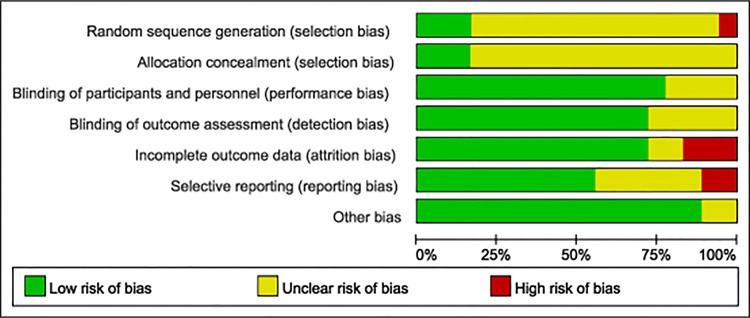
None of the included trials reported any significant differences in characteristics of subjects in the treatment or control arm. Allocation concealment was adequate in 5 studies and unclear in others. Sixteen studies were double-blind design, one study [22] only masked paticipants, and five studies were unclear. Risk-of-bias graph and risk of bias summary are shown in Figs 2 and 3.
Fig 2. Risk-of-bias graph.
The review of judgments of authors about each risk-of-bias item is presented as percentages across all included studies.
Fig 3. Risk of bias summary.
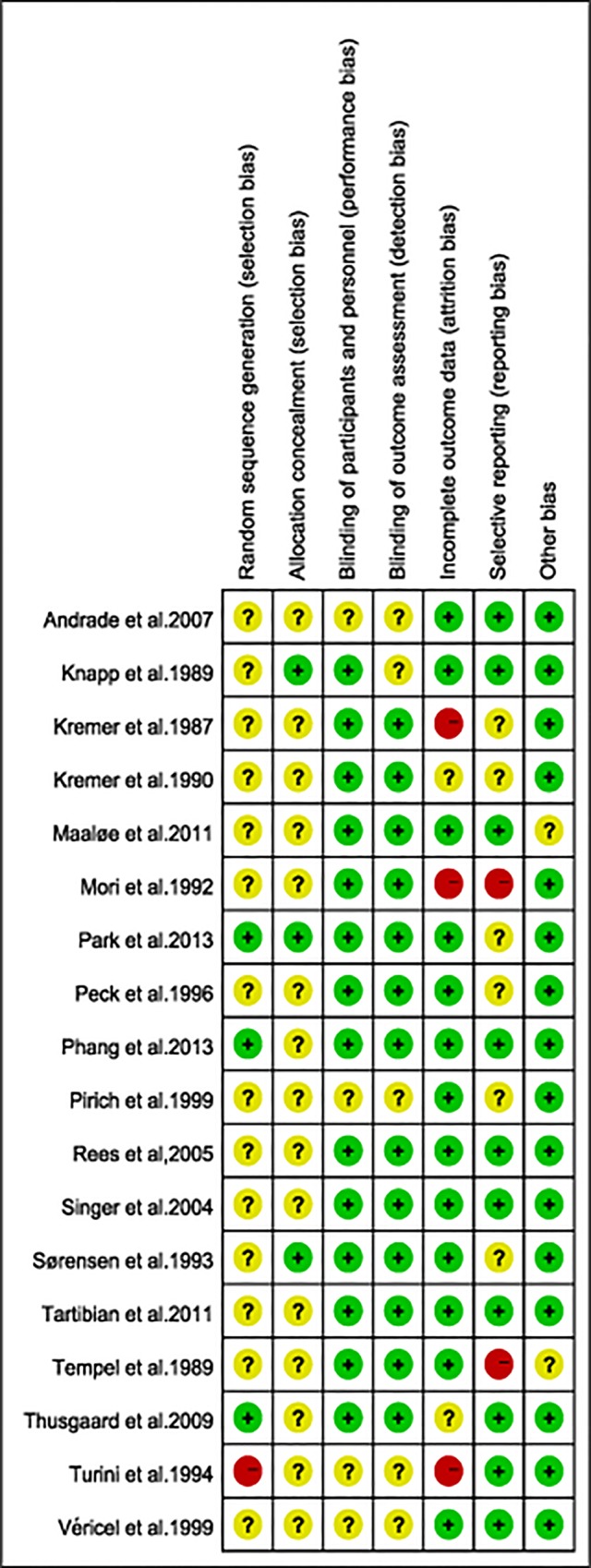
Review author’s judgements about each risk of bias item for each included study.
Effect of marine-derived n-3 PUFA supplementation on PGE2
Five studies reported the effect of marine-derived n-3 PUFA on PGE2 [16, 25–28], among which two studies [26, 28] included in the meta-analysis showed a significant reduction on PGE2 in plasma of healthy subjects with the supplementation of marine-derived n-3 PUFA compared with placebo supplementation (SMD:-1.27; 95% CI: -1.90,-0.63).
