Abstract
Background
Dopamine (DA) may be involved in central obesity (CO), an inflammatory condition, through its role in the central nervous system and in periphery, where it may affect immune cell function through five different DA receptors (DR). Whether dopaminergic pathways in peripheral immune cells are implicated in the inflammatory condition linked to CO is however unknown.
Methods
In a cohort of blood donors with and without CO, categorized by waist circumference (WC) (CO: WC ≥0.80 m in women and ≥0.94 m in men), we studied the expression of DR and tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of DA, in peripheral blood mononuclear cells (PBMCs) and their relation with anthropometric and metabolic/endocrine and inflammatory parameters. DR D1-5 and TH expression was assessed by semi quantitative real-time PCR. As inflammatory markers we investigated the immunophenotype of monocyte subsets by flow cytometry, staining for CD14, CD16, CD11b and CD36.
Results
CO individuals showed higher plasma levels of leptin and higher inflammatory pattern of monocytes compared with non-CO. PBMC expression of DR D2, DR D4 and DR D5 as well as of TH were lower in CO in comparison with non-CO. DR D2, and DR D5 expression correlated with lower WC and weight, and with lower inflammatory pattern of monocytes, and TH expression correlated with lower WC. DR D4 expression correlated with lower plasma levels of glycosylated hemoglobin, and DR D2 expression correlated with lower CO.
Conclusions
Results show that CO is associated with peripheral inflammation and downregulation of dopaminergic pathways in PBMCs, possibly suggesting DR expressed on immune cells as pharmacological targets in obesity for better metabolic outcome.
Introduction
The modern obesity epidemic is a major public health issue, with the principal cause of morbidity due to metabolic dysfunction such as insulin resistance, type 2 diabetes, dyslipidemia and cardiovascular disease [1]. Although it is widely accepted that a state of chronic low grade inflammation is responsible for the metabolic dysfunction of obesity, its precise etiology is not completely characterized.
The expression of common receptors and signaling networks in immune cells and adipocytes is the basis of the immunological metabolic cross-talk that explains the inflammatory comorbidities of obesity [2]. On the one hand, immune cells play a central role in adipose tissue biology and, on the other hand, adipocytes have been recently proposed as “immune cells” since they express, cytokines, chemokines, multiple receptors and cell molecules involved in the immune response [3]. Visceral or central obesity (CO) is indeed regarded as an inflammatory disease [1], and recent research has been focused on the study of peripheral blood mononuclear cells (PBMCs) in obesity. Indeed, PBMCs are exposed to systemic factors, such nutrients and inflammatory molecules [4], and may constitute potential biomarkers of early homeostatic energy imbalance, and reducing inflammation could be useful in preventing the occurrence of obesity and its consequences [5].
Dopamine (DA), a classical brain neurotransmitter involved in various vital central nervous system functions, including feeding, reward and cognition, has been reported to modulate peripheral immune function [6–8]. Indeed, DA-induced immunomodulation is currently the focus of intense experimental research and dopaminergic pathways are increasingly considered a target for drug development in immune disease [9]. Immune cells themselves produce endogenous DA, and possibly uptake DA from other sources, using this transmitter as an autocrine/paracrine mediator [10–12]. The first and rate limiting step of DA biosynthesis is the hydroxylation of the L-tyrosine to L-DOPA via tyrosine hydroxylase (TH). Once released, DA can bind to five distinct G protein-coupled receptors [13]. Dopaminergic receptors (DR) are grouped into two families according to their pharmacological profile and main second messenger coupling: the D1-like (D1 and D5) which activate adenylate cyclase and the D2-like (D2, D3 and D4) which inhibit adenylate cyclase [13].
While several lines of evidence support the involvement of DA in obesity through its role in the central nervous system (CNS) [14–16] no information exists so far regarding dopaminergic pathways in peripheral immune cells of obese subjects. We therefore decided to study the expression of DR and TH in PBMCs from a cohort of blood donors and their relation with anthropometric, metabolic and inflammatory parameters in central obesity.
