Abstract
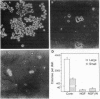
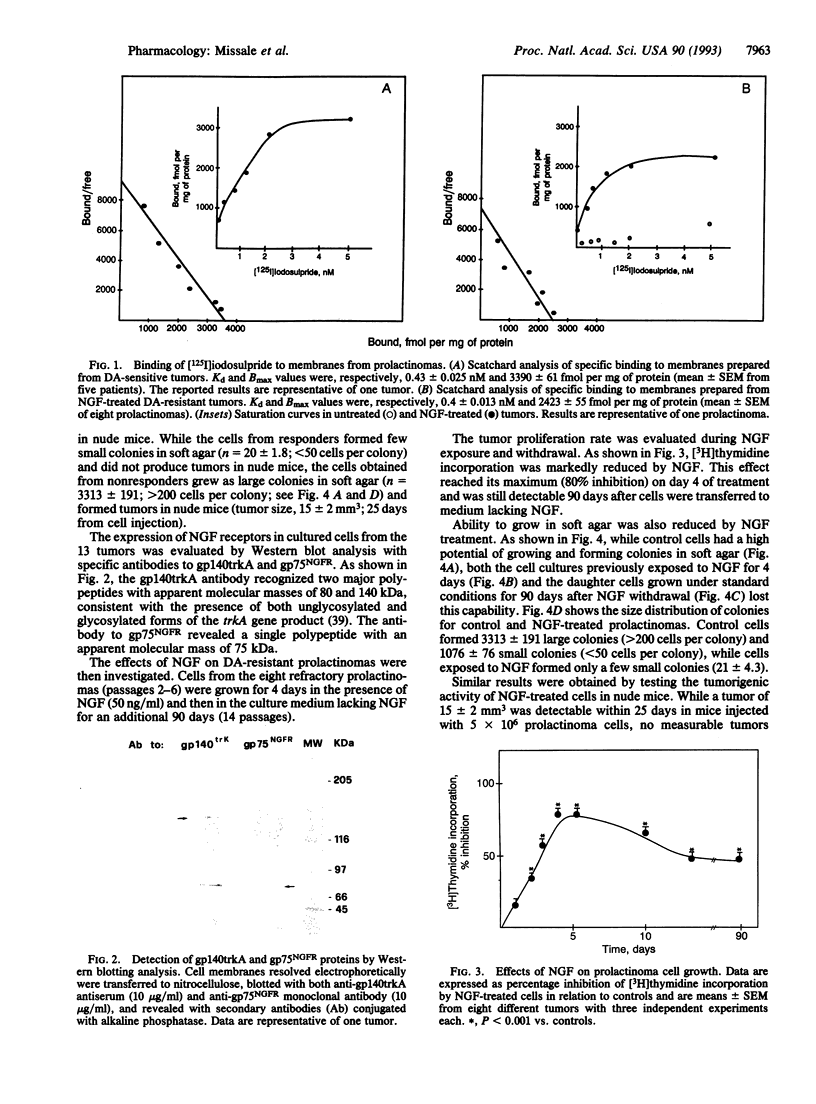
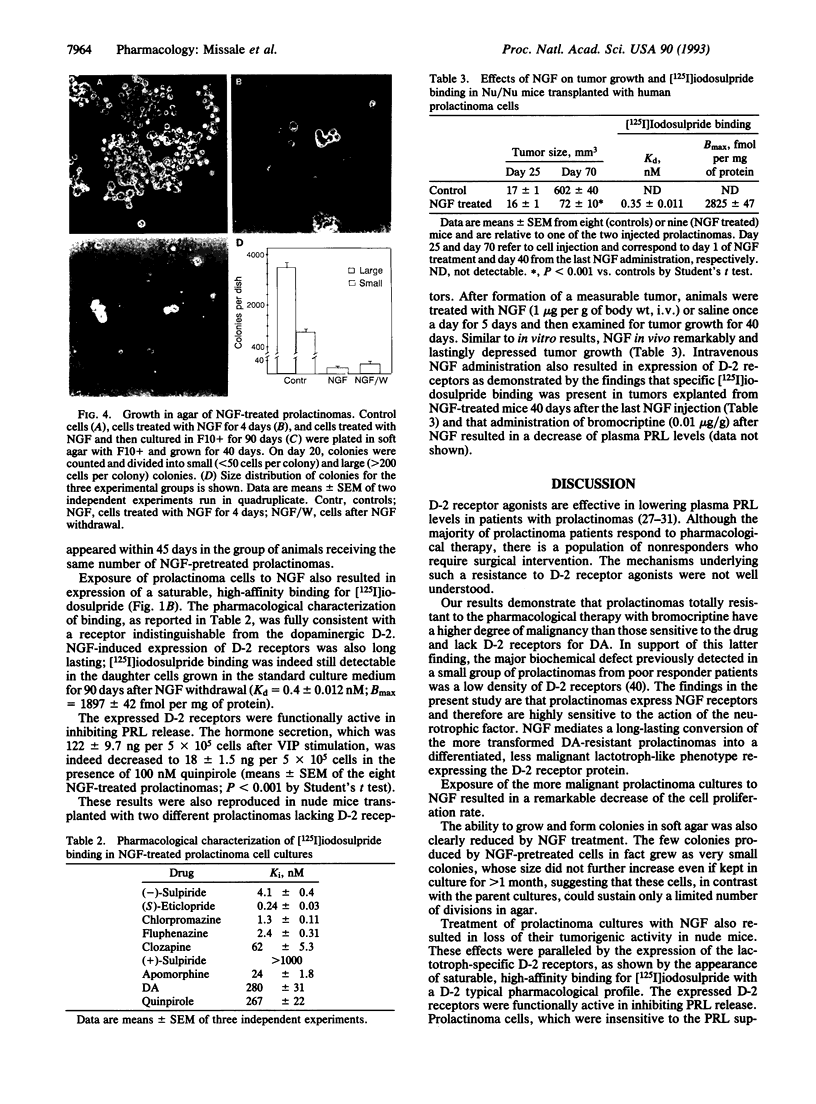
The most effective therapy of human prolactinomas is represented by dopamine D-2 receptor agonists; there is, however, a population of nonresponder patients who require surgical intervention. In the present study, we report that prolactinomas totally resistant to pharmacological therapy have a high potential of both growing in soft agar and forming tumors in nude mice and lack D-2 receptors for dopamine. These tumors express the receptors for nerve growth factor (NGF) and are sensitive to its differentiating activity. After exposure to NGF for 4 days, prolactinoma cells decreased their proliferation rate, lost their capability to form colonies in soft agar, lost their tumorigenic activity in nude mice, and reexpressed the lactotroph-specific D-2 receptor protein inhibiting prolactin release. These effects were permanent after NGF withdrawal and were reproducible in vivo in nude mice transplanted with the tumors. NGF in fact remarkably and lastingly depressed tumor growth and induced expression of D-2 receptors when injected intravenously once a day for 5 days into prolactinoma-bearing nude mice. These data suggest that NGF may induce a long-lasting switch of gene expression in human prolactinomas, modifying their transforming phenotype and reverting them to more differentiated, less malignant, dopamine-sensitive lactotroph-like cells. The possibility thus arises that short-term treatment with NGF may restore the refractory patients to conventional pharmacological therapy with D-2 agonists.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTIA M. A., DEOME K. B., WEISS D. W. IMMUNOLOGY OF SPONTANEOUS MAMMARY CARCINOMAS IN MICE. II. RESISTANCE TO A RAPIDLY AND A SLOWLY DEVELOPING TUMOR. Cancer Res. 1965 May;25:451–457. [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985 Fall;6(4):564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chandler C. E., Parsons L. M., Hosang M., Shooter E. M. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984 Jun 10;259(11):6882–6889. [PubMed] [Google Scholar]
- Chiodini P., Liuzzi A., Cozzi R., Verde G., Oppizzi G., Dallabonzana D., Spelta B., Silvestrini F., Borghi G., Luccarelli G. Size reduction of macroprolactinomas by bromocriptine or lisuride treatment. J Clin Endocrinol Metab. 1981 Oct;53(4):737–743. doi: 10.1210/jcem-53-4-737. [DOI] [PubMed] [Google Scholar]
- Cronin M. J., Faure N., Martial J. A., Weiner R. I. Absence of high affinity dopamine receptor in GH3 cells: a prolactin-secreting clone resistant to the inhibitory action of dopamine. Endocrinology. 1980 Mar;106(3):718–723. doi: 10.1210/endo-106-3-718. [DOI] [PubMed] [Google Scholar]