Effect of marine-derived n-3 PUFA supplementation on TXB2
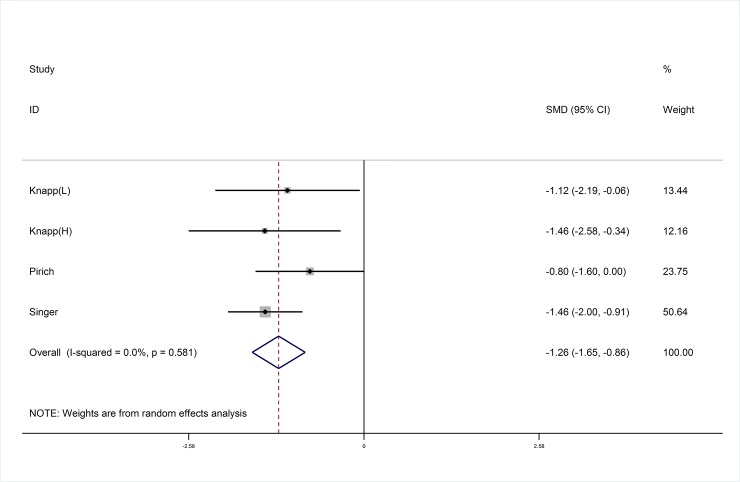
Six studies [21, 22, 24, 29–31] were included in this systematic review and meta-analysis, and data from three studies [22, 30–31] with four arms were pooled to demonstrate that marine-derived n-3 PUFA showed a siginificant difference in reducing TXB2 in subjects with high risk of CVD compared with placebo (SMD:-1.26; 95% CI: -1.65, -0.86; I2 = 0.0%) (Fig 4).
Fig 4. Pooled effect size of marine-derived PUFA supplementation on TXB2.in subjects with high risk of CVD.
CVD, cardiovascular disease. SMD, standard mean difference.
Effect of marine-derived n-3 PUFA supplementation on LTB4
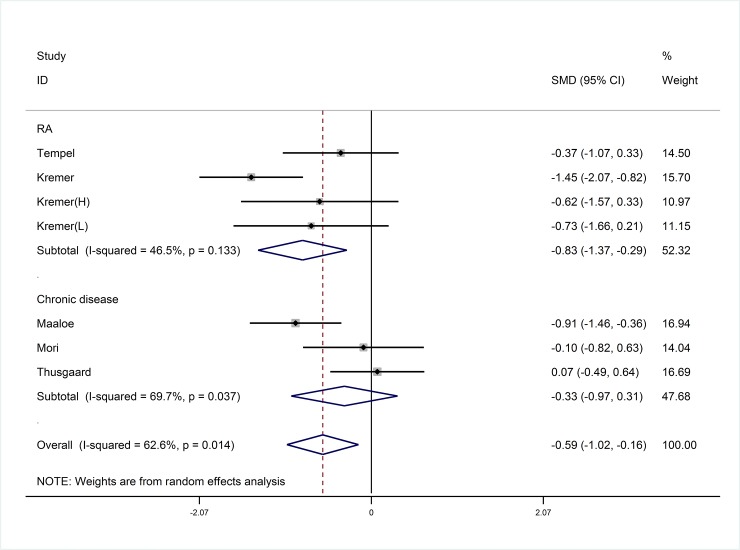
Six studies [23, 32–36] with seven study arms reported the effect of marine-derived n-3 PUFA on LTB4 in unhealthy subjects and the other one [37] reported in healthy subjects. Marine-derived n-3 PUFA was more effective in reducing the concentration of LTB4 in neutrophils of unhealthy subjects compared with placebo (SMD:-0.59; 95% CI: -1.02, -0.16; I2 = 62.6%) (Fig 5).
Fig 5. Pooled effect size of marine-derived n-3 PUFA supplementation on LTB4 in unhealthy subjects including RA and Chronic diseases patients.
RA, rheumatoid arthritis. SMD, standard mean difference.
Subgroup analysis and meta-regression
Subgroup analyses and meta-regression analyses were conducted to evaluate the effects of the confounding factors on primary outcomes of marine-derived n-3 PUFA on LTB4 for each potential variable including dose of total marine-derived n-3 PUFA, EPA and DHA, ratio of EPA to DHA, duration and design of study, body mass index (BMI) and age of subjects and type of intervention and placebo. Full details are shown in Table 2. Subgroup analyses showed a more significant reduction on LTB4 in RA patients with supplementation of marine-derived n-3 PUFA (SMD: -0.83; 95% CI: -1.37, -0.29), but not in non-autoimmune chronic disease patients (SMD: -0.33; 95% CI: -0.97, 0.31) (Fig 5). No significant reduction on LTB4 was found when the duration of intervention with marine-derived n-3 PUFA was less than 14 weeks (SMD: -0.34; 95% CI: -0.81, 0.13) (Table 2). There was no significant difference in serum (SMD: -1.05; 95% CI: -1.61, -0.49) and plasma (SMD: -1.46; 95% CI: -2.00, -0.91) concerning the effect of marine-derived n-3 PUFA on TXB2. The use of anti-inflammatories failed to significantly change the results for the effect of marine-derived n-3 PUFA on LTB4 (P = 0.286).