Methods
Ethics statement
This work was approved by the Ethical Committee of Centro Hospitalar of Porto (Porto, Portugal). All participants signed a written informed consent as described in the consent procedure of the study protocol approved by the Ethics committee. The clinical study is registered at the local research department with the identifier 072/09 (047-DEFI/065-CES).
Study Population
Thirty blood donors from the blood bank of Clinical Haematology department of Centro Hospitalar of Porto were enrolled in the study. The individuals followed the selection criteria for blood donation and were not under any medicines for at least one month before enrolment. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice and the mean values were used in the analyses; readings were taken on the right arm, with subjects in the supine position after they had rested for 5 min.
Anthropometrics
Height (in m) was based on an identification document and confirmed by medical record. Body weight was measured to the nearest 100 g on electronic weight scales. Body mass index (BMI) was calculated by dividing weight by height squared and expressed in kg x m-2 and categorized as described before [17]. Waist circumference (WC) was measured with a flexible tape at the level midway between the lowest rib and the iliac crest. CO was defined following International Diabetes Federation criteria by using WC (≥0.80 m for women and ≥0.94 m for men) [18].
Biochemical analysis
Blood samples were taken under standardized conditions. Fasting plasma glucose, triacylglycerol (TAG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterokg.m-2l (LDL-C), very low-density lipoprotein-cholesterol (VLDL-C), glycosylated hemoglobin (HbA1c) were measured using standard techniques. Plasma catecholamines (CA) [adrenaline (AD) and noradrenaline (NA)] were assayed by high pressure liquid chromatography with electrochemical detection (HPLC-ED) (CROMSYSTEMS). Leptin was measured in serum by solid phase two-site enzyme immunoassay (Merecodia Leptin ELISA) and high-sensitivity C-reactive protein (hsCRP) by nephelometry (CardioPhase hsCRP–BnProSpec Siemens).
Flow Cytometry assay of monocytes
Monocytes were analyzed in whole blood samples by means of flow cytometry following a previously described technique [19]. The enumeration and the imunophenotypic analysis of monocytes were performed in fresh EDTA-K3 anti-coagulated blood samples. Immunophenotypic studies were done using a whole blood stain-lyse-and-then-wash method and a direct immunofluorescence technique using the following four-color panel of monoclonal antibodies: mouse anti-human CD36 conjugated with FITC (clone FA6.152, IgG1), mouse anti-human CD16 conjugated with PE (clone 3G8, IgG1), mouse anti-human CD14 conjugated with PE-Cy5 (IgG2a, clone RMO52) all obtained from Beckman Coulter (catalogue numbers IM0766U, IM1238U and IM2640U, respectively), and mouse anti-human CD11b conjugated with APC (IgG2a, clone D12) obtained from Becton Dickinson (BD) (catalogue number 333143).
Data acquisition was carried out on a FACS-Calibur flow cytometer (BD), using the Cell Quest software program (BD). Information on a minimum of 2x105 events was acquired for each staining and stored as list mode data. For data analysis, the Paint-a-Gate Pro software program (BD) was used. Monocytes were quantified based on the CD14 expression, while CD16 was used to differentiate classical (CD16-) and non-classical (CD16+) monocyte populations. The median fluorescence intensity (MFI) of CD14, CD36 and CD11b was assessed in each subset and expressed as fluorescence arbitrary units (AU). The forward scatter (FSC) and side scatter (SSC) of the cell subsets were also measured. In order to overcome inter-individual variations, we calculated the ratio for each parameter between non-classical CD14+CD16+ and classical CD14+CD16- monocytes in each individual.
