Abstract
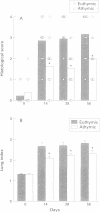
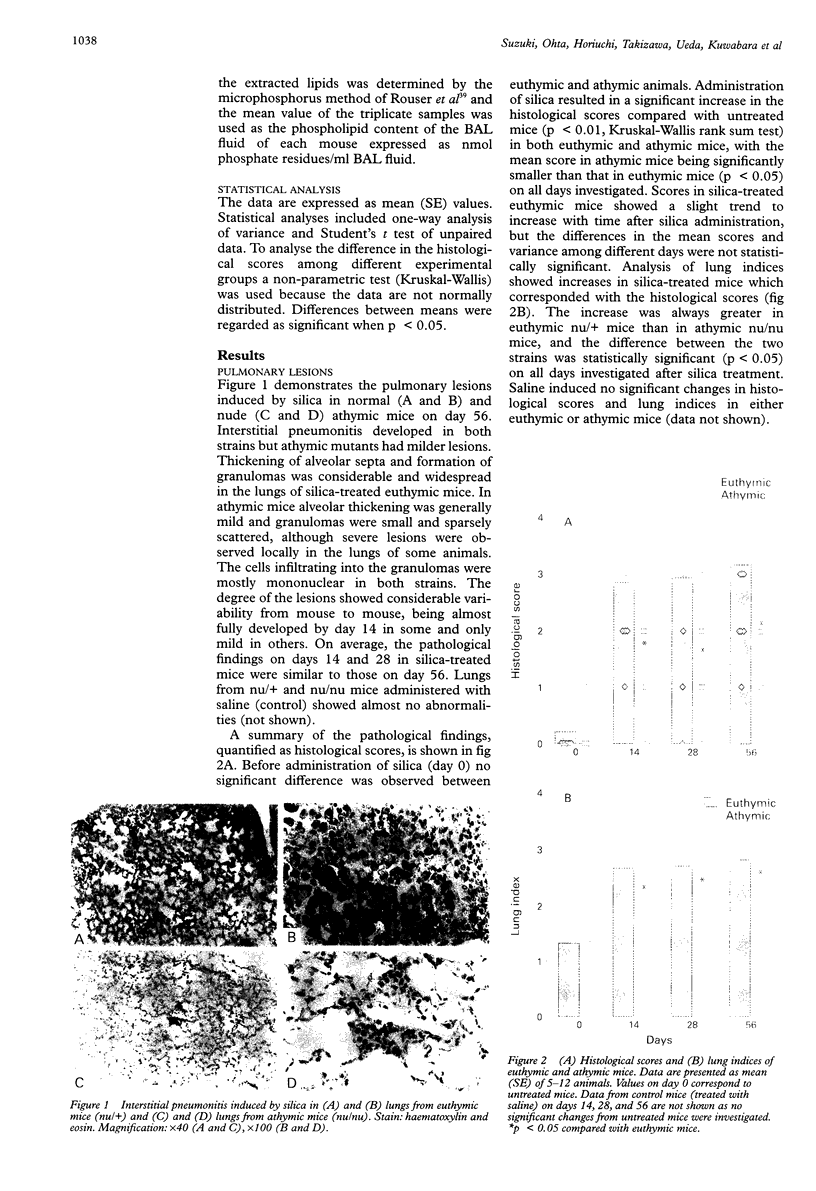
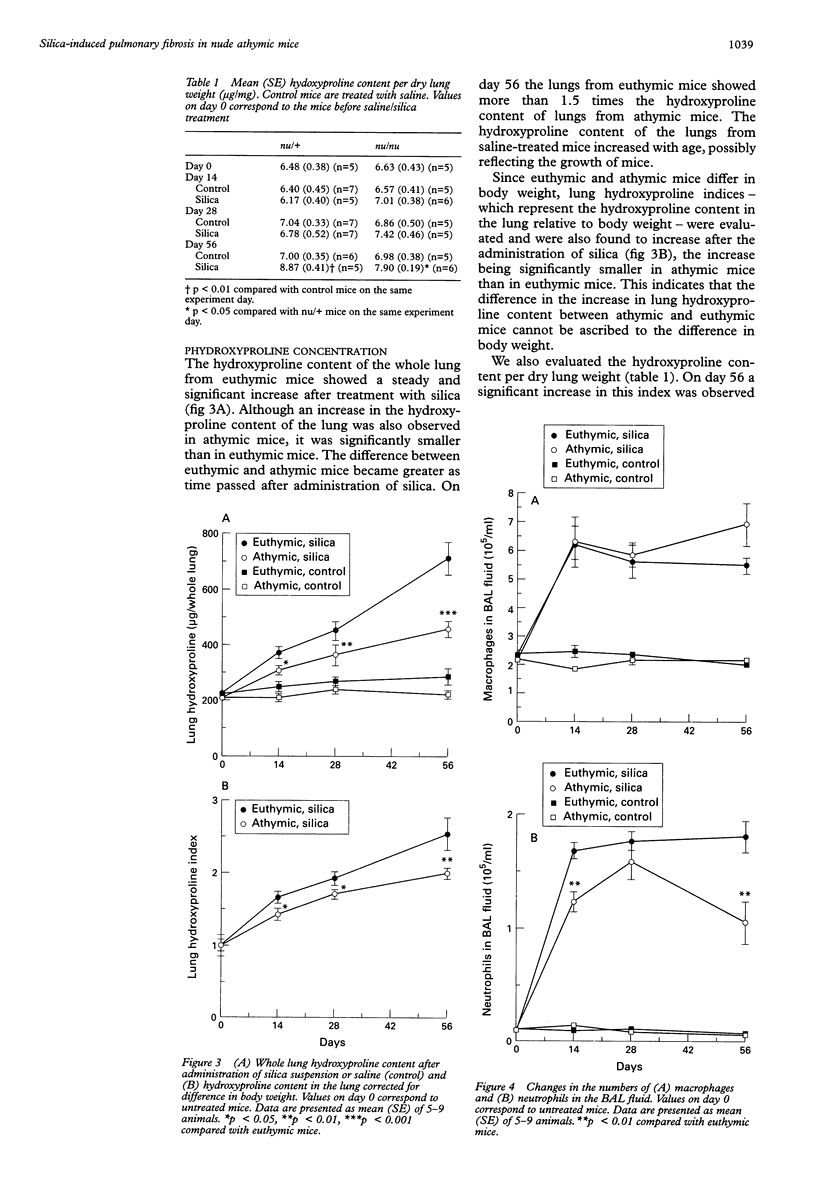
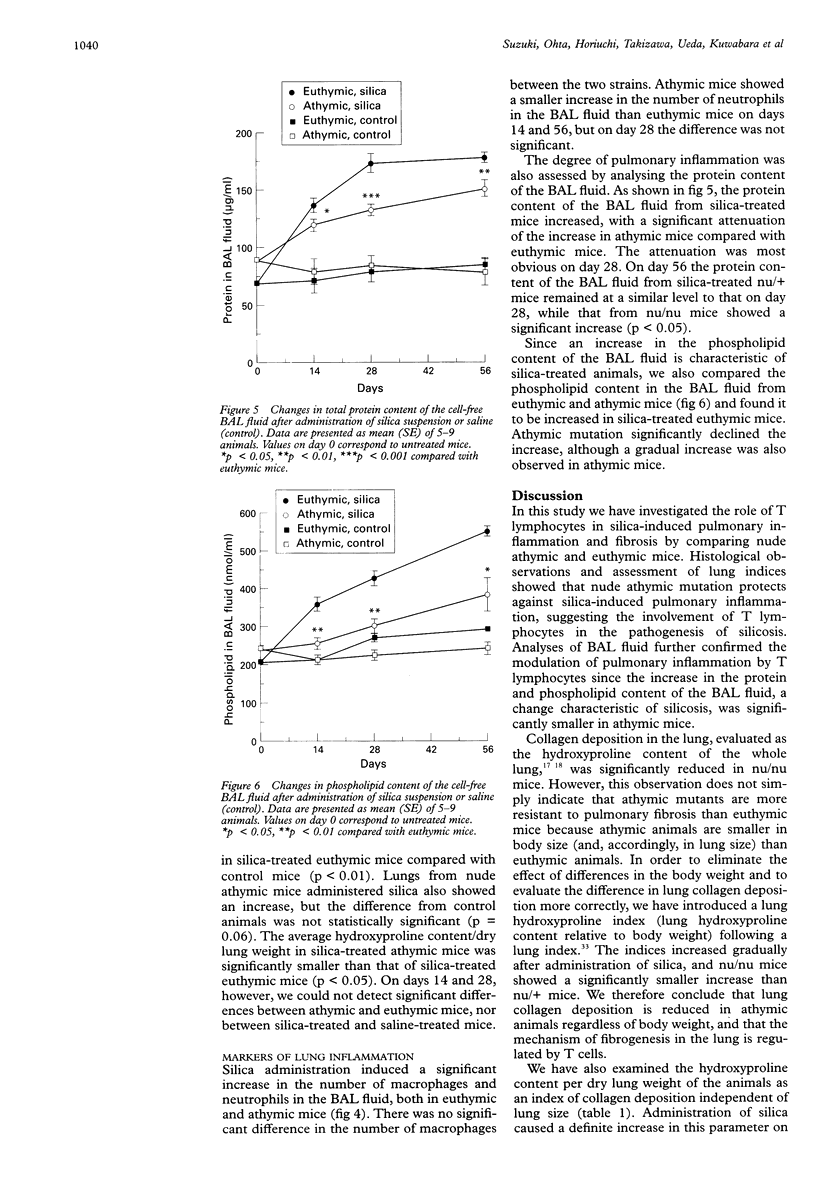
BACKGROUND: Silica-induced pulmonary inflammation and fibrosis in animals provides a good model for chronic pulmonary inflammation and fibrosis. Although lymphocytes are implicated in the pathogenesis of pulmonary fibrosis, experimental models using silica-treated athymic nude mice have not been successful in showing the fibrogenic mechanism regulated by T cells. The aim of this study was to re-evaluate the role of T lymphocytes in the development of silicosis by comparing the response to silica administration of nude athymic mutants with that of euthymic animals. METHODS: Suspensions of silica particles were transnasally administered to nude athymic mice (Balb/c nu/nu) as well as to their euthymic littermates (Balb/c nu/+). The degree of pulmonary inflammation and fibrosis was assessed on days 14, 28, and 56 based upon histological observation, analysis of collagen deposition in the lungs, and analysis of the cellular constituent, protein, and phospholipid content in the bronchoalveolar lavage fluid. RESULTS: Histologically, athymic mice developed less severe interstitial pneumonitis than euthymic mice. In euthymic mice the lung hydroxyproline content increased with time after silica administration from 6.48 (0.38) micrograms hydroxyproline/mg dry lung weight on day 0 to 8.87 (0.41) micrograms/mg on day 56. A gradual increase in lung hydroxyproline content was also observed in athymic mice but the increase was significantly smaller than in euthymic mice (6.63 (0.43) micrograms/mg on day 0, 7.90 (0.19) micrograms/mg on day 56). Administration of silica resulted in an increase in the number of macrophages and neutrophils and in the total protein and phospholipid content of the bronchoalveolar lavage (BAL) fluid in both mouse strains. No significant difference was detected between athymic