Abstract
The link between cytomegalovirus (CMV) infection and inflammatory bowel diseases remains an important subject of debate. CMV infection is frequent in ulcerative colitis (UC) and has been shown to be potentially harmful. CMV reactivation needs to be diagnosed using methods that include in situ detection of viral markers by immunohistochemistry or by nucleic acid amplification techniques. Determination of the density of infection using quantitative tools (numbers of infected cells or copies of the genome) is particularly important. Although CMV reactivation can be considered as an innocent bystander in active flare-ups of refractory UC, an increasing number of studies suggest a deleterious role of CMV in this situation. The presence of colonic CMV infection is possibly linked to a decreased response to steroids and other immunosuppressive agents. Some treatments, notably steroids and cyclosporine A, have been shown to favor CMV reactivation, which seems not to be the case for therapies using anti-tumor necrosis factor drugs. According to these findings, in flare-ups of refractory UC, it is now recommended to look for the presence of CMV reactivation by using quantitative tools in colonic biopsies and to treat them with ganciclovir in cases of high viral load or severe disease.
Keywords: Human cytomegalovirus, Ulcerative colitis, Inflammatory bowel disease, Ganciclovir, Viral load, Flare-up, Inflammation, Intestinal mucosa, Quantitative polymerase chain reaction
Core tip: There is increasing evidence for the deleterious effect of in situ cytomegalovirus (CMV) reactivation in flare-ups of refractory ulcerative colitis. In patients aged > 30 years with a high density of infection in the colonic tissue, or with stigmata of severe disease associated with colonic markers of CMV reactivation (whatever the density of infection), treatment with ganciclovir is highly recommended, together with anti-tumor necrosis factor monoclonal antibody therapy in the absence of any contraindication to these drugs. For validating the present strategy based on our experience and the in-depth analysis of the available literature presented in this review, prospective randomized controlled studies are urgently needed.
INTRODUCTION
Cytomegalovirus (CMV) belongs to the Herpesviridae family. The viral genome consists of linear double-stranded DNA protected by a capsid and an envelope. After primary infection, which may or may not be symptomatic, the virus is known to maintain a persistent, life-long infection of the host, often as a latent form that can be found in several cell types. These cells are mainly myeloid progenitors, monocytes and endothelial cells, meaning that CMV could be latent in several organs or tissues, and especially in the colon[1,2]. During the latent stage, the CMV genome is present as an episomal circular form in the cell nucleus, with minimal viral expression and without viral particle production. CMV can reactivate from the latent stage, leading to the production of new viral particles. CMV reactivation is triggered by inflammation or immunosuppression. Beside reactivation from an endogenous latent virus, reinfection can be induced by an exogenous strain present in a tissue/organ graft or blood transfusion.
The host immune response is critical in controlling CMV infection. Cellular immunity, especially natural killer cells, and interferons play a major role at the stage of primary infection and in long-term control of the infection. Consequently, the clinical expression of CMV infection is generally absent in an immunocompetent host, even if some severe infections, especially colitis[1-5], have been reported in the literature. In contrast, the most preoccupant manifestations of CMV infection are observed in immunocompromised patients with altered cellular immunity, that is, after transplantation of solid organ grafts or hematopoietic stem cells, in cases of HIV infection, in patients undergoing chemotherapy or immunotherapy, and during pregnancy. Clinical manifestations may vary from acute febrile illness to organ disease (e.g., retinitis, pneumonitis, encephalitis, colitis and hepatitis)[6].
CMV infection is of particular interest in inflammatory bowel diseases (IBD) that combine inflammation in the colon and the long-term maintenance of immunosuppressive therapy; both of which can reactivate latent CMV[7]. Local inflammation in the bowel wall leads to the secretion of proinflammatory cytokines, including tumor necrosis factor (TNF)-α. As a consequence, these cytokines are able to activate CMV replication and the migration of CMV-infected monocytes and macrophages in the inflamed tissue to propagate infection further, generating a vicious cycle of pathology[2]. However, IBD is a complex entity that involves different clinical situations dominated by Crohn’s disease (CD) and ulcerative colitis (UC). In CD, severe CMV primary infections have been reported; some of them being complicated further by hemophagocytic lymphohistiocytosis[8]. The administration of ganciclovir was shown to contribute to clinical remission[9]. However, and although the seroprevalence to CMV is similar in CD and UC patients[10], CMV reactivation was shown to be much less frequent in CD than in UC patients, with no significant impact on clinical evolution[10-20]. This observation can be attributed to the different cytokine profiles observed in these two IBDs: CD is most likely attributed to T helper (Th)1 and Th17 CD4+ T-cell differentiation with secretion of interferon-γ that exerts an inhibiting effect on CMV replication. In contrast, UC exhibits a Th2 profile with limited secretion of antiviral cytokines, which could favor viral reactivation or tolerance[21]. Consequently, CD will be excluded from the scope of this review.
The use of various virological methods for diagnosing CMV reactivation affects the results obtained when exploring the role of this agent in UC and has led to controversial theories. Once the role of CMV is established in the evolution of UC, several predictive factors can be selected in order to identify those patients who are more likely at risk of developing CMV reactivation in the colon, and who may therefore benefit from antiviral therapy. Accordingly, the aim of this review is to answer four successive questions: (1) how to diagnose CMV reactivation accurately in colonic tissue of UC patients; (2) what is the impact of colonic CMV infection in the evolution of UC; (3) what are the predictive factors that may help to identify those patients at risk of unfavorable evolution; and (4) in this population, can antiviral therapy be of any use in improving the long-term evolution of UC?
HOW TO DIAGNOSE CMV REACTIVATION ACCURATELY IN COLONIC TISSUE OF UC PATIENTS
Figure 1 shows the different techniques that are presently used for the diagnosis of CMV infection. Only a few techniques are indicated for the current diagnosis of CMV reactivation in the colonic tissue of UC patients.
Figure 1.
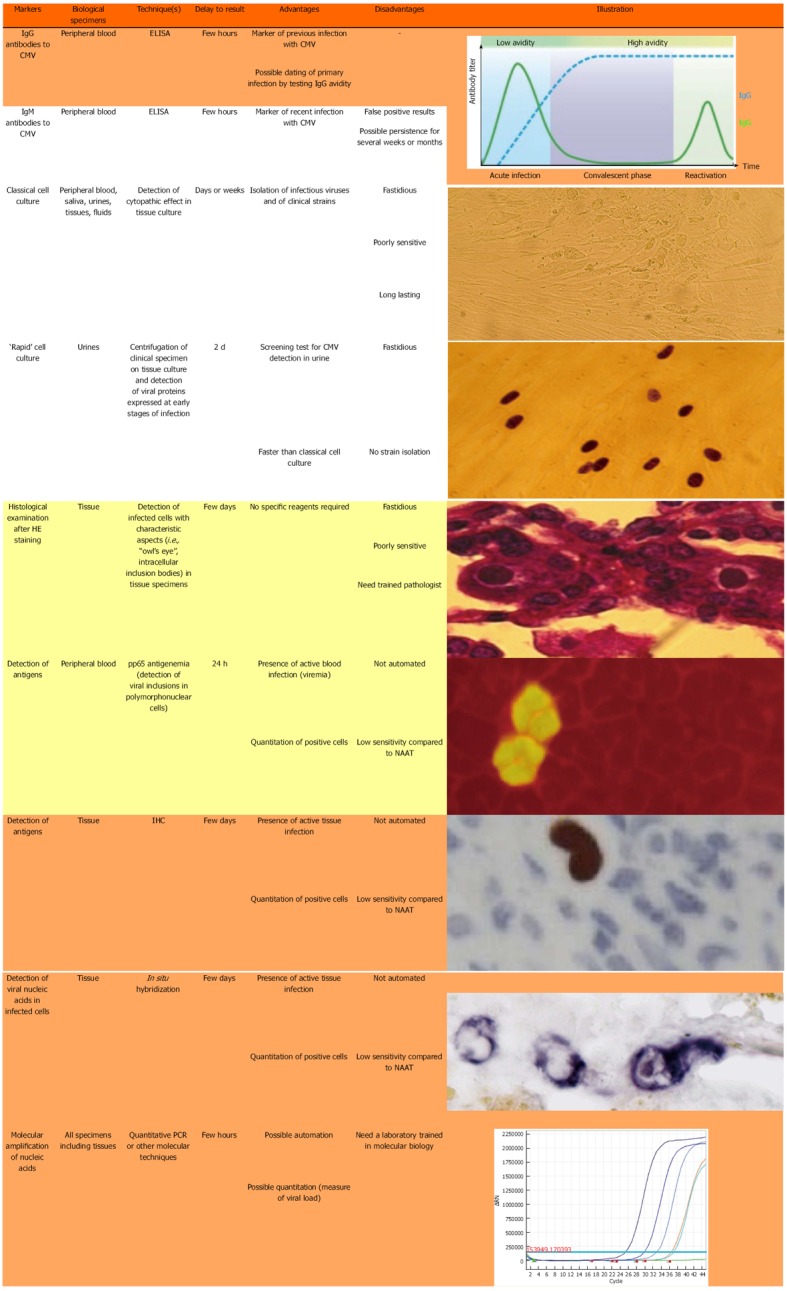
Techniques currently used for the detection of markers of cytomegalovirus infection. Analyses highlighted in orange are very useful, those highlighted in yellow of little use, and those without color of no use, for the diagnosis of CMV reactivation in inflammatory bowel diseases. ELISA: Enzyme-linked immunosorbent assay; IHC: Immunohistochemistry; CMV: Cytomegalovirus; HE staining: Hematoxylin and eosin staining; NAAT: Nucleic acid amplification test.