Table 2. Subgroup analysis for the effect of marine-derived n-3 PUFAs on LTB4 in unhealthy subjects (subjects with non-autoimmune chronic diseases or auto-immune diseases).
| Subgroup analysis | LTB4 | ||||
|---|---|---|---|---|---|
| N | SMD (95%CI) | Heterogeneity | P2 | ||
| I2 (%) | P1 | ||||
| Overall | 7 | -0.59 (-1.12, -0.25) | 62.6 | 0.014 | |
| Sex ratio(male/female) | 0.286 | ||||
| <0.5 | 3 | -0.33 (-0.97, 0.31) | 69.7 | 0.037 | |
| > = 0.5 | 4 | -0.83 (-1.37, -0.29) | 46.5 | 0.133 | |
| Age | 0.965 | ||||
| <median | 3 | -0.58 (-1.50, 0.34) | 84.4 | 0.002 | |
| > = median | 4 | -0.63 (-1.01, -0.25) | 3.80 | 0.374 | |
| Health status | 0.163 | ||||
| Chronic diseases | 3 | -0.33 (-0.97, 0.31) | 69.7 | 0.037 | |
| Rheumatoid arthritis | 4 | -0.83 (-1.37, -0.29) | 46.5 | 0.133 | |
| Duration | 0.138 | ||||
| <14weeks | 4 | -0.34 (-0.81, 0.13) | 54.6 | 0.086 | |
| > = 14 weeks | 3 | -1.04 (-1.59, -0.48) | 27.8 | 0250 | |
| Daily dose of total n-3 PUFA(g) | 0.987 | ||||
| <median | 2 | -0.69 (-1.21, -0.16) | 30.0 | 0.232 | |
| > = median | 3 | -0.49 (-1.46, 0.48) | 85.6 | 0.001 | |
| NR | 2 | -0.68 (-1.34, -0.06) | 0 | 0.880 | |
| daily dose of EPA(g) | 0.679 | ||||
| <median | 2 | -0.42 (-1.39, 0.54) | 83.2 | 0.015 | |
| > = median | 3 | -0.65 (-1.49, 0.18) | 77.8 | 0.011 | |
| NR | 2 | -0.68 (-1.34, -0.01) | 0 | 0.880 | |
| daily dose of DHA(g) | 0.987 | ||||
| <median | 2 | -0.69 (-1.21, -0.16) | 30.0 | 0.232 | |
| > = median | 3 | -0.49 (-1.46, 0.48) | 85.6 | 0.001 | |
| NR | 2 | -0.68 (-1.34, -0.01) | 0 | 0.880 | |
| Ratio of EPA/DHA | 0.765 | ||||
| < 1.55 | 3 | -0.76 (-1.62, 0.10) | 84.8 | 0.001 | |
| > = 1.55 | 2 | -0.24 (-0.74, 0.27) | 0 | 0.594 | |
| NR | 2 | -0.68 (-1.34, -0.01) | 0 | 0.880 | |
| Region | 0.927 | ||||
| Europe | 3 | -0.41 (-1.01, -0.19) | 66.6 | 0.500 | |
| America | 3 | -1.04 (-1.59, -0.48) | 27.8 | 0.250 | |
| Australia | 1 | -0.10 (-0.82, 0.63) | NA | NA | |
N, number of included studies (or comparisons); NR, not reported; NA, not associated with this item; P1 value for heterogeneity within each subgroup; P2 value for heterogeneity between subgroups with meta-regression analysis.