Real time PCR analysis for the expression of DR and TH in PBMCs
Peripheral blood mononuclear cells were isolated by density gradient centrifugation (Ficoll method) and DR and TH mRNA were assayed by real time PCR as previously described [20]. Briefly, total RNA was extracted from PBMCs by PerfectPure RNA Cell & Tissue kit (5Prime), and the amount of extracted RNA was estimated by spectrophotometry at 260 nm. Total RNA was then reverse transcribed using the High-capacity cDNA Archive Kit (Applied Biosystems, Foster City, USA), according to the manufacturer’s instructions. Real-time PCR was performed with an ABI prism 7000 apparatus (Applied Biosystems) using the Assay on demand kits for the genes of interest (Applied Biosystems), according to the manufacturer’s instructions. Gene sequence data were obtained from the Reference Sequence collection (RefSeq; www.ncbi.nlm.nih.gov/projects/RefSeq). The thermal profile for each gene was: stage 1, 2 min at 50°C; stage 2, 10 min at 95°C; stage 3, 40 cycles including 15 s at 95°C and 1 min at 60°C. Further details about real-time PCR conditions are shown in Table 1. Linearity of real-time PCR assays were tested by constructing standard curves by use of serial 2-fold dilutions of a standard calibrator cDNA and regression coefficients (r2) were always >0.900 (data not shown). Relative expression was determined by normalization to 18S rRNA (housekeeping gene) by means of AB Prism 7000 SDS software. S1 Fig shows HKG levels (expressed as Ct) for the different genes, and also the comparison between the two groups of subjects. No difference is statistically significant and the range of the individual values is very narrow (about one cycle) and clearly superimposed between groups and throughout genes. In addition, HKG minor variations are not influential and likely due to small variations in the amount of cDNA pipetted for the PCR analysis. Gene expression levels in a given sample were represented as 2-ΔCt where ΔCt = [Ct (gene)–Ct (18S rRNA)]. The ratio (R) was calculated for DR and TH mRNA expression between individuals with and without CO. R<0.5 was conventionally considered as underexpression and R>2.0 as overexpression.
Table 1. Real-Time PCR gene expression.
Data are from RefSeq—NCBI Reference Sequence Database (http://www.ncbi.nlm.nih.gov/refseq/).
| Gene | UniGene ID | Interrogated Sequence | Translated Protein | Exon Boundary | Assay Location | Amplicon Length |
|---|---|---|---|---|---|---|
| TH | Hs.435609 | NM_199292.2 | NP_954986.2 | 3–4 | 424–422 | 63 |
| DRD1 | Hs.2624 | NM_000794.3 | NP_000785.1 | 1–2 | 462–1620 | 110 |
| DRD2 | Hs.73893 | NM_000795.3 | NP_000786.1 | 2–3 | 524 | 64 |
| DRD3 | Hs.121478 | NM_033663.3 | NP_387512.3 | 3–4 | 809–725 | 73 |
| DRD4 | Hs.99922 | NM_000797.3 | NP_000788.2 | 1–2 | 285–283 | 99 |
| DRD5 | Hs.380681 | NM_000798.4 | NP_000789.1 | 1–1 | 1092–744 | 88 |
| 18SrRNA | X03205.1 | N/A | N/A | N/A | N/A | 187 |
Abbreviations: TH, tyrosine hydroxylase; DRD1, dopaminergic receptor type1; DRD2, dopaminergic receptor type 2; DRD3, dopaminergic receptor type 3; DRD4, dopaminergic receptor type 4; DRD5, dopaminergic receptor type 5.
All the parameters were assessed in 30 (17 with and 13 without CO) individuals, except leptin and CD11b, assessed in 21 (11 with and 10 without CO) and 13 (8 with and 5 without CO), respectively.
Statistical Analysis
The modified Kolmogorov-Smirnov test with the correction of Lilliefors was used to evaluate the fit of the data to a normal distribution. Variables were summarized using relative and absolute frequencies or means±standard error of the mean (SEM), as appropriate. Non-normally distributed data were summarized as median, 25th and 75th percentiles. To compare the quantitative independent variables we used bivariate ANOVA or Mann-Whitney tests for normally and non-normally distributed data, respectively. The Pearson Chi-Square test was used to compare qualitative independent variables. Correlations were assessed by non-parametric Spearman rank analysis. Only those correlations with significance lower or equal to 0.01 were considered. The strength of association between variables was estimated by odds ratio (OR) and their respective 95% confidence interval (CI) using multiple logistic regression. Variables that in the univariate analysis showed statistical significance below 10% (p <0.10) were included in the logistic regression model. Data analysis was performed with the version 22.0 software Predictive Analytics Software (PASW Statistics Software).