and euthymic mice in the numbers of macrophages, but the increase in neutrophils in the BAL fluid of athymic mice was significantly smaller than in euthymic mice on days 14 and 56. The total protein and phospholipid content of the BAL fluid from athymic mice was lower than that from euthymic mice. CONCLUSIONS: T lymphocytes appear to be involved in the pathogenesis of silica-induced pneumonitis. Since pulmonary fibrosis develops even in nude athymic mice, T cells do not seem to play a primary part in fibrogenic response but they regulate, at least to some extent, the response of inflammatory cells and fibrogenesis of the lung.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Role of polymorphonuclear leukocytes in silica-induced pulmonary fibrosis. Am J Pathol. 1984 Oct;117(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis A. H., Sohnle P. G., Mandel G. S., Wiessner J., Mandel N. S. Kinetics of inflammatory and fibrotic pulmonary changes in a murine model of silicosis. J Lab Clin Med. 1985 May;105(5):547–553. [PubMed] [Google Scholar]
- Carré P. C., Mortenson R. L., King T. E., Jr, Noble P. W., Sable C. L., Riches D. W. Increased expression of the interleukin-8 gene by alveolar macrophages in idiopathic pulmonary fibrosis. A potential mechanism for the recruitment and activation of neutrophils in lung fibrosis. J Clin Invest. 1991 Dec;88(6):1802–1810. doi: 10.1172/JCI115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. S. Pathogenesis of silicosis: current concepts and hypotheses. Lung. 1986;164(3):139–154. doi: 10.1007/BF02713638. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekimoto H., Aikawa M., Ohnuki T., Takahashi K., Matsuda A., Takita T., Umezawa H. Immunological involvement in pulmonary fibrosis induced by peplomycin. J Antibiot (Tokyo) 1985 Jan;38(1):94–98. doi: 10.7164/antibiotics.38.94. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Mizel S. B., Farrar J. J. Participation of lymphocyte activating factor (Interleukin 1) in the induction of cytotoxic T cell responses. J Immunol. 1980 Mar;124(3):1371–1377. [PubMed] [Google Scholar]