- Cunnah D., Besser M. Management of prolactinomas. Clin Endocrinol (Oxf) 1991 Mar;34(3):231–235. doi: 10.1111/j.1365-2265.1991.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Nye S. H., Hantzopoulos P., Macchi M. J., Squinto S. P., Goldfarb M., Yancopoulos G. D. TrkB mediates BDNF/NT-3-dependent survival and proliferation in fibroblasts lacking the low affinity NGF receptor. Cell. 1991 Jul 26;66(2):405–413. doi: 10.1016/0092-8674(91)90629-d. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikka J., Hefti F. Development of septal cholinergic neurons in culture: plating density and glial cells modulate effects of NGF on survival, fiber growth, and expression of transmitter-specific enzymes. J Neurosci. 1988 Aug;8(8):2967–2985. doi: 10.1523/JNEUROSCI.08-08-02967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Higgins G. A., Koh S., Chen K. S., Gage F. H. NGF induction of NGF receptor gene expression and cholinergic neuronal hypertrophy within the basal forebrain of the adult rat. Neuron. 1989 Aug;3(2):247–256. doi: 10.1016/0896-6273(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Johnson D., Lanahan A., Buck C. R., Sehgal A., Morgan C., Mercer E., Bothwell M., Chao M. Expression and structure of the human NGF receptor. Cell. 1986 Nov 21;47(4):545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Jumblatt J. E., Tischler A. S. Regulation of muscarinic ligand binding sites by nerve growth factor in PC12 phaeochromocytoma cells. Nature. 1982 May 13;297(5862):152–154. doi: 10.1038/297152a0. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R., Lamballe F., Bryant S., Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992 May;8(5):947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Klein R., Nanduri V., Jing S. A., Lamballe F., Tapley P., Bryant S., Cordon-Cardo C., Jones K. R., Reichardt L. F., Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991 Jul 26;66(2):395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Liuzzi A., Dallabonzana D., Oppizzi G., Verde G. G., Cozzi R., Chiodini P., Luccarelli G. Low doses of dopamine agonists in the long-term treatment of macroprolactinomas. N Engl J Med. 1985 Sep 12;313(11):656–659. doi: 10.1056/NEJM198509123131103. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Coughlin M. D., Bienenstock J., Denburg J. A. Nerve growth factor promotes human hemopoietic colony growth and differentiation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6508–6512. doi: 10.1073/pnas.85.17.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A. M., Scanlon M. F., Hall K., Cook D. B., Hall R. Reduction in size of a pituitary tumor by bromocriptine therapy. N Engl J Med. 1979 Feb 8;300(6):291–293. doi: 10.1056/NEJM197902083000606. [DOI] [PubMed] [Google Scholar]
- Missale C., Boroni F., Castelletti L., Dal Toso R., Gabellini N., Sigala S., Spano P. Lack of coupling of D-2 receptors to adenylate cyclase in GH-3 cells exposed to epidermal growth factor. Possible role of a differential expression of Gi protein subtypes. J Biol Chem. 1991 Dec 5;266(34):23392–23398. [PubMed] [Google Scholar]
- Missale C., Castelletti L., Boroni F., Memo M., Spano P. Epidermal growth factor induces the functional expression of dopamine receptors in the GH3 cell line. Endocrinology. 1991 Jan;128(1):13–20. doi: 10.1210/endo-128-1-13. [DOI] [PubMed] [Google Scholar]