Specific IgG serology, usually performed by ELISA, is necessary to identify those patients who have already been in contact with CMV and consequently could be at risk of an endogenous reactivation at the colonic level. In seropositive patients, two kinds of techniques can identify CMV reactivation at this level.
The first group of techniques relies on histological examination of colonic tissue. The direct examination of colon biopsies after hematoxylin and eosin (HE) staining can show typical aspects of “owl’s eye” images (Figure 1) but this technique is poorly sensitive and frequently leads to false-negative results. Immunohistochemistry (IHC) on colonic tissue is much more sensitive and can be quantitative by numbering the infected cells[22-25]. However, a recent paper from McCurdy et al[26] indicated that a great number of biopsy samples must be examined in order to achieve adequate sensitivity.
The second group of techniques is based on the detection of CMV DNA in colonic tissue. In situ hybridization can be used for this purpose but, as for HE staining, this technique lacks sensitivity and can only identify severe CMV reactivation episodes. It has largely been replaced by molecular techniques based on nucleic acid amplification tests (NAATs). Although very sensitive, qualitative PCR with two rounds of amplification (nested PCR) should be avoided because of the risk of cross-contamination and false-positive results[27,28]. In contrast, real-time quantitative PCR (qPCR) assays are very sensitive, allowing the detection of low-level reactivation, and accurate determination of the viral load, and can be automated. In contrast with IHC, they give no information on the infectious potential of the detected genome, nor on the stage (latent or productive) of CMV infection. To optimize the predictive value of these tests, it is necessary to determine the thresholds of CMV DNA load that would require initiating antiviral therapy[6,29]. One of the main difficulties with NAATs is the inter-laboratory standardization of quantitative data[6,30,31], together with the harmonization of viral load expression in tissue specimens (copies[10,25,29,32-34] or international units[6], per mg of tissue[10,25], µg of DNA[14,34] or number of cells[29,32,33]). This lack of standardization makes the comparison of results between studies difficult and universally accepted cut-off values of CMV DNA load for assessing CMV disease have still to be defined[24,32,33]. Another important feature with NAATs is the risk of a false-negative result if the biopsy is performed at a distance from an inflamed focus; indeed, CMV markers are detected in inflamed tissue only[10,14,25,34] and inflammation[35-40] is present in the mucosa as foci that are sometimes difficult to identify during colonoscopy. To minimize this risk, it is our experience to measure CMV DNA on a couple of biopsies taken at the same time and to use the result exhibiting the highest viral load (manuscript in press). As detailed below, the presence of ulcers is correlated with that of viral stigmata[20,25,41,42], which indicates that these areas must be privileged in performing the biopsies. As an alternative to colonic biopsies, some authors have proposed the determination of viral load in feces[43-45]; however, this technique was recently shown to be poorly sensitive for the detection of CMV colitis in immunosuppressed patients[46].
IHC is still considered to be the gold standard for the identification of CMV in tissue sections[26,47,48]. However, the choice between IHC and NAAT (mainly qPCR) for detecting CMV reactivation in colon biopsy of UC patients is a matter of ongoing debate[25], even in current international recommendations[49], although an increasing number of laboratories are switching from histology-based techniques to qPCR assays for the quantification of CMV load in colonic tissue, due to the simplicity and rapidity of the latter tests. Indeed, with current NAATs, the results of viral load can be recovered within one working day. Due to the absence of any indication on the infectivity of a detected genome, the use of viral load thresholds avoids the useless treatment of latent infection.
WHAT IS THE IMPACT OF COLONIC CMV INFECTION ON THE EVOLUTION OF UC?
General considerations
The implication of CMV reactivation in colonic tissue on the clinical evolution of UC has been highly debated[22,27,50]. Table 1[10,12-17,20,22,41,42,51-85] lists, in chronological order, the main studies that have tried to explore this relationship. Some of them have reported CMV markers in patients without an impact on IBD evolution, which has led to the idea that CMV infection could be considered as an “innocent bystander”[27] or byproduct of the pathology. Many others have shown a negative impact of CMV infection in UC evolution and, in some of them, an improvement of clinical status when antiviral therapy was initiated, suggesting an active role of CMV.
Table 1.
Main studies recording the impact of cytomegalovirus on inflammatory bowel diseases course
| Studies by chronological order | No. of studied patients by type of IBD | Method used for CMV detection | Main results of the study | Impact of CMV |
| Vega et al[51], 1999 | 7 UC and 2 CD | Histology and IHC | Ganciclovir allowed clinical remission in 5/7 patients, with absence of CMV markers after antiviral therapy | Unfavorable |
| Cottone et al[52], 2001 | 55 UC and 7 CD | Histology and IHC | Antiviral treatment (3 with ganciclovir and 2 with foscarnet) allowed clinical remission in 5/7 patients | Unfavorable |
| PCR in PBMC | ||||
| Papadakis et al[53], 2001 | 5 UC, 3 CD, 2 indeterminate colitis; all medically refractory | Heterogeneous (serology, histology, IHC, ISH, PCR, cell culture) | Ganciclovir improved clinical outcome in 8/9 patients | Unfavorable |
| Wada et al[54], 2003 | 47 moderate to severe UC | pp65 antigenemia and IHC | Association of CMV infection with steroid resistance [13/16 (81.3%) vs 9/31 (29%), P = 0.001] and severe endoscopic score (P < 0.05); ganciclovir effective in 8/12 patients (66.7%) | Unfavorable |
| Criscuoli et al[55], 2004 | 38 UC and 4 CD with severe disease | pp65 antigenemia, qualitative PCR in leucocytes, histology and IHC | No clear association with steroid resistance, no need for antiviral therapy | None |
| Kambham et al[56], 2004 | 80 UC | IHC | CMV detected in 10 of 40 (25%) patients with refractory UC vs 1 of 40 (2.5%) patients with nonrefractory UC | Unfavorable |
| Kishore et al[57], 2004 | 61 UC and 2 CD | Serology (IgM), qualitative PCR in biopsy | CMV infection associated with poor outcome, with surgical treatment (4/10 vs 4/53, P < 0.05) and death (3/10 vs 0/53, P < 0.005) | Unfavorable |
| Alain et al[58], 2005 | 63 CD and 28 UC | Serology (IgM), viruria, pp65 antigenemia, detection of mRNA in blood, tissue cell culture of blood and tissue, histology and IHC | 8/14 patients with CMV infection experienced high dose steroid or azathioprine; ganciclovir improved 4/4 treated patients | Unfavorable |
| Maconi et al[59], 2005 | 77 UC with colectomy | Histology and IHC | Trend for an association between CMV reactivation and corticoresistance (15/55, 27.3% vs 2/22, 9.1%, P = 0.123) | Unfavorable |
| Dimitroulia et al[12], 2006 | 58 UC and 27 CD | PCR in blood and IHC | No association with disease severity | None |
| Kojima et al[60], 2006 | 126 UC with colectomy | Histology and IHC | CMV markers in surgical specimens more frequently detected in patients with severe or refractory disease | Unfavorable |
| Lavagna et al[61], 2006 | 24 refractory UC leading to colectomy | IHC and PCR in tissue | No pouchitis in CMV positive patients (compared to 3/21 of CMV negative ones) | None |
| Kuwabara et al[13], 2007 | 34 UC and 16 CD | IHC | CMV positive cell density associated with steroid resistance and colectomy rate | Unfavorable |
| Minami et al[62], 2007 | 23 severe UC | Heterogeneous (serology or histology or IHC or PCR in blood) | 18 out 23 patients receiving CyA exhibited CMV infection; 15/18 (83.3%) CMV positive required colectomy; colectomy could be avoided in the 3 remaining patients by administration of ganciclovir | Unfavorable |
| Matsuoka et al[63], 2007 | 69 moderate to severe UC | pp65 antigenemia and qPCR in plasma, histology | Low peripheral viral load observed in 25/48 patients; none exhibited CMV markers in tissue. No impact on clinical outcome and spontaneous clearance of CMV markers in blood without ganciclovir | None |
| Yoshino et al[14], 2007 | 30 UC refractory to immunosuppressive therapies | qPCR in tissue | Clinical remission after ganciclovir alone in 4/12 treated, the remaining 8 required additional anti-inflammatory treatment | Unfavorable |
| Domènech et al[64], 2008 | 114 active UC | pp65 antigenemia tissue: histology, IHC and detection of pp67 mRNA | Steroid and CyA treatment predisposes to CMV reactivation in colon (6/19); ganciclovir associated to remission in 3/6 patients; CMV markers detected in 2 surgical specimens | Unfavorable |
| Maher et al[65], 2009 | 49 UC and 23 CD with active disease | Serology, histology and IHC | CMV infection more frequent in steroid resistant patients (8/23, 34.8% vs 1/31, 3.2%) | Unfavorable |
| Kim et al[17], 2010 | 122 UC | IHC | CMV-positive patients required hospitalization (OR = 4.9; 95%CI: 1.2-19.0) and were hospitalized ≥ 7 d (OR = 5.0; 95%CI: 1.6-21.3) | Unfavorable |
| Lévêque et al[16], 2010 | 33 CD and 20 UC | qPCR in tissue | CMV infection more frequent after corticoid or azathioprine therapy; no relation with disease severity; no need of antiviral therapy | None |
| Omiya et al[42], 2010 | 20 UC | PCR in tissue | Absence of large ulcer in case of CMV infection | None |