Sensitivity analysis
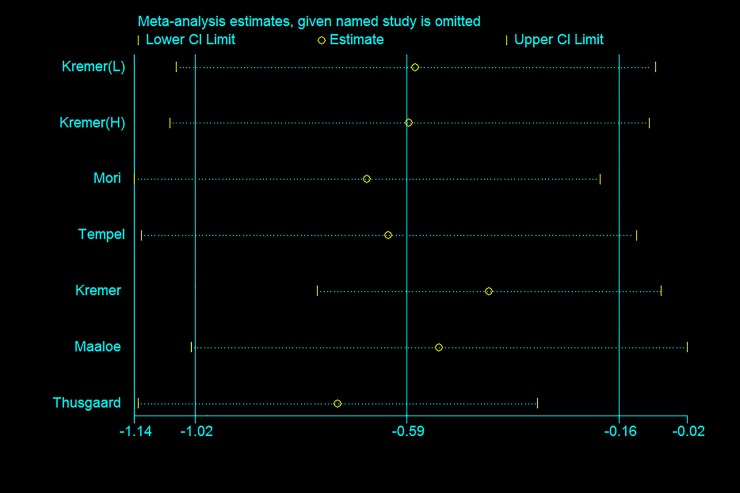
We also conducted sensitivity analyses to explore the source of heterogeneity. Sensitivity analysis was performed by studying whether effect size changes significantly after excluding special trials one by one. Some trials were not consistent with others in terms of intervention method, measurement method, samples and volunteers. Rees et al [25] detected PGE2 concentration in mononuclear cell; special volunteers were recruited such as elite swimmers [28] and pregnant women [21]; Radioimmunoassay[16, 22, 30] and ELISA [24–29, 31] were used to determine the concentrations of PGE2 and LTB4. Moreover, the studies by Mori et al [33], Pirich et al [30] and Turini et al [37] had low quality and high risk bias. However, the results of the sensitivity analysis on PGE2, TXB2 and LTB4 (Fig 6) showed that the pooled effects remained non-significant when analyses were limited to high-quality trials and conducted after excluding special trials.
Fig 6. Sensitive analysis with pseudo 95% confidence limits for studies with LTB4.
In the study by Knapp [22], the high dose arm was compared with mixed oil arm labelled Knapp L, whereas the low dose arm was compared with safflower oil arm labelled Knapp H. The pooled effects remained non-significant when we compared the high dose arm with safflower oil and low dose arm with mixed oil arm.
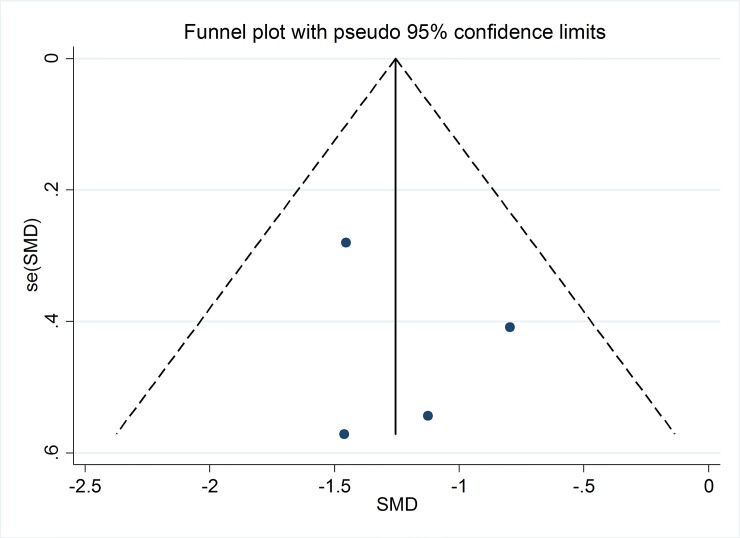
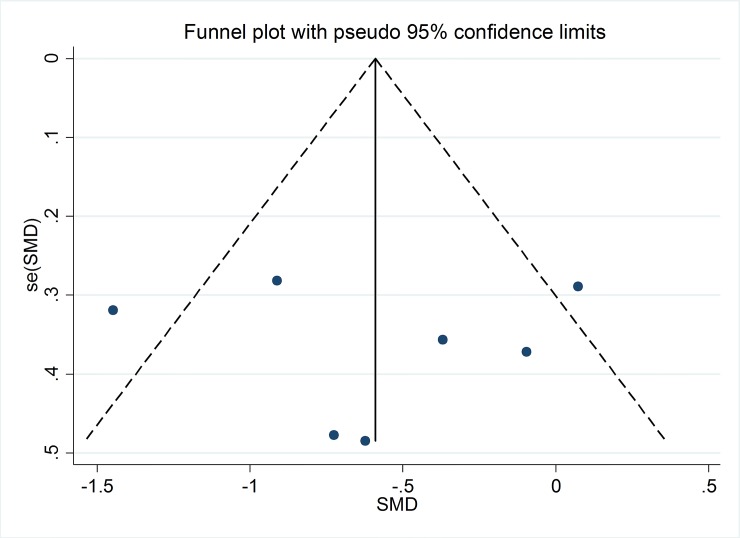
Publication bias
On the basis of funnel plots (Figs 7 and 8) and Egger’s test, no significant publication bias was shown in the meta-analysis of TXB2 in serum or plasma of subjects with high risk of CVD (P = 0.59) and LTB4 in neutrophils of unhelthy subjects (P = 0.60) with supplementation of marine-derived n-3 PUFA.
Fig 7. Funnel plot with pseudo 95% confidence limits for studies with TXB2.