Results
Characteristics of the study population
In this cohort of blood donors the prevalence of central obesity was 57.7%, and the mean BMI was 30±1.5 and 24±0.7 kg.m-2, respectively in individuals with and without CO (Table 2). There were no differences between subjects without and with CO, with the exception of plasma leptin which was higher in CO.
Table 2. Characteristics of the participants (n = 30) and comparison between groups defined by Waist Circumference.
| Overall | Waist Circumference | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Reference values (range) | mean± SEM/* | F | df | No CO (n = 13) | CO (n = 17) | P |
| Age (years) | 41±2 | 0.795 | 1, 28 | 39±3 | 42±2 | 0.380 | |
| Weight (Kg) | 76±3 | 3.302 | 1, 28 | 70±2 | 80±4 | 0.080 | |
| Height (m) | 1.67±0.02 | 5.780 | 1, 28 | 1.71±0.01 | 1.64±0.02 | 0.023 | |
| BMI (kg.m-2) | 27.2±1.0 | 10.448 | 1, 28 | 24±0.7 | 30±1.5 | 0.003 | |
| WC (m) | 0.95±0.03 | 13.492 | 1, 28 | 0.86±0.02 | 1.02±0.04 | 0.001 | |
| SBP (mmHg) | <130 | 134±3 | 0.120 | 1, 28 | 133±4 | 135±5 | 0.732 |
| DBP (mmHg) | <85 | 79±2 | 0.362 | 1, 28 | 77±2 | 80±3 | 0.552 |
| Glycemia (mg/dL) | 70–105 | 85±1 | 0.200 | 1, 28 | 86±1 | 84±2 | 0.659 |
| HgA1c (%) | 3.8–5.6 | 5.2±0.06 | 2.070 | 1, 28 | 5.1±0.08 | 5.3±0.07 | 0.161 |
| TC (mg/dL) | 0–200 | 188±7 | 0.807 | 1, 28 | 181±10 | 193±9 | 0.377 |
| LDL-C (mg/dL) | 0–130 | 117±6 | 0.084 | 1, 28 | 115±9 | 118±8 | 0.774 |
| HDL-C (mg/dL) | 35–55 | 51±3 | 0.080 | 1, 28 | 50±4 | 52±4 | 0.780 |
| VLDL-C (mg/dL) | 3–56 | 20±2 | 2.901 | 1, 28 | 16±2 | 23±3 | 0.100 |
| TAG (mg/dL) | 40–160 | 99±10 | 2.855 | 1, 28 | 80±9 | 113±16 | 0.102 |
| NA (pmoL/L) | 709–4019 | 879±131 | 0.444 | 1, 28 | 778±185 | 956±185 | 0.511 |
| AD (pmoL/L) | <328 | 167±17 | 0.172 | 1, 28 | 175±31 | 160±18 | 0.682 |
| Cortisol (μg/dL) | 6.2–19.4 | 15±0.8 | 0.439 | 1, 28 | 16±1 | 15±1 | 0.513 |
| Leptin (ng/mL)** | 2.0–5.6 | 0.9±0.3 | 6.779 | 1, 19 | 0.138±0.047 | 1.626±0.542 | 0.017 |
| Leucocytes (cells/μL) | 4500–13000 | 6403±280 | 1.823 | 1, 28 | 5977±483 | 6729±319 | 0.188 |
| Monocytes (cells/μL) | 400–500 | 446±31 | 1.361 | 1, 28 | 487±55 | 415±34 | 0.253 |
| Lymphocytes (cells/μL) | 1000–4800 | 1951±96 | 3.729 | 1, 28 | 1748±123 | 2106±131 | 0.064 |
Abbreviations: BMI, Body Mass Index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein-cholesterol; VLDL-C, very low-density lipoprotein-cholesterol; TAG, triacylglycerol; NA, noradrenaline; AD, adrenaline; F Snedcor’s distribution; df default freedom; p level of significance. Data are presented as mean ± standard error of the mean (SEM). Differences between groups were analyzed by ANOVA.