- Heppleston A. G., Styles J. A. Activity of a macrophage factor in collagen formation by silica. Nature. 1967 Apr 29;214(5087):521–522. doi: 10.1038/214521a0. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Wright N. A., Stewart J. A. Experimental alveolar lipo-proteinosis following the inhalation of silica. J Pathol. 1970 Aug;101(4):293–307. doi: 10.1002/path.1711010402. [DOI] [PubMed] [Google Scholar]
- Hubbard A. K. Role for T lymphocytes in silica-induced pulmonary inflammation. Lab Invest. 1989 Jul;61(1):46–52. [PubMed] [Google Scholar]
- Hunninghake G. W., Glazier A. J., Monick M. M., Dinarello C. A. Interleukin-1 is a chemotactic factor for human T-lymphocytes. Am Rev Respir Dis. 1987 Jan;135(1):66–71. doi: 10.1164/arrd.1987.135.1.66. [DOI] [PubMed] [Google Scholar]
- Kimura R., Hu H., Stein-Streilein J. Delayed-type hypersensitivity responses regulate collagen deposition in the lung. Immunology. 1992 Dec;77(4):550–555. [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J., Kelley J. Lymphokine regulation of macrophage-derived growth factor secretion following pulmonary injury. Am J Pathol. 1985 Nov;121(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Kumar R. K., Li W., O'Grady R. Activation of lymphocytes in the pulmonary inflammatory response to silica. Immunol Invest. 1990 Aug;19(4):363–372. doi: 10.3109/08820139009050776. [DOI] [PubMed] [Google Scholar]
- Kumar R. K. Quantitative immunohistologic assessment of lymphocyte populations in the pulmonary inflammatory response to intratracheal silica. Am J Pathol. 1989 Oct;135(4):605–614. [PMC free article] [PubMed] [Google Scholar]