| Suzuki et al[66], 2010 | 73 UC | pp65 antigenemia | Irregular ulceration associated to 100% of CMV infection | Unfavorable |
| Criscuoli et al[67], 2011 | 28 UC with CMV reactivation | Histology, IHC and nested PCR in tissue | Persistence of CMV markers in colon after acute colitis flare-up despite remission | None |
| Nguyen et al[22], 2011 | 26 UC and 17 CD | Histology and IHC | Higher colectomy rate in patients exhibiting high grade infection; decreased colectomy rate with ganciclovir use | Unfavorable |
| Roblin et al[10], 2011 | 42 moderate to severe UC | qPCR in tissue | The tissue CMV DNA load is predictive of resistance to immunosuppressive therapy; ganciclovir treatment cleared CMV DNA in tissue and improved outcome in 7/8 patients | Unfavorable |
| Al-Zafiri et al[20], 2012 | 13 CD and 18 UC with CMV reactivation | IHC | Colectomy rate higher (9/31, 29%) in CMV positive than in CMV negative (65/581, 11.2%) IBD patients | Unfavorable |
| Kim et al[68], 2012 | 72 moderate to severe UC treated with IV steroids | PCR in tissue | Association of CMV infection with steroid resistance; clinical improvement after ganciclovir (11/14) | Unfavorable |
| Yoshino et al[69], 2012 | 17 UC refractory to tacrolimus | qPCR in tissue | Colectomy-free time lower in CMV positive patients than in CMV-negative ones (35.7% at 17.7 mo vs 88.9% at 45.9 mo respectively, log-rank test P < 0.005) | Unfavorable |
| Fukuchi et al[70], 2013 | 51 active UC | IHC or qPCR in tissue | CMV DNA became negative after GMAA in patients with clinical remission | Unfavorable |
| IIda et al[71], 2013 | 187 active UC | pp65 antigenemia | CMV infection more frequent in steroid refractory patients (27/82, 32.9% vs 6/105, 5.7%) | Unfavorable |
| Kopylov et al[72], 2013 | 13 UC with CMV reactivation | IHC | The disease was more severe in the 7 patients requiring ganciclovir therapy, including 1 death and 3 colectomies | Unfavorable |
| Delvincourt et al[73], 2014 | 26 UC and 110 IBD hospitalized | qPCR in blood or tissue | No alteration of the course of IBD flare | None |
| Do Carmo et al[74], 2014 | 249 CD+151 UC | Qualitative PCR in stools | CMV infection is rare (only 9 patients) and is not associated with IBD disease activity | None |
| Inokuchi et al[75], 2014 | 118 UC | pp65 antigenemia | Delay to clinical remission higher in CMV positive patients (21 d vs 16 d, P < 0.01); ganciclovir decreased the rate of colectomy in multivariate analysis | Unfavorable |
| Kim et al[76], 2014 | 72 moderate to severe UC | Heterogeneous (serology or histology or IHC or PCR) | Cumulative colectomy (log rank, P = 0.025) and disease flare-up rates (log-rank, P = 0.048) higher in CMV positive patients | Unfavorable |
| Kim et al[77], 2014 | 229 moderate to severe UC | IHC and pp65 antigenemia | Association between positive pp65 antigenemia and rate of colectomy (13/39, 33.3% vs 5/44, 11.4%, P < 0.05) | Unfavorable |
| Maconi et al[78], 2014 | 30 UC and 8 CD with active colitis and CMV infection | Histology/IHC | Antiviral therapy associated with a higher clinical remission rate at 12 mo (77.8% vs 45%, P < 0.05, and 77.8% vs 19.4%, P < 0.05) in UC patients and patients with steroid-dependent/refractory disease, respectively | Unfavorable |
| Matsumoto et al[79], 2014 | 222 UC | Antigenemia, histology, PCR | CMV infection as a risk factor for hospitalization because of UC aggravation (OR = 8.2, 95%CI: 1.91-35.33, P < 0.005) | Unfavorable |
| Olaisen et al[80], 2014 | 77 patients undergoing colectomy | IHC | CMV positive patients received higher doses of corticoids and were at higher risk of postoperative complications | Unfavorable |
| Yamada et al[81], 2014 | 33 refractory UC | qPCR in tissue | Induction remission rate by infliximab lower (54.5%) in CMV-positive patients than in CMV-negative ones (81.8%) although not statistically significant | Unfavorable |
| Chun et al[82], 2015 | 43 moderate to severe UC | pp65 antigenemia | Positive antigenemia associated with steroid refractoriness (11/12, 91.7% vs 12/31, 38.7%, P < 0.005); ganciclovir improved outcome: colectomy in 2/8 (25%) vs 2/4 (50%) | Unfavorable |
| Ciccocioppo et al[32], 2015 | 24 UC and 16 CD | qPCR in tissue | In refractory patients, more frequent CMV infection and higher viral load; efficacy of ganciclovir in all refractory patients | Unfavorable |
| Jones et al[83], 2015 | 1111 IBD patients | Histology, IHC, ISH | Antiviral therapy improved surgery-free survival outcome | Unfavorable |
| Gauss et al[84], 2015 | 166 UC and 131 CD | IHC and PCR in tissue | CMV reactivation associated to longer hospital stay (P < 0.001) | Unfavorable |
| McCurdy et al[41], 2015 | 45 UC, 21 CD and 2 indeterminate IBD colitis | Histology, ISH, IHC | CMV reactivation associated to medically refractory disease (OR = 3.69, P < 0.001) and endoscopic ulcers (OR = 2.95, P < 0.001) | Unfavorable |
| Minami et al[85], 2015 | 29 severe UC treated either with tacrolimus or infliximab | qPCR in tissue | Colectomy rate higher in patients with CMV infection (5/6, 83.3% vs 8/23, 34.8%, P < 0.05) | Unfavorable |
GMAA: Granulocyte/monocyte adsorptive apheresis; IHC: Immunohistochemistry; ISH: In situ hybridization; NAAT: Nucleic acid amplification test; PBMC: Peripheral blood monocular cells; PCR: Polymerase chain reaction; qPCR: Quantitative real-time PCR; IBD: Inflammatory bowel diseases; CD: Crohn’s disease; UC: Ulcerative colitis; CMV: Cytomegalovirus.
In our opinion, many of these discrepancies are related to misleading definitions of the populations of patients included in the studies or to the use of inadequate tools for the evaluation CMV reactivation in the gut. First, patients with CMV primary infection exhibiting CMV colitis are sometimes mixed with patients with CMV reactivation, notably in historic studies, which introduced a bias in the evaluation of prognosis[53,58,86-90]. Second, several studies, including some recent ones[41,74,78,84], have evaluated CMV markers in both UC and CD patients, although these two IBDs are very different in terms of the risks of CMV reactivation, as discussed above. Third, a few studies used peripheral blood markers, and notably pp65 antigenemia (Figure 1), to evaluate CMV reactivation in UC patients; positive antigenemia was associated with steroid refractoriness and UC exacerbation in one study[71], corticoresistance in another[5], and the presence of ulcer and risk of colectomy in a third[76]. However, viremia is poorly sensitive[10,14,19,64,77,78,82]; no threshold has been established for starting therapy; and the search for CMV should be performed in colonic biopsy in order to evaluate the risk of reactivation at an early stage of infection, corresponding to an increased chance of successful antiviral treatment. Finally, as stated in the previous section, the comparison of clinical results between studies is rendered difficult by the diversity of techniques that are used to determine CMV reactivation at the colonic level (IHC vs NAATs) and the lack of standardization of the different tests used for quantifying the viral load.
Despite these discrepancies, there is an increasing consensus for considering CMV reactivation as a marker of poor prognosis in UC patients, as illustrated by the results of the studies listed in Table 1 and by the recommendations of international guidelines[47,49,91] for the systematic detection of CMV reactivation in flare-ups of UC patients, and in using antiviral drugs in particular circumstances that will be detailed later in this review.
Factors implicated in the occurrence of CMV reactivation in UC patients
Role of immunotherapy: Administration of steroids is a known predisposing factor for CMV reactivation by suppressing anti-CMV T-cell specific function[92] and by directly activating viral replication[93,94]. Indeed, many studies have documented this risk in UC patients[14,17,32,52,59,80]. It has been shown that administration of steroids over a period of at least 3 mo at a dose of at least 10 mg is associated with a risk of CMV reactivation, without any effect of cumulative doses[52]. The prevalence of CMV reactivation increased with the exposition of high-dose steroid therapy for 7-14 d[17].
With regards to immunomodulatory therapy other than steroids, cyclosporine (CyA) is also associated with the risk of active CMV infection[62,64,83]. In a study including 23 patients with severe UC undergoing CyA treatment, 18 of them developed CMV infection, as illustrated by the presence of IgM antibody, CMV DNA or inclusion bodies by histology after approximately 8 d of treatment[62]. In a prospective study, CMV infection was observed in five of six UC patients after 7-10 d CyA treatment[64]. Consequently, the risk of CMV infection should be carefully monitored when this drug is used as an alternative to other contraindicated immunomodulatory agents. In contrast, the use of azathioprine or anti-TNF monoclonal antibodies (mAbs) was not associated with an increased risk of CMV reactivation[10,41,52,64,95-99]. We recently reported 109 consecutive flares-up of UC in patients undergoing anti-TNF maintenance therapy; these patients were not at a higher risk of CMV reactivation and, reciprocally, the occurrence of CMV reactivation had no effect on the further evolution of UC. These results plead for the preferential use of these molecules in cases of refractory flare-up associated with CMV reactivation[100]. However, in a recent study combining CD and UC patients, the use of immunomodulators, including thiopurines or methotrexate, was significantly associated with occurrence of CMV disease[41]. Tacrolimus was recently proposed as an alternative to previous treatments, especially in cases of refractory flare-up[85,98]; further studies are needed to appreciate the risk of developing CMV reactivation in this context[101].