*Data presented as median, 25th and 75th percentiles and statistical analysis performed with Mann- Whitney U test for the differences between the 2 groups.
**Leptin was assessed in 21 individuals (11 with and 10 without CO).
Plasma CA levels were similar between WC established groups, but in CO AD correlated with leptin (r = 0.860; P = 0.001). In the whole population, total monocytes were 446±31 cells/μL and no differences were noticed between subjects with and without CO (415±34 vs 487±55, respectively; p = 0.253).
Total monocyte count as well as CD16+ and CD16- monocytes were not different between subjects with and without CO (Tables 2 and 3) however, in comparison to monocytes from subjects without CO, cells from subjects with CO showed lower SSC, CD14, CD11b, and CD36 ratios (Table 3), defining a higher inflammatory phenotype for pro-inflammatory monocytes.
Table 3. Immunophenotypic characteristics of CD16+ and CD16- monocytes in overall participants and categorized by waist circumference.
| Parameter | Overall | *p | Without CO | With CO | p |
|---|---|---|---|---|---|
| cells/μL | |||||
| CD16+ | 54±12 | 57±21 | 53±13 | ||
| CD16- | 392±25 | 430±43 | 362±28 | ||
| CD16+/CD16- | 0.14±0.02 | <0.001 | 0.12±0.03 | 0.15±0.03 | ns |
| Cells % | |||||
| CD16+ | 11.4±1.4 | 10±2 | 12±2 | ||
| CD16- | 88.6±1.4 | 90±2 | 88±2 | ||
| CD16+/CD16- | 0.14±0.02 | <0.001 | 0.13±0.03 | 0.15±0.03 | ns |
| FSC | |||||
| CD16+ | 557±11 | 546±18 | 560±13 | ||
| CD16- | 558±11 | 548±17 | 565±13 | ||
| CD16+/CD16- | 1±0.005 | ns | 1.00±0.01 | 1.00±0.01 | ns |
| SSC | |||||
| CD16+ | 418±10 | 435±17 | 405±11 | ||
| CD16- | 486±8 | 488±13 | 483±10 | ||
| CD16+/CD16- | 0.86±0.01 | <0.001 | 0.89±0.02 | 0.84±0.01 | 0.022 |
| CD14 | |||||
| CD16+ | 875±109 | 893±163 | 860±151 | ||
| CD16- | 2340±322 | 1874±353 | 2561±476 | ||
| CD16+/CD16- | 0.41±0.03 | <0.001 | 0.51±0.05 | 0.34±0.03 | 0.009 |
| CD36 | |||||
| CD16+ | 54±12 | 332±59 | 282±28 | ||
| CD16- | 392±25 | 671±78 | 781±45 | ||
| CD16+/CD16- | 0.43±0.03 | <0.001 | 0.51±0.05 | 0.38±0.03 | 0.023 |
| CD11b** | |||||
| CD16+ | 11.4±1.4 | 90±35 | 115±35 | ||
| CD16- | 88.6±1.4 | 143±54 | 405±174 | ||
| CD16+/CD16- | 0.49±0.05 | 0.050 | 0.64±0.05 | 0.40±0.06 | 0.015 |
Abbreviations: CO, central obesity; FSC, forward scatter; SSC, side scatter; ns, not significant. Values of CD14, CD36 and CD11b expressed as fluorescence arbitrary units (AU). Data are presented as mean ± standard error of the mean (SEM). Differences between groups analyzed by ANOVA p, level of significance; p* significance between CD16+ and CD16- monocytes subsets.