- Li W., Kumar R. K., O'Grady R., Velan G. M. Role of lymphocytes in silicosis: regulation of secretion of macrophage-derived mitogenic activity for fibroblasts. Int J Exp Pathol. 1992 Dec;73(6):793–800. [PMC free article] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Elias J. A., Bashey R. I., Jimenez S. A., Daniele R. P. The regulation of lung fibroblast proliferation by alveolar macrophages in experimental silicosis. Am Rev Respir Dis. 1984 May;129(5):767–771. doi: 10.1164/arrd.1984.129.5.767. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- O'Garra A. Interleukins and the immune system 2. Lancet. 1989 May 6;1(8645):1003–1005. doi: 10.1016/s0140-6736(89)92640-8. [DOI] [PubMed] [Google Scholar]
- Pernis B., Vigliani E. C. The role of macrophages and immunocytes in the pathogenesis of pulmonary diseases due to mineral dusts. Am J Ind Med. 1982;3(2):133–137. doi: 10.1002/ajim.4700030203. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Sappino A. P., Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990 Mar 15;344(6263):245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G. A., Szapiel S., Ferrans V. J., Crystal R. G. Susceptibility to experimental interstitial lung disease is modified by immune- and non-immune-related genes. Am Rev Respir Dis. 1987 Feb;135(2):448–455. doi: 10.1164/arrd.1987.135.2.448. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H., McGarry B. M. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am Rev Respir Dis. 1983 May;127(5):614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Seggev J. S., Goren M. B., Kirkpatrick C. H. The pathogenesis of trehalose dimycolate-induced interstitial pneumonitis. III. Evidence for a role for T lymphocytes. Cell Immunol. 1984 May;85(2):428–435. doi: 10.1016/0008-8749(84)90256-9. [DOI] [PubMed] [Google Scholar]
- Shaw R. J., Benedict S. H., Clark R. A., King T. E., Jr Pathogenesis of pulmonary fibrosis in interstitial lung disease. Alveolar macrophage PDGF(B) gene activation and up-regulation by interferon gamma. Am Rev Respir Dis. 1991 Jan;143(1):167–173. doi: 10.1164/ajrccm/143.1.167. [DOI] [PubMed] [Google Scholar]