Age > 30 years: Two recent studies have documented the risk of CMV reactivation in IBD patients older than 30 years. In a retrospective case-control study performed on 68 IBD patients (66% with UC) exhibiting CMV infection by tissue analysis, who were each matched to three controls without stigmata of CMV infection, McCurdy et al[41] showed that CMV disease was significantly associated with age > 30 years. No stratification was performed by type of IBD (CD or UC). In another retrospective study, Gauss et al[84] recorded positive CMV markers in 21 IBD patients - 18 with CMV DNA in colonic biopsy and three with positive blood antigenemia (the PCR assay was not done) - out of 100 patients, and most of them (17/21) exhibited UC. The presence of CMV markers was significantly associated with age ≥ 30 years (OR = 14.26; 95%CI: 2.89-118.57). Despite the high significance of these data, they relied only on two studies with a low number of patients, which implies that further trials are required to consolidate these observations.
Other predictive factors of CMV infection in IBD patients: The two retrospective studies mentioned in the above paragraph also documented other predictive factors of CMV infection in IBD patients. In addition to age > 30 years, McCurdy et al[41] identified four additional risk factors: medically refractory IBD; the presence of ulcers at endoscopy; treatment with corticosteroids; and treatment with immunomodulators (with the exception of anti-TNF mAb). After adjustment in a multivariate model, refractory disease, treatment with immunomodulators and age > 30 years remained independently associated with CMV infection. The authors propose a CMV risk score based on these criteria for the prediction of CMV infection in IBD patients. Furthermore, in addition to age > 30 years, the case-control study of Gauss et al[84] identified a blood leukocyte count < 11000/mL, disease duration at admission < 60 mo, and the presence of immunosuppressive therapy at admission as significant predictors of CMV infection in IBD patients. As no stratification was done by type of IBD in these two retrospective studies, it would be interesting to re-evaluate specifically these predictors in UC patients who are most at risk of CMV infection among IBD patients.
WHAT ARE THE PREDICTIVE FACTORS OF UNFAVORABLE EVOLUTION IN UC PATIENTS WITH CMV INFECTION?
Resistance to steroids and other immunosuppressive agents
CMV reactivation was recorded as one of the most important risk factors for steroid-refractory UC. A retrospective study that investigated CMV infection by IHC in 77 surgical specimens reported a rate of CMV infection of 27.3% in samples from steroid-refractory UC patients compared to 9.1% in those from steroid-sensitive ones[102]. In the prospective study that we conducted on 42 consecutive patients hospitalized for moderate to severe UC and treated with IV steroids, the only factor associated by multivariate analysis with CMV DNA in inflammatory tissue was resistance to steroids (OR = 4.7; 95%CI: 1.2-22.5)[10]. Two other prospective studies reported the same association between resistance to steroids and CMV reactivation[52,64]. Recent studies[71,78] including two multivariate analyses[41,84] confirmed the link between CMV reactivation and steroid resistance. In a meta-analysis published last year and summarizing 11 studies involving 867 IBD patients, the relative risk for steroid resistance was significantly higher in CMV-positive patients (OR = 2.07; 95%CI: 1.80-2.39)[103].
As shown in our work on flare-ups of refractory UC, CMV reactivation affects the response to immunosuppressive therapy, including anti-TNF mAbs[10]. In a similar context, Yamada et al[81] showed that the induction remission rate by infliximab was lower (54.5%) in CMV-positive than in CMV-negative patients (81.8%), although the difference was not statistically significant.
Acute severe colitis and requirement of colectomy
Since the first description of CMV markers in surgical specimens[104], a higher rate of colectomy has been observed in cases of CMV reactivation vs CMV-negative groups[20,69,76,85]. In the prospective study published by Domènech et al[64], colectomy was performed in 3/6 patients exhibiting CMV reactivation compared to 2/12 patients without markers of CMV infection. The prevalence of CMV markers detected using IHC in surgical specimens was also shown to be higher in severe UC than in refractory UC (25% vs 8.3% and 25% vs 2.5%[56,60], respectively). In a recent report, Yoshino et al[69] showed that the colectomy-free time was higher in patients without CMV colitis. Finally, Matsumoto and Yoshida reported recently that CMV infection and steroid use were independent risk factors for hospitalization because of UC aggravation and the need for surgery[79]. By retrospective analysis of a surgery database including 1100 patients, Uchino et al[105] recorded seven cases exhibiting UC-related lesions in the stomach and small intestine after colectomy; six of seven exhibited CMV infection either with positive antigenemia or CMV markers in tissue (IHC or PCR). These severe CMV infections were all refractory to ganciclovir treatment.
Presence of ulcers with endoscopic examination
Several studies have argued for a link between the presence of ulcers after endoscopic examination, CMV reactivation and unfavorable evolution. In a study of UC patients hospitalized due to exacerbation of symptoms, colonoscopic findings were compared between 15 CMV-positive and 58 CMV-negative patients, as determined by blood antigenemia: more abnormalities (irregular ulceration, wide mucosal defect) were observed in patients with UC complicated by CMV infection[66]. More recently, the retrospective study mentioned previously[41] reported a trend towards severe endoscopic disease in CMV-infected IBD patients (OR = 1.67; 95%CI: 0.85-3.32). In the subgroup of UC patients, the presence of endoscopic ulcers was significantly associated with CMV disease (OR = 3.0; 95%CI: 1.38-6.51). In another study, the absence of large ulcers was predictive of non-active CMV infection in UC patients positive for the presence of colonic CMV DNA: the 10 patients exhibiting this profile attained remission without antiviral therapy at 2 mo and maintained remission[42]. However, other studies, including ours[10,54], did not identify stigmata of tissue injury as a marker of CMV infection. It may depend upon the severity of UC in the studied populations that may have been lower in the latter studies.
Density of viral infection
Using either molecular or histological assays to evaluate the density of viral infection, this quantitative or semi-quantitative marker was shown to be related to the severity of colonic lesions in UC patients. Using histopathology, Nguyen et al[22] distinguished low-grade CMV infection (when IHC was positive only) from high-grade infection (detected by HE staining): colectomy rates were 29% and 83%, respectively, in untreated patients. Jones et al[83] defined high-grade CMV density by the presence of more than four typical inclusions in biopsy specimens. Similarly, Kuwabara et al[13] proposed that dense CMV disease, defined as > 10 inclusions per histological section, was shown to be predictive of significantly higher final daily doses of steroids before surgery, and showed increased steroid resistance. In addition, the frequency of emergency surgery was higher and postoperative hospital stay was significantly longer in the dense CMV group.
By using qPCR in colon biopsies, we performed a random sensitivity analysis for correlating the presence of CMV in tissue with the occurrence of resistance to the successive lines of treatment[10]. A positive colonic CMV load was associated with an increased risk of steroid resistance [likelihood ratio (LR+) of 3.0], with a sensitivity of 50% and a specificity of 100% [area under the receiver operating characteristic curve (AUROC) = 0.54, P < 0.05]. A viral load of > 250 copies/mg of tissue was predictive of resistance to three successive lines of treatment with a sensitivity of 100% and specificity of 66.6% (LR+ 4.33; AUROC = 0.85, P < 0.05). In contrast, the absence of CMV DNA in tissue was predictive of a favorable response to any treatment with a sensitivity of 100% and specificity of 50% (LR+ 2.21; AUROC = 0.65, P < 0.05).
WHAT IS THE BENEFIT OF ANTI-CMV THERAPY ON THE EVOLUTION OF UC IN PATIENTS WITH CMV REACTIVATION?
Systematic review of literature
Regarding the management of CMV infection in UC patients, the guidelines of the European Crohn’s and Colitis Organization in 2014 are as follows: “Screening for CMV infection is not necessary before starting immunomodulator therapy. In patients with acute steroid-resistant colitis, CMV should be excluded, preferably by tissue PCR or immunohistochemistry, before increasing immunomodulator therapy. In case of severe steroid-resistant colitis with CMV detected in the mucosa during immunomodulator therapy, antiviral therapy should be initiated and discontinuation of immunomodulators considered until colitis symptoms improve. In case of systemic CMV disease, immunomodulator therapy must be discontinued”[49]. However, randomized controlled trials would be useful in reinforcing the level of evidence supporting these guidelines.
If most of the gastroenterology societies recommend antiviral treatment of severe flare-ups of UC exhibiting CMV markers in inflamed tissue, no recommendations are given on which antiviral drug should be used and for what duration. No study has compared ganciclovir and foscarnet in this indication and no data are available on the pharmacokinetics of antiviral drugs in colonic tissue; notably, regarding the difference between ganciclovir and valganciclovir and the role of possible malabsorption in inflamed tissue. In contrast to transplant recipients[106], the overall incidence of CMV resistance to ganciclovir in IBD has never been analyzed. In this context, most authors use ganciclovir to treat CMV reactivation in UC patients (reviewed in Shukla et al[48]). In our clinical practice, we use empirically IV ganciclovir for 1 wk followed by oral valganciclovir for 2 wk but the relevance of this strategy has not been evaluated.
A lot of case reports, as well as punctual prospective studies, have reported a clinical improvement associated with a reduction of colectomy rate when UC patients with CMV reactivation received ganciclovir (or exceptionally, foscarnet). In a previous review paper[50], we collected seven prospective studies[14,34,51,64,67,68,95] that analyzed the efficacy of treatment of CMV reactivation by ganciclovir in UC patients: from a total of 58 treated patients, 46 presented a clinical improvement and 11 justified colectomy (18%).