**CD11b was assessed in 13 individuals (8 with and 5 without CO).
Expression of DR and TH in PBMCs
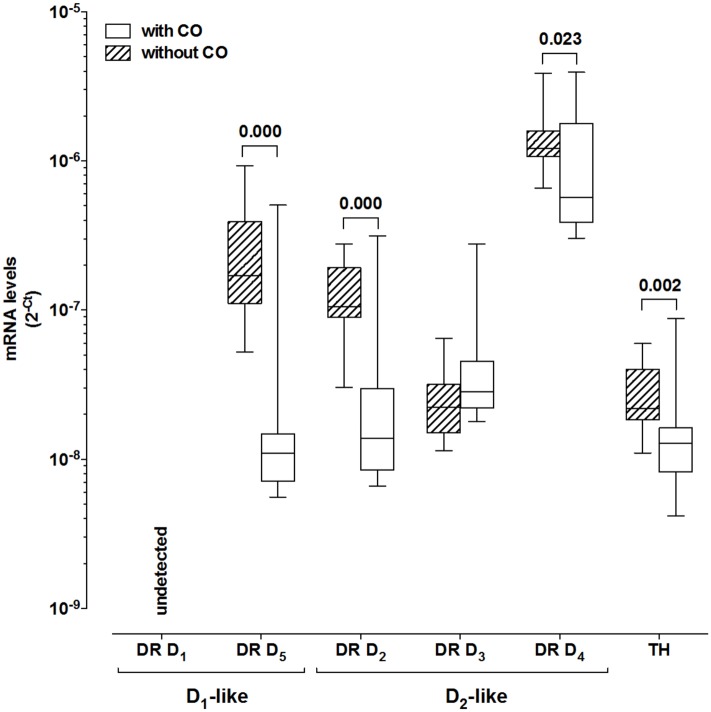
Compared to PBMCs from subjects without CO, cells from individuals with CO expressed lower D1-like DR D5 (R = 0.29) and D2-like DR D2 (R = 0.13) and D4 (R = 0.47), as well as lower TH mRNA (R = 0.58) (Fig 1). In centrally obese individuals, the comparison between obese and non-obese BMI defined did not show differences in mRNA levels of TH and DR in PBMCs (S1 Table).
Fig 1. Comparison of the expression of Dopaminergic receptors in Peripheral Blood Mononuclear Cells between Groups with and without Central Obesity.
Boxes indicate medians with 25th–75th percentiles and whiskers indicate minimum and maximum values. Mann-Whitney test used for comparison between the two groups. Abbreviations: Tyrosine hydroxylase (TH), Central Obesity (CO), DRD1 dopaminergic receptor type1, DRD2 dopaminergic receptor type 2, DRD3 dopaminergic receptor type 3, DRD4 dopaminergic receptor type 4, DRD5 dopaminergic receptor type 5, Significant differences are indicated (P value).
Correlation between DR and TH mRNA expression in PBMCs and anthropometric, metabolic/endocrine and inflammatory markers
As shown in Table 4, in the whole cohort, the expression of DR D2 and DR D5 mRNA in PBMCs was negatively correlated with weight, BMI, WC and leptin levels, and positively correlated with CD11b ratio in monocytes. Moreover, DR D4 mRNA was negatively correlated with HbA1c and TH mRNA was correlated with lower WC and leptin levels.