Fig 8. Funnel plot with pseudo 95% confidence limits for studies with LTB4.
Discussion
The results of this meta-analysis supported the effect of marine-derived n-3 PUFA on suppressing TXB2 in subjects with high risk of CVD and LTB4 in neutrophils in unhealthy subjects. TXB2 can induce vasoconstriction and promote aggregation of platelets as well as adhesiveness of PMNs, and LTB4 is involved in modulating the intensity and duration of inflammatory responses. On one hand, n-3 PUFAs, especially marine-derived n-3 PUFAs, directly inhibit AA metabolism to produce eicosanoids by replacing AA as the substrate, and indirectly alter the expression of inflammatory genes through effects on transcription factor genes [12]. On the other hand, marine-derived n-3 PUFAs, especially EPA, can also act as a substrate catalyzed by 5-lipoxygenase enzymes and cyclooxygenase enzymes to generate several eicosanoids. These eicosanoids, such as LTB5 and LTE5, have slightly different structures and less potent pro-inflammatory function compared with those formed from AA [38]. Furthermore, EPA and DHA can also generate a novel genus of mediators, termed specialized pro-resolving mediators.
Subgroup analysis results showed a significant effect on LTB4 in RA patients in neutrophils with supplementation of marine-derived n-3 PUFA, Park et al [27] also found that n-3 PUFA supplementation significantly decreased LTB4 levels in serum of RA patients who weighed more than 55kg. For subjects with chronic diseases, the function of marine-derived n-3 PUFA did not reach statistical significance. However, PGE2 was significantly increased in hemodialysis patients compared with control group following supplementation of marine-derived n-3 PUFA [16], and a slightly but not significant increase was also found in RA patients [27]. In addition, marine-derived n-3 PUFA did not result in significantly decreased formation of plasma PGE2 in mildly hypertriacylglycerolemic subjects [39]. A hypothesis was put forward to explain the different results between PGE2 and LTB4: a decrease in proteinuria and an improvement in glomerular filtration rate was reported [40], which may be relevant to the function of n-3 PUFA on immunoglobulin A nephropathy. Increased DHA and EPA could result in the production of inactive LTB5 along with decreased synthesis of the inflammatory LTB4. These eicosanoids were produced through different pathways, LTB4 was generated through lipoxygenase pathway with 5-lipoxygenases enzymes, while PGE2 and TXA2 /TXB2 were from cyclo-oxygenase (COX) pathway. So LTB5 blocked the lipoxygenase pathway and arachidonic acid was metabolized to produce PGE2 through the cyclooxygenase pathway, which led to a decrease in LTB4 and corresponding increase in PGE2.
However, for another product of the cyclooxygenase pathway, TXB2 showed a reduction with supplementation of marine-derived n-3 PUFA in populations with high risk of CVD. Mehta et al. [41] found that EPA supplementation had no effect on concentrations of PGE2 in Barrett’s mucosa. This study also suggested no significant alteration in AA and increase in phospholipase A2 activity. Hishinuma et al. [42] demonstrated that an increased availability of AA resulted in an increase in 2-series eicosanoid production in murine mast cells, and the predominant COX2 pathway products were PGE2 > PGF2α > TXB2 > 6-keto-PGF1α [43, 44] when AA is in abundance. Therefore, it may lead to no significant change in PGE2 and a reduction in TXB2 concentration.
An animal experiment by Kang et al [45] examined this issue from another aspect. They measured the inflammatory indicators in wild type (WT) mice and fat-1 mice (a kind of transgenic mice rich in endogenous n-3 PUFA), both of which were induced to have colitis. Results showed that in colon tissue, no significant differences in the content of AA, LTB4 and PGE2 were found, but there was a remarkable increase in the amounts of EPA and DHA as well as their potent bioactive products including resolvins and protectins (RvE1, RvD3, and PD1/NPD1), and the less potent products including LTB5 and PGE3. The author assumed that marine-derived n-3 PUFA exerts anti-inflammatory functions by the potent bioactive rather than AA-derived eicosanoids. This hypothesis could explain why there was no difference in PGE2 levels after marine-derived n-3 PUFA supplementation, but this conflicted with our results for TXB2 and LTB4. The inconsistency may be most likely due to the difference in the ratio of n-3/n-6 PUFA. In the study by Kang et al [45], the n-3/n-6 ratio was so high in fat-1 mice that it could supply limited AA as substrate to rise PGE2, TXB2 and LTB4. More animal experiments are needed to explore the mechanism of how marine-derived n-3 PUFA exerts its effect on inflammation.