Several studies analyzed the benefit of ganciclovir on colectomy rate according to the density of CMV infection. In the study of Nguyen et al[22], the antiviral treatment did not change colectomy rate for the patients with low-grade CMV infection (31% vs 29% without CMV treatment) but it significantly decreased the colectomy rate for those with high-grade CMV infection (44% vs 83% without CMV treatment). Similarly, Jones et al[83] argued that antiviral treatment significantly reduced the risk of surgery (OR = 0.31; 95%CI: 0.14-0.70); patients with high-grade infection showed a significant benefit of antiviral therapy, whereas those with low-grade infection presented higher rates of colectomy. In a study performed in our hospital[10], eight patients with a high CMV DNA load in the colon, and who had failed to respond to at least two lines of treatment, were treated with ganciclovir for 15 d in addition to their ongoing immunosuppressive therapy. For seven of them, clinical remission was obtained with a sustained response to the last therapeutic line after a follow-up of 6 mo, which resulted in a step-down therapeutic strategy for all of them[10].
Recently, a meta-analysis was performed to determine the impact of antiviral therapy on the colectomy rate in UC patients presenting with CMV infection[48]. Fifteen studies were included in this meta-analysis with a total of 333 patients; 43.2% were treated with antiviral therapy and 56.8% were not. The diagnosis was made primarily by HE and/or IHC in seven studies and by tissue PCR in four. No difference was noticed in terms of colectomy between patients treated with antiviral therapy and those without treatment (OR = 0.92; 95%CI: 0.31-2.76), with moderate heterogeneity (I2 = 65%). There was no significant difference in the risk of colectomy based on the method of CMV diagnosis. Next, the authors analyzed the risk of colectomy in those patients with corticosteroid-refractory UC related to CMV reactivation; eight studies were available concerning 139 patients, 77 of whom received antiviral therapy. The risk of colectomy was significantly lower in patients with corticosteroid-refractory UC treated with antiviral therapy than in patients not treated with antiviral therapy (OR = 0.20; 95%CI: 0.08-0.49), with no heterogeneity (I2 = 0). When the analysis was limited to studies that defined refractory disease as failure to respond to 1 wk of intravenous corticosteroids, the benefit of antiviral therapy remained significant (OR = 0.23; 95%CI: 0.06-0.82). Finally, when the analysis was further stratified on the method of CMV diagnosis, the risk of colectomy remained significantly lower only when CMV infection was based on histological criteria (3 studies; OR = 0.06; 95%CI: 0.01-0.34) but not on tissue PCR (4 studies; OR = 0.31; 95%CI: 0.09-1.11). The latter observation may be related to the fact that the analysis was not adequately powered and that three of the four studies based on tissue PCR reported only qualitative results.
Place of granulocyte/monocyte adsorptive apheresis in the treatment of CMV-related flare-ups of UC
Granulocyte/monocyte adsorptive apheresis (GMAA) is a biological therapy comprising removal of granulocytes/macrophages producing inflammatory cytokines. This strategy was evaluated in a randomized, double-blind, sham-controlled study for the treatment of UC flare-ups. The treatment was well tolerated but did not demonstrate efficacy for induction of clinical remission or response in patients with moderate-to-severe flare-ups[107]. More recently, Japanese studies have investigated the efficacy of GMAA in active UC flare-ups associated or not with colonic CMV reactivation. In a retrospective study, 11 UC patients in clinical failure under steroid and immunomodulatory therapy were treated with additional GMAA: nine achieved remission and two underwent colectomy[108]. Fukuchi et al[70] tested this strategy in 51 active UC flare-up episodes, and 15 of them were associated with in situ CMV infection. In the absence of steroid treatment, the clinical remission rate did not differ between UC patients, whether positive and negative for CMV (73.3% vs 69.4%). CMV DNA became negative in all UC patients positive for CMV who achieved clinical remission 1 wk after completion of intensive GMAA but no data on long-term evolution were reported. Presently GMAA is not recommended in the treatment of UC flare-ups by American and European guidelines. Additional studies are needed to evaluate the benefit of GMAA in UC patients with flare-ups associated with CMV reactivation.
Discussion of therapeutic algorithms
At least three therapeutic algorithms have been proposed for the intake of refractory flare-ups of UC according to the presence of CMV reactivation in the gut[48,50,109]. These algorithms are all similar but do not take into consideration the risk factors listed above together with the density of CMV infection[83] and the absence of reciprocal deleterious effects between anti-TNF mAbs and CMV reactivation[100]. The therapeutic algorithm that we propose in Figure 2 integrates these relatively new concepts. Of note, as recommended by the European guidelines[49], the antiviral therapy must be initiated after discontinuation of immunomodulators that will be reintroduced at the end of the flare-up.
Figure 2.
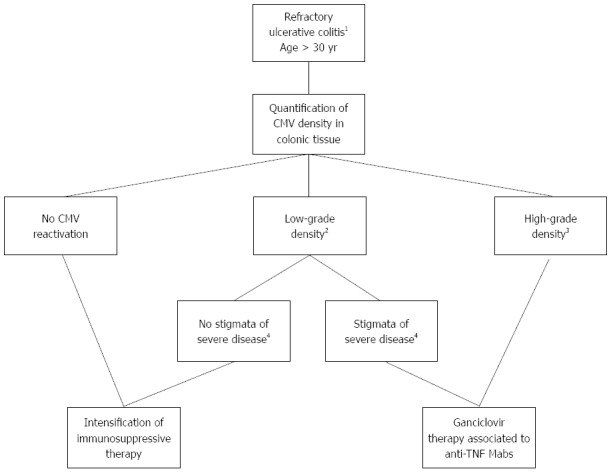
Therapeutic algorithm for the intake of flare-ups of refractory ulcerative colitis in patients aged > 30 years according to the quantification of cytomegalovirus density in colonic tissue. 1Defined by steroid resistance or immunosuppressive treatment or anti-TNF drugs; 2Defined by quantification of CMV DNA in intestinal tissue of 10-250 copies/mg of inflamed tissue or low-grade CMV density by IHC in biopsy specimens (4 inclusions or less); 3Defined by quantification of CMV DNA in intestinal tissue of > 250 copies/mg of inflamed tissue or high-grade CMV density by IHC in biopsy specimens (more than 4 inclusions); 4Defined by a need for hospitalization and a Lichtiger score > 10. CMV: Cytomegalovirus; IHC: immunohistochemistry; TNF: Tumor necrosis factor.
CONCLUSION
Despite conflicting results, there is increasing evidence, notably in recent studies, for the deleterious effect of in situ CMV reactivation in flare-ups of refractory UC. In patients aged > 30 years with a high density of infection in the colonic tissue or with stigmata of severe disease associated with colonic markers of CMV reactivation (whatever the density of infection), treatment with ganciclovir appears to be recommended with anti-TNF mAb therapy in the absence of explicit contraindications to these drugs. In order to validate the present strategy based on our experience and the in-depth analysis of the available literature presented in this review, prospective randomized controlled studies are urgently needed.
ACKNOWLEDGEMENTS
The authors acknowledge Philip Lawrence for his careful revision of the English style of the manuscript. They are indebted to the five reviewers for their constructive remarks that helped to improve considerably the quality of the manuscript.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 10, 2015
First decision: October 14, 2015
Article in press: December 19, 2015
P- Reviewer: Annese V, Caviglia RD, Goenka MK, Maltz C, Miheller P S- Editor: Yu J L- Editor: Kerr C E- Editor: Ma S
References
- 1.You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14:334–342. doi: 10.1007/s11894-012-0266-4. [DOI] [PubMed] [Google Scholar]
- 2.Goodman AL, Murray CD, Watkins J, Griffiths PD, Webster DP. CMV in the gut: a critical review of CMV detection in the immunocompetent host with colitis. Eur J Clin Microbiol Infect Dis. 2015;34:13–18. doi: 10.1007/s10096-014-2212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galiatsatos P, Shrier I, Lamoureux E, Szilagyi A. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig Dis Sci. 2005;50:609–616. doi: 10.1007/s10620-005-2544-6. [DOI] [PubMed] [Google Scholar]
- 4.Seo TH, Kim JH, Ko SY, Hong SN, Lee SY, Sung IK, Park HS, Shim CS, Han HS. Cytomegalovirus colitis in immunocompetent patients: a clinical and endoscopic study. Hepatogastroenterology. 2012;59:2137–2141. doi: 10.5754/hge10825. [DOI] [PubMed] [Google Scholar]
- 5.Ko JH, Peck KR, Lee WJ, Lee JY, Cho SY, Ha YE, Kang CI, Chung DR, Kim YH, Lee NY, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60:e20–e26. doi: 10.1093/cid/ciu969. [DOI] [PubMed] [Google Scholar]
- 6.Pillet S, Roblin X, Cornillon J, Mariat C, Pozzetto B. Quantification of cytomegalovirus viral load. Expert Rev Anti Infect Ther. 2014;12:193–210. doi: 10.1586/14787210.2014.870887. [DOI] [PubMed] [Google Scholar]