Table 4. Correlations between dopaminergic receptors and tyrosine hydroxylase expression and anthropometric, metabolic/endocrine and inflammatory parameters.
| TH | DRD2 | DRD3 | DRD4 | DRD5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Weight | ns | -0.502 | 0.005 | ns | ns | -0.483 | 0.007 | |||
| BMI | ns | -0.577 | 0.001 | ns | ns | -0.557 | 0.001 | |||
| WC | -0.529 | 0.003 | -0.681 | <0.001 | ns | ns | -0.621 | <0.001 | ||
| HbA1c | ns | ns | ns | -0.605 | <0.001 | ns | ||||
| Leptin | -0.555 | 0.009 | -0.739 | <0.001 | ns | ns | -0.618 | 0.003 | ||
| Ratio CD11b | ns | 0.927 | <0.001 | ns | ns | 0.748 | 0.003 | |||
Abbreviations: TH tyrosine hydroxylase, DRD2 dopaminergic receptor type 2, DRD3 dopaminergic receptor type 3, DRD4 dopaminergic receptor type 4, DRD5 dopaminergic receptor type 5, BMI, Body mass index; WC waist circumference; HbA1c glycosylated hemoglobin; Ratio CD11b, ratio between the expression of CD11b in CD16+ monocytes and the expression of CD11b in CD16- monocytes and the median fluorescence intensity of CD11b was assessed in each subset and expressed as fluorescence arbitrary units; r correlation coefficients, calculated by Spearman’s rho test; P level of significance; ns not significant if P>0.01.
In the model of CO, the logistic regression analysis showed a lower association for the development of central obesity for DR D2 mRNA expression ≥0.0000000455 (odds ratio [OR] 0.018 confidence interval [CI] 95% 0002–0.195).
All data are included in the supporting information (S1 Dataset).
Discussion
This is the first work studying the expression of DR and TH in peripheral immune cells in obesity. Our findings show that CO is associated with inflammation, as shown by higher plasma levels of leptin and a more inflammatory pattern of non-classical monocytes, and that in CO PBMCs exhibit a distinct pattern of DR and TH expression. In particular: (i) PBMCs from subjects with CO show a reduction of the expression of DR D2, DR D4 and DR D5 as well as lower levels of TH mRNA in comparison to cells from the non-obese group; (ii) DR D2 and DR D5 expression in PBMCs strongly correlates with lower weight, BMI and WC, lower plasma levels of leptin and with a lower inflammatory pattern, while DR D4 mRNA correlates with less HbA1c and TH mRNA correlates with lower WC and leptin levels; (iii) the expression of DR D2 may have a protective role against the presence of CO.
The relationship between DA and obesity has been subject of extensive research in the nervous system [14–16, 21]. Scientific evidence has established an association between obesity and hyposensitivity of dopaminergic systems, both within the CNS [22] and in peripheral tissues [23]. While DA brain functions are well known, its peripheral role is an emerging subject of study. An association between the intake of a high fat diet and alterations in brain DA levels [24, 25] has been already described, and Wang et al. (2001) reported that in obese DR D2 availability correlated negatively with BMI [22].
Till now, knowledge about the role of DA in peripheral tissues during obesity has been limited to its influence on pancreatic β cells regulating insulin release [26] and to the modulation of insulin effects on adipocytes [21]. Indeed, Borcherding et al (2011) suggested a regulatory role of peripheral DA in adipose tissue functions as human adipocytes cell lines express DR [27].
In our study, we found decreased DR D2, D4 and D5 as well as TH mRNA levels in PBMCs from subjects with CO. Decreased expression of DR D2 in PBMCs has been already reported in other inflammatory immune mediated diseases such as Crohn’s disease [28] and systemic lupus erythematosus (SLE) [29]. Indeed, administration of DR D2 agonists reduced disease activity in patients with rheumatoid arthritis [30, 31] and decreased serum immunoglobulin and anti-DNA antibody levels in SLE patients [30]. Single-nucleotide polymorphisms of DR D2, associated to lower expression and function, increase inflammation in human renal proximal tubule cells [32]. In human lymphocytes, DR D2 agonists increase the secretion of anti-inflammatory cytokines [33]. As regards DR D5, their reduction in PBMCs has been previously reported in patients suffering from multiple sclerosis [34, 35], an immune-mediated inflammatory disease in which DR D5 expression in PBMCs has been also suggested to predict the therapeutic response to immunomodulating treatment with interferon- β [35].