In healthy subjects, marine-derived n-3 PUFA supplementation had no significant effect on TXB2 [21, 24], which is consistent with the result from Murphy et al. [46], who found that levels of TXB2 tended to decrease with n-3 PUFA supplementation in both groups, but this did not reach statistical significance. It was most likely due to the low production of eicosanoids at baseline in healthy subjects. However, Andrade et al. found swimming athletes who had supplementation of marine-derived n-3 PUFA showed decreased PGE2 in plasma [28], which was also confirmed in common healthy subjects reported by Tartibian et al [26], and EPA also led to a significant reduction in the levels of PGE2 in mononuclear cell (MNC) [25] and skin unexposed to ultraviolet radiation [47]. The different results between PGE2 and TXB2 may result from the different dose of n-3 PUFA. Individuals with a higher concentrations of EPA and DHA into erythrocyte membranes also showed the greatest reduction in erythrocyte AA content [48]. A substantial increase in the EPA content of MNC phospholipids (> 4-fold) and a corresponding decrease in AA (25%) was required to affect PGE2 production [25].
We only included studies for meta-analysis where PGE2 were measured in plasma of healthy subjects, and TXB2 were tested in in serum/plasma in subjects with high risk of CVD (Fig 4) and LTB4 were measured in neutrophils of unhealthy subjects (Fig 5). Others were only included for systematic review and reported in discussion. Additionally, LTB4 was detemined by HPLC, while PGE2 and TXB2 were measured by radioimmunoassay or ELISA. We conducted SMD as the estimated effect size and random effects model was used for meta-analysis.
We first utilized pooled data to quantitatively assess the effect of marine-derived n-3 PUFA on AA-derived major eicosanoids (PGE2, TXA2/TXB2 and LTB4), and subgroup analyses were conducted to evaluate the effects of confounding factors on overall effect size of LTB4 in unhealthy subjects. In addition, we discovered the effects of marine-derived n-3 PUFA on PGE2 and TXB2 were different, and future studies can recruit volunteers with different health status to explore the effect of n-3 PUFA on eicosanoids. Lastly, no significant publication bias was shown in the meta-analysis according to the results of funnel plots and Egger’s test.
There are several limitations in the present study. First, to explore the potential influence on effect size by several confounding factors (such as age of subjects and dose of n-3 PUFA), ‘shared’ control group was split into two or more control groups to couple the intervention group according to the method by Cochrane handbook [18]. Studies included in one meta-analysis were so limited that all trials in a subgroup were likely to be from one study in subsequent subgroup analysis. Secondly, the placebo in control group showed variety including olive oil, safflower, mineral oil, corn oil and others, and subgroup analysis by the type of placebo couldn’t be performed for too few trials in each subgroup. Lastly, dose-response of the effect of marine-derived n-3 PUFA on major eicosanoids was also not conducted due to limited trials. Therefore, more well-controlled RCTs to investigate the effect of marine-derived n-3 PUFA on major eicosanoids are needed to solve the problems above.
Conclusions
In conclusion, this systematic review and meta-analysis provided evidence that marine-derived n-3 PUFA had a significant benefit on the reduction of TXB2 content in subjects with high risk of CVD and LTB4 concentrations in unhealthy subjects. Subgroup analyses showed that marine-derived n-3 PUFA significantly decreased LTB4 in RA patients, but not in non-autoimmune chronic disease patients, and duration of intervention with marine-derived n-3 PUFA also affected the overall effect size. High quality RCTs are needed to explore the effects of marine-derived n-3 PUFA on different eicosanoids in subjects with different health status.
Supporting Information
(DOC)
(DOC)
Acknowledgments
This study was funded by the National Basic Research Program of China (973 Program: 2015CB553604); by National Natural Science Foundation of China (NSFC: 81273054); and by the Ph.D. Programs Foundation of Ministry of Education of China (20120101110107). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the National Basic Research Program of China (973 Program: 2015CB553604); by National Natural Science Foundation of China (NSFC: 81273054); and by the Ph.D. Programs Foundation of Ministry of Education of China (20120101110107).