- Snider G. L. Interstitial pulmonary fibrosis--which cell is the culprit? Am Rev Respir Dis. 1983 May;127(5):535–539. doi: 10.1164/arrd.1983.127.5.535. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Chensue S. W., Basha M. A., Standiford T. J., Lynch J. P., Baggiolini M., Kunkel S. L. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990 Apr;2(4):321–326. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- Struhar D., Harbeck R. J., Mason R. J. Lymphocyte populations in lung tissue, bronchoalveolar lavage fluid, and peripheral blood in rats at various times during the development of silicosis. Am Rev Respir Dis. 1989 Jan;139(1):28–32. doi: 10.1164/ajrccm/139.1.28. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Horiuchi T., Ohta K., Yamaguchi M., Ueda T., Takizawa H., Hirai K., Shiga J., Ito K., Miyamoto T. Mast cells are essential for the full development of silica-induced pulmonary inflammation: a study with mast cell-deficient mice. Am J Respir Cell Mol Biol. 1993 Nov;9(5):475–483. doi: 10.1165/ajrcmb/9.5.475. [DOI] [PubMed] [Google Scholar]
- Takizawa H., Ohta K., Hirai K., Misaki Y., Horiuchi T., Kobayashi N., Shiga J., Miyamoto T. Mast cells are important in the development of hypersensitivity pneumonitis. A study with mast-cell-deficient mice. J Immunol. 1989 Sep 15;143(6):1982–1988. [PubMed] [Google Scholar]
- Takizawa H., Ohta K., Horiuchi T., Suzuki N., Ueda T., Yamaguchi M., Yamashita N., Ishii A., Suko M., Okudaira H. Hypersensitivity pneumonitis in athymic nude mice. Additional evidence of T cell dependency. Am Rev Respir Dis. 1992 Aug;146(2):479–484. doi: 10.1164/ajrccm/146.2.479. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Gately C. L. Modulation of fibroblast growth by a lymphokine of human T cell continuous T cell line origin. J Immunol. 1983 Mar;130(3):1226–1230. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]
- Weissler J. C. Idiopathic pulmonary fibrosis: cellular and molecular pathogenesis. Am J Med Sci. 1989 Feb;297(2):91–104. doi: 10.1097/00000441-198902000-00005. [DOI] [PubMed] [Google Scholar]