- 7.Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–2865. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 8.N’Guyen Y, Baumard S, Salmon JH, Lemoine L, Lévêque N, Servettaz A, Jaussaud R, Strady C, Andreoletti L. Cytomegalovirus associated hemophagocytic lymphohistiocytosis in patients suffering from Crohn’s disease treated by azathioprine: a series of four cases. Inflamm Bowel Dis. 2011;17:E116–E118. doi: 10.1002/ibd.21770. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian V, Finlayson C, Harrison T, Rice P, Pollok R. Primary cytomegalovirus infectious colitis complicating Crohn’s disease successfully treated with oral valganciclovir. J Crohns Colitis. 2010;4:199–202. doi: 10.1016/j.crohns.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Roblin X, Pillet S, Oussalah A, Berthelot P, Del Tedesco E, Phelip JM, Chambonnière ML, Garraud O, Peyrin-Biroulet L, Pozzetto B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001–2008. doi: 10.1038/ajg.2011.202. [DOI] [PubMed] [Google Scholar]
- 11.Roblin X, Pillet S, Berthelot P, Del Tedesco E, Phelip JM, Chambonnière ML, Peyrin-Biroulet L, Pozzetto B. Prevalence of cytomegalovirus infection in steroid-refractory Crohn’s disease. Inflamm Bowel Dis. 2012;18:E1396–E1397. doi: 10.1002/ibd.21907. [DOI] [PubMed] [Google Scholar]
- 12.Dimitroulia E, Spanakis N, Konstantinidou AE, Legakis NJ, Tsakris A. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:879–884. doi: 10.1097/01.mib.0000231576.11678.57. [DOI] [PubMed] [Google Scholar]
- 13.Kuwabara A, Okamoto H, Suda T, Ajioka Y, Hatakeyama K. Clinicopathologic characteristics of clinically relevant cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol. 2007;42:823–829. doi: 10.1007/s00535-007-2103-3. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516–1521. doi: 10.1002/ibd.20253. [DOI] [PubMed] [Google Scholar]
- 15.Nakase H, Yoshino T, Honzawa Y, Chiba T. Low prevalence of CMV infection in patients with Crohn’s disease in comparison with ulcerative colitis: effect of different immune response on prevalence of CMV infection. Dig Dis Sci. 2010;55:1498–1499. doi: 10.1007/s10620-010-1162-0. [DOI] [PubMed] [Google Scholar]
- 16.Lévêque N, Brixi-Benmansour H, Reig T, Renois F, Talmud D, Brodard V, Coste JF, De Champs C, Andréoletti L, Diebold MD. Low frequency of cytomegalovirus infection during exacerbations of inflammatory bowel diseases. J Med Virol. 2010;82:1694–1700. doi: 10.1002/jmv.21877. [DOI] [PubMed] [Google Scholar]
- 17.Kim JJ, Simpson N, Klipfel N, Debose R, Barr N, Laine L. Cytomegalovirus infection in patients with active inflammatory bowel disease. Dig Dis Sci. 2010;55:1059–1065. doi: 10.1007/s10620-010-1126-4. [DOI] [PubMed] [Google Scholar]
- 18.Aarnio MT, Böhm JP, Nuorva KP, Pitkänen RI, Kuopio TH, Voutilainen ME. Absence of cytomegalovirus from the gastrointestinal tract of patients with active Crohn’s disease. In Vivo. 2012;26:151–155. [PubMed] [Google Scholar]
- 19.Antonelli E, Baldoni M, Giovenali P, Villanacci V, Essatari M, Bassotti G. Intestinal superinfections in patients with inflammatory bowel diseases. J Crohns Colitis. 2012;6:154–159. doi: 10.1016/j.crohns.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Al-Zafiri R, Gologan A, Galiatsatos P, Szilagyi A. Cytomegalovirus complicating inflammatory bowel disease: a 10-year experience in a community-based, university-affiliated hospital. Gastroenterol Hepatol (N Y) 2012;8:230–239. [PMC free article] [PubMed] [Google Scholar]
- 21.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen M, Bradford K, Zhang X, Shih DQ. Cytomegalovirus Reactivation in Ulcerative Colitis Patients. Ulcers. 2011;2011:pii 282507. doi: 10.1155/2011/282507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills AM, Guo FP, Copland AP, Pai RK, Pinsky BA. A comparison of CMV detection in gastrointestinal mucosal biopsies using immunohistochemistry and PCR performed on formalin-fixed, paraffin-embedded tissue. Am J Surg Pathol. 2013;37:995–1000. doi: 10.1097/PAS.0b013e31827fcc33. [DOI] [PubMed] [Google Scholar]
- 24.Langner C, Magro F, Driessen A, Ensari A, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, et al. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Arch. 2014;464:511–527. doi: 10.1007/s00428-014-1543-4. [DOI] [PubMed] [Google Scholar]
- 25.Zidar N, Ferkolj I, Tepeš K, Štabuc B, Kojc N, Uršič T, Petrovec M. Diagnosing cytomegalovirus in patients with inflammatory bowel disease--by immunohistochemistry or polymerase chain reaction? Virchows Arch. 2015;466:533–539. doi: 10.1007/s00428-015-1741-8. [DOI] [PubMed] [Google Scholar]
- 26.McCurdy JD, Enders FT, Jones A, Killian JM, Loftus EV, Bruining DH, Smyrk TC. Detection of Cytomegalovirus in Patients with Inflammatory Bowel Disease: Where to Biopsy and How Many Biopsies? Inflamm Bowel Dis. 2015;21:2833–2838. doi: 10.1097/MIB.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16:1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 28.Nakase H, Yoshino T, Matumura K, Honzawa Y, Yamamoto S, Matsuura M, Chiba T. Positive finding of colonic polymerase chain reaction for cytomegalovirus DNA is not false positive but a warning for treating patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2011;17:E13–E14. doi: 10.1002/ibd.21401. [DOI] [PubMed] [Google Scholar]
- 29.Ganzenmueller T, Henke-Gendo C, Schlué J, Wedemeyer J, Huebner S, Heim A. Quantification of cytomegalovirus DNA levels in intestinal biopsies as a diagnostic tool for CMV intestinal disease. J Clin Virol. 2009;46:254–258. doi: 10.1016/j.jcv.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Pang XL, Fox JD, Fenton JM, Miller GG, Caliendo AM, Preiksaitis JK. Interlaboratory comparison of cytomegalovirus viral load assays. Am J Transplant. 2009;9:258–268. doi: 10.1111/j.1600-6143.2008.02513.x. [DOI] [PubMed] [Google Scholar]
- 31.Waggoner JJ, Pinsky BA. Comparison of automated nucleic acid extraction methods for the detection of cytomegalovirus DNA in fluids and tissues. PeerJ. 2014;2:e334. doi: 10.7717/peerj.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciccocioppo R, Racca F, Paolucci S, Campanini G, Pozzi L, Betti E, Riboni R, Vanoli A, Baldanti F, Corazza GR. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: need for mucosal viral load measurement. World J Gastroenterol. 2015;21:1915–1926. doi: 10.3748/wjg.v21.i6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciccocioppo R. Letter: cytomegalovirus infection in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:127–129. doi: 10.1111/apt.13234. [DOI] [PubMed] [Google Scholar]
- 34.Kou T, Nakase H, Tamaki H, Kudo T, Nishio A, Chiba T. Cytomegalovirus infection in patients with ulcerative colitis diagnosed by quantitative real-time PCR analysis. Dig Dis Sci. 2006;51:1052–1055. doi: 10.1007/s10620-006-8006-y. [DOI] [PubMed] [Google Scholar]
- 35.Kleer CG, Appelman HD. Ulcerative colitis: patterns of involvement in colorectal biopsies and changes with time. Am J Surg Pathol. 1998;22:983–989. doi: 10.1097/00000478-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Kim B, Barnett JL, Kleer CG, Appelman HD. Endoscopic and histological patchiness in treated ulcerative colitis. Am J Gastroenterol. 1999;94:3258–3262. doi: 10.1111/j.1572-0241.1999.01533.x. [DOI] [PubMed] [Google Scholar]
- 37.Joo M, Odze RD. Rectal sparing and skip lesions in ulcerative colitis: a comparative study of endoscopic and histologic findings in patients who underwent proctocolectomy. Am J Surg Pathol. 2010;34:689–696. doi: 10.1097/PAS.0b013e3181db84cd. [DOI] [PubMed] [Google Scholar]
- 38.Shah SN, Amarapurkar AD, Shrinivas N, Rathi PM. Atypical histological features of ulcerative colitis. Trop Gastroenterol. 2011;32:107–111. [PubMed] [Google Scholar]
- 39.DeRoche TC, Xiao SY, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol Rep (Oxf) 2014;2:178–192. doi: 10.1093/gastro/gou031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SH, Yang SK, Park SK, Kim JW, Yang DH, Jung KW, Kim KJ, Ye BD, Byeon JS, Myung SJ, et al. Atypical distribution of inflammation in newly diagnosed ulcerative colitis is not rare. Can J Gastroenterol Hepatol. 2014;28:125–130. doi: 10.1155/2014/834512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCurdy JD, Jones A, Enders FT, Killian JM, Loftus EV, Smyrk TC, Bruining DH. A model for identifying cytomegalovirus in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:131–17; quiz e7. doi: 10.1016/j.cgh.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Omiya M, Matsushita M, Tanaka T, Kawamata S, Okazaki K. The absence of large ulcer predicts latent cytomegalovirus infection in ulcerative colitis with positive mucosal viral assay. Intern Med. 2010;49:2277–2282. doi: 10.2169/internalmedicine.49.3657. [DOI] [PubMed] [Google Scholar]
- 43.Boom R, Sol C, Weel J, Lettinga K, Gerrits Y, van Breda A, Wertheim-Van Dillen P. Detection and quantitation of human cytomegalovirus DNA in faeces. J Virol Methods. 2000;84:1–14. doi: 10.1016/s0166-0934(99)00127-5. [DOI] [PubMed] [Google Scholar]