References
- 1.Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423: 356–61. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444: 860–7. [DOI] [PubMed] [Google Scholar]
- 3.Libby P (2006) Inflammation and cardiovascular disease mechanisms. The American journal of clinical nutrition 83: 456s–60s. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. (2000) Inflammation and Alzheimer’s disease. Neurobiology of aging 21: 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST (2003) Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proceedings of the National Academy of Sciences of the United States of America 100: 1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstraete M (1983) Introduction: Thromboxane in biological systems and the possible impact of its inhibition. British journal of clinical pharmacology 15: 7s–11s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson WR (1994) The role of leukotrienes in inflammation. Annals of internal medicine 121: 684–97. [DOI] [PubMed] [Google Scholar]
- 8.Bucher HC, Hengstler P, Schindler C, Meier G (2002) N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. The American journal of medicine 112: 298–304. [DOI] [PubMed] [Google Scholar]
- 9.Browning LM (2003) n-3 Polyunsaturated fatty acids, inflammation and obesity-related disease. The Proceedings of the Nutrition Society 62: 447–53. [DOI] [PubMed] [Google Scholar]
- 10.Jicha GA, Markesbery WR (2010) Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clinical interventions in aging 5: 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calder PC (2012) Mechanisms of action of (n-3) fatty acids. The Journal of nutrition 142: 592s–9s. 10.3945/jn.111.155259 [DOI] [PubMed] [Google Scholar]
- 12.Calder PC (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American journal of clinical nutrition 83: 1505s–19s. [DOI] [PubMed] [Google Scholar]
- 13.Trebble TM, Wootton SA, Miles EA, Mullee M, Arden NK, Ballinger AB, et al. (2003) Prostaglandin E2 production and T cell function after fish-oil supplementation: response to antioxidant cosupplementation. The American journal of clinical nutrition 78: 376–82. [DOI] [PubMed] [Google Scholar]
- 14.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ (1996) The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. The American journal of clinical nutrition 63: 116–22. [DOI] [PubMed] [Google Scholar]
- 15.Sperling R, Benincaso A, Knoell C, Larkin J, Austen K, Robinson D (1993) Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. Journal of Clinical Investigation 91: 651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peck LW, Monsen ER, Ahmad S (1996) Effect of three sources of long-chain fatty acids on the plasma fatty acid profile, plasma prostaglandin E2 concentrations, and pruritus symptoms in hemodialysis patients. The American journal of clinical nutrition 64: 210–4. [DOI] [PubMed] [Google Scholar]
- 17.Zulyniak MA, Perreault M, Gerling C, Spriet LL, Mutch DM (2013) Fish oil supplementation alters circulating eicosanoid concentrations in young healthy men. Metabolism: clinical and experimental 62: 1107–13. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J (2014) Green S. Cochrane handbook for systematic reviews of interventions Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration, 2011. Available: www.cochrane-handbook.org. [Google Scholar]
- 19.Follmann D, Elliott P, Suh I, Cutler J (1992) Variance imputation for overviews of clinical trials with continuous response. Journal of clinical epidemiology 45: 769–73. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. British medical journal 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorensen JD, Olsen SF, Pedersen AK, Boris J, Secher NJ, FitzGerald GA (1993) Effects of fish oil supplementation in the third trimester of pregnancy on prostacyclin and thromboxane production. American journal of obstetrics and gynecology 168: 915–22. [DOI] [PubMed] [Google Scholar]
- 22.Knapp HR, FitzGerald GA (1989) The antihypertensive effects of fish oil. A controlled study of polyunsaturated fatty acid supplements in essential hypertension. The New England journal of medicine 320: 1037–43. [DOI] [PubMed] [Google Scholar]
- 23.Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, et al. (1990) Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis and rheumatism 33: 810–20. [DOI] [PubMed] [Google Scholar]
- 24.Phang M, Lincz LF, Garg ML (2013) Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. The Journal of nutrition 143: 457–63. 10.3945/jn.112.171249 [DOI] [PubMed] [Google Scholar]
- 25.Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, et al. (2006) Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. The American journal of clinical nutrition 83: 331–42. [DOI] [PubMed] [Google Scholar]
- 26.Tartibian B, Maleki BH, Abbasi A (2011) Omega-3 fatty acids supplementation attenuates inflammatory markers after eccentric exercise in untrained men. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine 21: 131–7. [DOI] [PubMed] [Google Scholar]
- 27.Park Y, Lee A, Shim SC, Lee JH, Choe JY, Ahn H, et al. (2013) Effect of n-3 polyunsaturated fatty acid supplementation in patients with rheumatoid arthritis: a 16-week randomized, double-blind, placebo-controlled, parallel-design multicenter study in Korea. The Journal of nutritional biochemistry 24: 1367–72. 10.1016/j.jnutbio.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 28.Andrade PM, Ribeiro BG, Bozza MT, Costa Rosa LF, Tavares do Carmo MG (2007) Effects of the fish-oil supplementation on the immune and inflammatory responses in elite swimmers. Prostaglandins, leukotrienes, and essential fatty acids 77: 139–45. [DOI] [PubMed] [Google Scholar]