- Otten U., Ehrhard P., Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10059–10063. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini I., Rasolonjanahary R., Gunz G., Bertrand P., Delivet S., Jedynak C. P., Kordon C., Peillon F., Jaquet P., Enjalbert A. Resistance to bromocriptine in prolactinomas. J Clin Endocrinol Metab. 1989 Sep;69(3):500–509. doi: 10.1210/jcem-69-3-500. [DOI] [PubMed] [Google Scholar]
- Post K. D., Biller B. J., Adelman L. S., Molitch M. E., Wolpert S. M., Reichlin S. Selective transsphenoidal adenomectomy in women with galactorrhea-amenorrhea. JAMA. 1979 Jul 13;242(2):158–162. [PubMed] [Google Scholar]
- Radeke M. J., Misko T. P., Hsu C., Herzenberg L. A., Shooter E. M. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987 Feb 12;325(6105):593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Ross R. J., Grossman A., Bouloux P., Rees L. H., Doniach I., Besser G. M. The relationship between serum prolactin and immunocytochemical staining for prolactin in patients with pituitary macroadenomas. Clin Endocrinol (Oxf) 1985 Sep;23(3):227–235. doi: 10.1111/j.1365-2265.1985.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Spark R. F., Baker R., Bienfang D. C., Bergland R. Bromocriptine reduces pituitary tumor size and hypersection. Requiem for pituitary surgery? JAMA. 1982 Jan 15;247(3):311–316. [PubMed] [Google Scholar]
- Squinto S. P., Stitt T. N., Aldrich T. H., Davis S., Bianco S. M., Radziejewski C., Glass D. J., Masiakowski P., Furth M. E., Valenzuela D. M. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991 May 31;65(5):885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Bandtlow C., Heumann R. The physiological function of nerve growth factor in the central nervous system: comparison with the periphery. Rev Physiol Biochem Pharmacol. 1987;109:145–178. doi: 10.1007/BFb0031026. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Tsuruta K., Frey E. A., Grewe C. W., Cote T. E., Eskay R. L., Kebabian J. W. Evidence that LY-141865 specifically stimulates the D-2 dopamine receptor. Nature. 1981 Jul 30;292(5822):463–465. doi: 10.1038/292463a0. [DOI] [PubMed] [Google Scholar]
- Tucker H. S., Grubb S. R., Wigand J. P., Taylon A., Lankford H. V., Blackard W. G., Becker D. P. Galactorrhea-amenorrhea syndrome: follow-up of forty-five patients after pituitary tumor removal. Ann Intern Med. 1981 Mar;94(3):302–307. doi: 10.7326/0003-4819-94-3-302. [DOI] [PubMed] [Google Scholar]
- Wass J. A., Williams J., Charlesworth M., Kingsley D. P., Halliday A. M., Doniach I., Rees L. H., McDonald W. I., Besser G. M. Bromocriptine in management of large pituitary tumours. Br Med J (Clin Res Ed) 1982 Jun 26;284(6333):1908–1911. doi: 10.1136/bmj.284.6333.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. F., Johnston J. M., Johnston D. G. Dopamine, the dopamine D2 receptor and pituitary tumours. Clin Endocrinol (Oxf) 1991 Dec;35(6):455–466. doi: 10.1111/j.1365-2265.1991.tb00928.x. [DOI] [PubMed] [Google Scholar]