- 44.Ganzenmueller T, Kluba J, Becker JU, Bachmann O, Heim A. Detection of cytomegalovirus (CMV) by real-time PCR in fecal samples for the non-invasive diagnosis of CMV intestinal disease. J Clin Virol. 2014;61:517–522. doi: 10.1016/j.jcv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Herfarth HH, Long MD, Rubinas TC, Sandridge M, Miller MB. Evaluation of a non-invasive method to detect cytomegalovirus (CMV)-DNA in stool samples of patients with inflammatory bowel disease (IBD): a pilot study. Dig Dis Sci. 2010;55:1053–1058. doi: 10.1007/s10620-010-1146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun YQ, Xu LP, Han TT, Zhang XH, Wang Y, Han W, Wang FR, Wang JZ, Chen H, Chen YH, et al. Detection of human cytomegalovirus (CMV) DNA in feces has limited value in predicting CMV enteritis in patients with intestinal graft-versus-host disease after allogeneic stem cell transplantation. Transpl Infect Dis. 2015;17:655–661. doi: 10.1111/tid.12420. [DOI] [PubMed] [Google Scholar]
- 47.Bitton A, Buie D, Enns R, Feagan BG, Jones JL, Marshall JK, Whittaker S, Griffiths AM, Panaccione R. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107:179–194; author reply 195. doi: 10.1038/ajg.2011.386. [DOI] [PubMed] [Google Scholar]
- 48.Shukla T, Singh S, Loftus EV, Bruining DH, McCurdy JD. Antiviral Therapy in Steroid-refractory Ulcerative Colitis with Cytomegalovirus: Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015;21:2718–2725. doi: 10.1097/MIB.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 49.Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Pillet S, Pozzetto B, Jarlot C, Paul S, Roblin X. Management of cytomegalovirus infection in inflammatory bowel diseases. Dig Liver Dis. 2012;44:541–548. doi: 10.1016/j.dld.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Vega R, Bertrán X, Menacho M, Domènech E, Moreno de Vega V, Hombrados M, Cabré E, Ojanguren I, Gassull MA. Cytomegalovirus infection in patients with inflammatory bowel disease. Am J Gastroenterol. 1999;94:1053–1056. doi: 10.1111/j.1572-0241.1999.01013.x. [DOI] [PubMed] [Google Scholar]
- 52.Cottone M, Pietrosi G, Martorana G, Casà A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A, Pagliaro L. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol. 2001;96:773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 53.Papadakis KA, Tung JK, Binder SW, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:2137–2142. doi: 10.1111/j.1572-0241.2001.03949.x. [DOI] [PubMed] [Google Scholar]
- 54.Wada Y, Matsui T, Matake H, Sakurai T, Yamamoto J, Kikuchi Y, Yorioka M, Tsuda S, Yao T, Yao S, et al. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003;46:S59–S65. doi: 10.1097/01.DCR.0000087486.21981.C6. [DOI] [PubMed] [Google Scholar]
- 55.Criscuoli V, Casà A, Orlando A, Pecoraro G, Oliva L, Traina M, Rizzo A, Cottone M. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis. 2004;36:818–820. doi: 10.1016/j.dld.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004;28:365–373. doi: 10.1097/00000478-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Kishore J, Ghoshal U, Ghoshal UC, Krishnani N, Kumar S, Singh M, Ayyagari A. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155–1160. doi: 10.1099/jmm.0.45629-0. [DOI] [PubMed] [Google Scholar]
- 58.Alain S, Ducancelle A, Le Pors MJ, Mazeron MC, de Saussure P, Bouhnik Y, Lavergne A. Cytomegalovirus infection in patients with active inflammatory bowel disease. J Clin Virol. 2005;33:180–182. doi: 10.1016/j.jcv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Maconi G, Colombo E, Zerbi P, Sampietro GM, Fociani P, Bosani M, Cassinotti A, Casini V, Russo A, Ardizzone S, et al. Prevalence, detection rate and outcome of cytomegalovirus infection in ulcerative colitis patients requiring colonic resection. Dig Liver Dis. 2005;37:418–423. doi: 10.1016/j.dld.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Kojima T, Watanabe T, Hata K, Shinozaki M, Yokoyama T, Nagawa H. Cytomegalovirus infection in ulcerative colitis. Scand J Gastroenterol. 2006;41:706–711. doi: 10.1080/00365520500408584. [DOI] [PubMed] [Google Scholar]
- 61.Lavagna A, Bergallo M, Daperno M, Sostegni R, Ravarino N, Crocellà L, Ramella A, Rocca R, Torchio B, Cavallo R, et al. The hazardous burden of Herpesviridae in inflammatory bowel disease: the case of refractory severe ulcerative colitis. Dig Liver Dis. 2006;38:887–893. doi: 10.1016/j.dld.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Minami M, Ohta M, Ohkura T, Ando T, Ohmiya N, Niwa Y, Goto H. Cytomegalovirus infection in severe ulcerative colitis patients undergoing continuous intravenous cyclosporine treatment in Japan. World J Gastroenterol. 2007;13:754–760. doi: 10.3748/wjg.v13.i5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, Okamoto S, Morohoshi Y, Izumiya M, Ichikawa H, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331–337. doi: 10.1111/j.1572-0241.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 64.Domènech E, Vega R, Ojanguren I, Hernández A, Garcia-Planella E, Bernal I, Rosinach M, Boix J, Cabré E, Gassull MA. Cytomegalovirus infection in ulcerative colitis: a prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008;14:1373–1379. doi: 10.1002/ibd.20498. [DOI] [PubMed] [Google Scholar]
- 65.Maher MM, Nassar MI. Acute cytomegalovirus infection is a risk factor in refractory and complicated inflammatory bowel disease. Dig Dis Sci. 2009;54:2456–2462. doi: 10.1007/s10620-008-0639-6. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol. 2010;16:1245–1251. doi: 10.3748/wjg.v16.i10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Criscuoli V, Rizzuto MR, Montalbano L, Gallo E, Cottone M. Natural history of cytomegalovirus infection in a series of patients diagnosed with moderate-severe ulcerative colitis. World J Gastroenterol. 2011;17:633–638. doi: 10.3748/wjg.v17.i5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim YS, Kim YH, Kim JS, Cheon JH, Ye BD, Jung SA, Park YS, Choi CH, Jang BI, Han DS, et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol. 2012;46:51–56. doi: 10.1097/MCG.0b013e3182160c9c. [DOI] [PubMed] [Google Scholar]
- 69.Yoshino T, Nakase H, Matsuura M. Letter: Mucosal PCR for cytomegalovirus in refractory ulcerative colitis. Aliment Pharmacol Ther. 2012;36:811–812; author reply 812. doi: 10.1111/apt.12009. [DOI] [PubMed] [Google Scholar]
- 70.Fukuchi T, Nakase H, Matsuura M, Yoshino T, Toyonaga T, Ohmori K, Ubukata S, Ueda A, Eguchi T, Yamashita H, et al. Effect of intensive granulocyte and monocyte adsorptive apheresis in patients with ulcerative colitis positive for cytomegalovirus. J Crohns Colitis. 2013;7:803–811. doi: 10.1016/j.crohns.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Iida T, Ikeya K, Watanabe F, Abe J, Maruyama Y, Ohata A, Teruyuki S, Sugimoto K, Hanai H. Looking for endoscopic features of cytomegalovirus colitis: a study of 187 patients with active ulcerative colitis, positive and negative for cytomegalovirus. Inflamm Bowel Dis. 2013;19:1156–1163. doi: 10.1097/MIB.0b013e31828075ce. [DOI] [PubMed] [Google Scholar]
- 72.Kopylov U, Sasson G, Geyshis B, Oikawa MT, Barshack I, Eliakim R, Ben-Horin S. Cytomegalovirus positive ulcerative colitis: A single center experience and literature review. World J Gastrointest Pathophysiol. 2013;4:18–23. doi: 10.4291/wjgp.v4.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delvincourt M, Lopez A, Pillet S, Bourrier A, Seksik P, Cosnes J, Carrat F, Gozlan J, Beaugerie L, Roblin X, et al. The impact of cytomegalovirus reactivation and its treatment on the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:712–720. doi: 10.1111/apt.12650. [DOI] [PubMed] [Google Scholar]
- 74.do Carmo AM, Santos FM, Ortiz-Agostinho CL, Nishitokukado I, Frota CS, Gomes FU, Leite AZ, Pannuti CS, Boas LS, Teixeira MG, et al. Cytomegalovirus infection in inflammatory bowel disease is not associated with worsening of intestinal inflammatory activity. PLoS One. 2014;9:e111574. doi: 10.1371/journal.pone.0111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inokuchi T, Kato J, Hiraoka S, Suzuki H, Nakarai A, Hirakawa T, Akita M, Takahashi S, Harada K, Okada H, et al. Long-term follow-up of ulcerative colitis patients treated on the basis of their cytomegalovirus antigen status. World J Gastroenterol. 2014;20:509–517. doi: 10.3748/wjg.v20.i2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim YS, Kim YH, Kim JS, Jeong SY, Park SJ, Cheon JH, Ye BD, Jung SA, Park YS, Choi CH, et al. Long-term outcomes of cytomegalovirus reactivation in patients with moderate to severe ulcerative colitis: a multicenter study. Gut Liver. 2014;8:643–647. doi: 10.5009/gnl13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JW, Boo SJ, Ye BD, Kim CL, Yang SK, Kim J, Kim SA, Park SH, Park SK, Yang DH, et al. Clinical utility of cytomegalovirus antigenemia assay and blood cytomegalovirus DNA PCR for cytomegaloviral colitis patients with moderate to severe ulcerative colitis. J Crohns Colitis. 2014;8:693–701. doi: 10.1016/j.crohns.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 78.Maconi G, Lombardini M, Furfaro F, Bezzio C, Zerbi P, Ardizzone S. Long-term outcome of inflammatory bowel diseases with cytomegalovirus colitis: effect of antiviral treatment. Eur J Gastroenterol Hepatol. 2014;26:1146–1151. doi: 10.1097/MEG.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 79.Matsumoto S, Yoshida Y. What are the factors that affect hospitalization and surgery for aggravation of ulcerative colitis? Eur J Gastroenterol Hepatol. 2014;26:282–287. doi: 10.1097/MEG.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 80.Olaisen M, Rydning A, Martinsen TC, Nordrum IS, Mjønes P, Fossmark R. Cytomegalovirus infection and postoperative complications in patients with ulcerative colitis undergoing colectomy. Scand J Gastroenterol. 2014;49:845–852. doi: 10.3109/00365521.2014.929172. [DOI] [PubMed] [Google Scholar]