- 29.Véricel E, Calzada C, Chapuy P, Lagarde M (1999) The influence of low intake of n-3 fatty acids on platelets in elderly people. Atherosclerosis. pp. 187–92. [DOI] [PubMed] [Google Scholar]
- 30.Pirich C, Gaszo A, Granegger S, Sinzinger H.(1999) Effects of fish oil supplementation on platelet survival and ex vivo platelet function in hypercholesterolemic patients. Thrombosis research pp. 219–27. [DOI] [PubMed] [Google Scholar]
- 31.Singer P, Wirth M (2004) Can n-3 PUFA reduce cardiac arrhythmias? Results of a clinical trial. Prostaglandins, leukotrienes, and essential fatty acids 71: 153–9. [DOI] [PubMed] [Google Scholar]
- 32.Kremer JM, Jubiz W, Michalek A, Rynes RI, Bartholomew LE, Bigaouette J, et al. (1987) Fish-Oil Fatty Acid Supplementation i n Active Rheumatoid Arthritis. Annals of internal medicine 106: 497–503. [DOI] [PubMed] [Google Scholar]
- 33.Mori TA, Vandongen R, Mahanian F, Douglas A (1992) Plasma lipid levels and platelet and neutrophil function in patients with vascular disease following fish oil and olive oil supplementation. Metabolism: clinical and experimental 41: 1059–67. [DOI] [PubMed] [Google Scholar]
- 34.van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH (1990) Effects of fish oil supplementation in rheumatoid arthritis. Annals of the rheumatic diseases 49: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maaloe T, Schmidt EB, Svensson M, Aardestrup IV, Christensen JH (2011) The effect of n-3 polyunsaturated fatty acids on leukotriene B(4) and leukotriene B(5) production from stimulated neutrophil granulocytes in patients with chronic kidney disease. Prostaglandins, leukotrienes, and essential fatty acids 85: 37–41. 10.1016/j.plefa.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 36.Thusgaard M, Christensen JH, Morn B, Andersen TS, Vige R, Arildsen H, et al. (2009) Effect of fish oil (n-3 polyunsaturated fatty acids) on plasma lipids, lipoproteins and inflammatory markers in HIV-infected patients treated with antiretroviral therapy: a randomized, double-blind, placebo-controlled study. Scandinavian journal of infectious diseases 41: 760–6. 10.1080/00365540903168056 [DOI] [PubMed] [Google Scholar]
- 37.Turini ME, Powell WS, Behr SR, Holub BJ (1994) Effects of a fish-oil and vegetable-oil formula on aggregation and ethanolamine-containing lysophospholipid generation in activated human platelets and on leukotriene production in stimulated neutrophils. The American journal of clinical nutrition 60: 717–24. [DOI] [PubMed] [Google Scholar]
- 38.Goldman DW, Pickett WC, Goetzl EJ (1983) Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochemical and biophysical research communications 117: 282–8. [DOI] [PubMed] [Google Scholar]
- 39.Dawczynski C, Massey KA, Ness C, Kiehntopf M, Stepanow S, Platzer M, et al. (2013) Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clinical nutrition (Edinburgh, Scotland) 32: 686–96. [DOI] [PubMed] [Google Scholar]
- 40.Holman RT, Johnson SB, Bibus D, Spencer DC, Donadio JV (1994) Essential fatty acid deficiency profiles in idiopathic immunoglobulin A nephropathy. American journal of kidney diseases 23: 648–54. [DOI] [PubMed] [Google Scholar]
- 41.Mehta SP, Boddy AP, Cook J, Sams V, Lund EK, Johnson IT, et al. (2008) Effect of n-3 polyunsaturated fatty acids on Barrett's epithelium in the human lower esophagus; The American journal of clinical nutrition pp. 949–56. [DOI] [PubMed] [Google Scholar]
- 42.Hishinuma T, Suzukia K, Saitoa M, Yamaguchi H, Suzuki N, Tomioka Y, et al. (2007) Simultaneous quantification of seven prostanoids using liquid chromatography/tandem mass spectrometry: The effects of arachidonic acid on prostanoid production in mouse bone marrow-derived mast cells. Prostaglandins Leukotrienes and Essential Fatty Acids 76: 321–9. [DOI] [PubMed] [Google Scholar]
- 43.Scott W, Pawlowski N, Andreach M, Cohn Z (1982) Resting macrophages produce distinct metabolites from exogenous arachidonic acid. The Journal of experimental medicine 155: 535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boughton-Smith N, Hawkey C, Whittle B (1983) Biosynthesis of lipoxygenase and cyclo-oxygenase products from [14C]-arachidonic acid by human colonic mucosa. Gut 24: 1176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, et al. (2006) Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proceedings of the National Academy of Sciences of the United States of America 103: 11276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy KJ, Galvin K, Kiely M, Morrissey PA, Mann NJ, Sinclair AJ (2006) Low dose supplementation with two different marine oils does not reduce pro-inflammatory eicosanoids and cytokines in vivo. Asia Pacific journal of clinical nutrition 15: 418–24. [PubMed] [Google Scholar]
- 47.Pilkington SM, Rhodes LE, Al-Aasswad NMI, Massey KA, Nicolaou A (2014) Impact of EPA ingestion on COX- and LOX-mediated eicosanoid synthesis in skin with and without a pro-inflammatory UVR challenge—report of a randomised controlled study in humans. Molecular nutrition & food research 58: 580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY (2006) Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clinical chemistry 52: 2265–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.