- 81.Yamada S, Yoshino T, Matsuura M, Minami N, Toyonaga T, Honzawa Y, Tsuji Y, Nakase H. Long-term efficacy of infliximab for refractory ulcerative colitis: results from a single center experience. BMC Gastroenterol. 2014;14:80. doi: 10.1186/1471-230X-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chun J, Lee C, Kwon JE, Hwang SW, Kim SG, Kim JS, Jung HC, Im JP. Usefulness of the cytomegalovirus antigenemia assay in patients with ulcerative colitis. Intest Res. 2015;13:50–59. doi: 10.5217/ir.2015.13.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones A, McCurdy JD, Loftus EV, Bruining DH, Enders FT, Killian JM, Smyrk TC. Effects of antiviral therapy for patients with inflammatory bowel disease and a positive intestinal biopsy for cytomegalovirus. Clin Gastroenterol Hepatol. 2015;13:949–955. doi: 10.1016/j.cgh.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 84.Gauss A, Rosenstiel S, Schnitzler P, Hinz U, Rehlen T, Kadmon M, Ehehalt R, Stremmel W, Zawierucha A. Intestinal cytomegalovirus infection in patients hospitalized for exacerbation of inflammatory bowel disease: a 10-year tertiary referral center experience. Eur J Gastroenterol Hepatol. 2015;27:712–720. doi: 10.1097/MEG.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 85.Minami N, Yoshino T, Matsuura M, Koshikawa Y, Yamada S, Toyonaga T, Madian A, Honzawa Y, Nakase H. Tacrolimus or infliximab for severe ulcerative colitis: short-term and long-term data from a retrospective observational study. BMJ Open Gastroenterol. 2015;2:e000021. doi: 10.1136/bmjgast-2014-000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Saussure P, Lavergne-Slove A, Mazeron MC, Alain S, Matuchansky C, Bouhnik Y. A prospective assessment of cytomegalovirus infection in active inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:1323–1327. doi: 10.1111/j.1365-2036.2004.02273.x. [DOI] [PubMed] [Google Scholar]
- 87.Chiba M, Abe T, Tsuda S, Ono I. Cytomegalovirus infection associated with onset of ulcerative colitis. BMC Res Notes. 2013;6:40. doi: 10.1186/1756-0500-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin SI, Sepehr A, Fishman JA. Primary infection with cytomegalovirus in ulcerative colitis. Dig Dis Sci. 2006;51:2184–2187. doi: 10.1007/s10620-006-9474-9. [DOI] [PubMed] [Google Scholar]
- 89.Streetz KL, Buhr T, Wedemeyer H, Bleck J, Schedel I, Manns MP, Göke MN. Acute CMV-colitis in a patient with a history of ulcerative colitis. Scand J Gastroenterol. 2003;38:119–122. [PubMed] [Google Scholar]
- 90.Hamlin PJ, Shah MN, Scott N, Wyatt JI, Howdle PD. Systemic cytomegalovirus infection complicating ulcerative colitis: a case report and review of the literature. Postgrad Med J. 2004;80:233–235. doi: 10.1136/pgmj.2003.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23; quiz 524. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 92.Widmann T, Sester U, Gärtner BC, Schubert J, Pfreundschuh M, Köhler H, Sester M. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. PLoS One. 2008;3:e3634. doi: 10.1371/journal.pone.0003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Damme E, Sauviller S, Lau B, Kesteleyn B, Griffiths P, Burroughs A, Emery V, Sinclair J, Van Loock M. Glucocorticosteroids trigger reactivation of human cytomegalovirus from latently infected myeloid cells and increase the risk for HCMV infection in D+R+ liver transplant patients. J Gen Virol. 2015;96:131–143. doi: 10.1099/vir.0.069872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inoue-Toyoda M, Kato K, Nagata K, Yoshikawa H. Glucocorticoids facilitate the transcription from the human cytomegalovirus major immediate early promoter in glucocorticoid receptor- and nuclear factor-I-like protein-dependent manner. Biochem Biophys Res Commun. 2015;458:180–185. doi: 10.1016/j.bbrc.2015.01.091. [DOI] [PubMed] [Google Scholar]
- 95.D’Ovidio V, Vernia P, Gentile G, Capobianchi A, Marcheggiano A, Viscido A, Martino P, Caprilli R. Cytomegalovirus infection in inflammatory bowel disease patients undergoing anti-TNFalpha therapy. J Clin Virol. 2008;43:180–183. doi: 10.1016/j.jcv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 96.Criscuoli V, Mocciaro F, Orlando A, Rizzuto MR, Renda MC, Cottone M. Cytomegalovirus disappearance after treatment for refractory ulcerative colitis in 2 patients treated with infliximab and 1 patient with leukapheresis. Inflamm Bowel Dis. 2009;15:810–811. doi: 10.1002/ibd.20742. [DOI] [PubMed] [Google Scholar]
- 97.Nakase H, Chiba T. TNF-alpha is an important pathogenic factor contributing to reactivation of cytomegalovirus in inflamed mucosa of colon in patients with ulcerative colitis: lesson from clinical experience. Inflamm Bowel Dis. 2010;16:550–551. doi: 10.1002/ibd.21047. [DOI] [PubMed] [Google Scholar]
- 98.Nakase H, Yamamoto S, Matsuura M, Honzawa Y, Chiba T. Cytomegalovirus affects clinical outcome of infliximab in ulcerative colitis refractory to tacrolimus. Aliment Pharmacol Ther. 2010;32:510–511. doi: 10.1111/j.1365-2036.2010.04372.x. [DOI] [PubMed] [Google Scholar]
- 99.Lavagna A, Bergallo M, Daperno M, Sostegni R, Costa C, Leto R, Crocellà L, Molinaro G, Rocca R, Cavallo R, et al. Infliximab and the risk of latent viruses reactivation in active Crohn’s disease. Inflamm Bowel Dis. 2007;13:896–902. doi: 10.1002/ibd.20131. [DOI] [PubMed] [Google Scholar]
- 100.Pillet S, Jarlot C, Courault M, Del Tedesco E, Chardon R, Saint-Sardos P, Presles E, Phelip JM, Berthelot P, Pozzetto B, et al. Infliximab Does Not Worsen Outcomes During Flare-ups Associated with Cytomegalovirus Infection in Patients with Ulcerative Colitis. Inflamm Bowel Dis. 2015;21:1580–1586. doi: 10.1097/MIB.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 101.Roblin X, Del Tedesco E. Tacrolimus in the management of hospitalized patients with steroid-refractory ulcerative colitis: don’t forget cytomegalovirus! Inflamm Bowel Dis. 2013;19:E67–E68. doi: 10.1097/MIB.0b013e318281013f. [DOI] [PubMed] [Google Scholar]
- 102.Barahona-Garrido J, Martínez-Benítez B, Espinosa-Cárdenas E, Sarti HM, Gutiérrez-Manjarrez JI, Aguirre-Gutiérrez R, Tellez-Avila FI, Coss-Adame E, García-Juárez I, Yamamoto-Furusho JK. Cytomegalovirus infection in patients who required colectomy for toxic megacolon or severe steroid-refractory ulcerative colitis. Dig Dis Sci. 2010;55:867–868. doi: 10.1007/s10620-009-1109-5. [DOI] [PubMed] [Google Scholar]
- 103.Wu XW, Wu L, Ji HZ, Wang FY. Relationship Between Cytomegalovirus Infection and Steroid Resistance in Inflammatory Bowel Disease: A Meta-Analysis. Dig Dis Sci. 2015;60:3203–3208. doi: 10.1007/s10620-015-3733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Powell RD, Warner NE, Levine RS, Kirsner JB. Cytomegalic inclusion disease and ulcerative colitis; report of a case in a young adult. Am J Med. 1961;30:334–340. doi: 10.1016/0002-9343(61)90105-x. [DOI] [PubMed] [Google Scholar]
- 105.Uchino M, Matsuoka H, Bando T, Hirata A, Sasaki H, Hirose K, Takesue Y, Nakamura S, Tomita N, Ikeuchi H. Clinical features and treatment of ulcerative colitis-related severe gastroduodenitis and enteritis with massive bleeding after colectomy. Int J Colorectal Dis. 2014;29:239–245. doi: 10.1007/s00384-013-1779-5. [DOI] [PubMed] [Google Scholar]
- 106.Hantz S, Garnier-Geoffroy F, Mazeron MC, Garrigue I, Merville P, Mengelle C, Rostaing L, Saint Marcoux F, Essig M, Rerolle JP, et al. Drug-resistant cytomegalovirus in transplant recipients: a French cohort study. J Antimicrob Chemother. 2010;65:2628–2640. doi: 10.1093/jac/dkq368. [DOI] [PubMed] [Google Scholar]
- 107.Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T, Wang T, Gustofson LM, Wong CJ, Vandervoort MK, Hanauer S. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–409. doi: 10.1053/j.gastro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 108.Yoshino T, Nakase H, Matsuura M, Matsumura K, Honzawa Y, Fukuchi T, Watanabe K, Murano M, Tsujikawa T, Fukunaga K, et al. Effect and safety of granulocyte-monocyte adsorption apheresis for patients with ulcerative colitis positive for cytomegalovirus in comparison with immunosuppressants. Digestion. 2011;84:3–9. doi: 10.1159/000321911. [DOI] [PubMed] [Google Scholar]
- 109.Sager K, Alam S, Bond A, Chinnappan L, Probert CS. Review article: cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:725–733. doi: 10.1111/apt.13124. [DOI] [PubMed] [Google Scholar]