In our study, PBMCs expression of DR D2 and D5 (and to a lesser extent also of D4 and TH) were shown to be associated with lower weight, with better metabolic/endocrine parameters and with a less inflammatory pattern. Moreover, logistic regression analysis suggests that DR D2 expression has a protective role for the existence of CO.
The correlations between the expression of DR and TH in PBMCs and more favorable metabolic/endocrine and inflammatory parameters suggest a possible role for dopaminergic pathways in the crosstalk between immunity and metabolism. Indeed, adipose tissue express DR and DA regulates adipose tissue functions [27]. So far, DA has been shown to have an inhibitory effect on leptin release from human adipocytes through D1-like DR receptors [27] and to modify the secretion of insulin in peripheral tissues [21]. Yu et al. (2014) described a negative effect through DR D4 activation on insulin action via decreasing insulin receptor expression [36]. Peripheral blockade of DR could therefore result in increased insulin release and sensitivity, thus promoting adipogenesis, weight gain, insulin resistance, and ultimately type 2 diabetes [21], adverse metabolic effects also described for neuroleptics [37]. DA inhibitory effects on the release and action of insulin may explain the inverse correlation between DR D4 expression in PBMCs and HbA1c plasma levels found in the present work. The inhibitory effect of DA on leptin release mediated by DR is in agreement with the relationship found between DR expression and leptin, considering PBMCs as early sensors for metabolic dysfunction. The major sources of DA in peripheral blood are neuronal fibers, adrenal medulla and neuroendocrine cells [38], and although the type of food ingested could affect blood DA levels [21, 39, 40] and putatively the expression and function of DR, in our study blood samples were collected in a fasted state.
Our work has some limitations. First, we did not analyze DR at protein level. Even though, Araki et al (2007) showed that DR mRNA and membrane-associated protein changes correspond with each other; moreover the same authors demonstrated that DR mRNA expression or receptor protein are valid surrogates for receptor function [41] and our group previously showed that DR responsiveness in human lymphocytes is better predicted by mRNA rather than membrane receptor expression [12].
Secondly, we do not know if the different pattern of DR and TH expression on PBMCs associated with CO is a cause or consequence of inflammatory obesity. This open question should be answered prospectively with further studies addressing the effect of weight loss on DR expression in peripheral immune cells. Finally, DR expression was assessed in PBMCs as a whole, and future studies should address the possible differential expression on distinct mononuclear cell subsets (e.g. lymphocytes vs monocytes, T helper (TH) 1 vs Th2, regulatory T cells vs Th17).
Conclusions
To our knowledge, this is the first work studying the expression of DR and TH in peripheral immune cells in obesity. CO is associated with under expression of DR D2, DR D4 and DR D5, lower levels of TH mRNA in PBMCs and with a higher innate inflammatory pattern. The expression of DR correlated with lower weight, with better metabolic profile and lower inflammatory pattern. The expression of DR D2 showed a protective role for the development of CO. Results thus indicate that CO is associated with peripheral inflammation and downregulation of dopaminergic pathways in PBMCs, and warrant further research to establish whether DR expressed on immune cells could represent pharmacological targets in obesity for better metabolic outcome.
Supporting Information
(XLSX)
(DOCX)
(DOCX)
Acknowledgments
The authors are grateful to Massimiliano Legnaro (Center of Research in Medical Pharmacology, University of Insubria) for technical assistance in real-time PCR assays and in flow cytometry assays to Ana Santos (Department of Clinical Haematology, Centro Hospitalar of Porto, Portugal).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by FCT funding UID/BIM/04293/2013-Instituto de Investigação e Inovação em Saúde (I3S), University of Porto, Porto, Portugal and Pest-OE/SAU/UI0215/2014-Unidade Multidisciplinar de Investigação Biomédica-UMIB/ICBAS/UP.
References
- 1.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444: 875–880. [DOI] [PubMed] [Google Scholar]
- 2.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13: 707–712. 10.1038/ni.2343 [DOI] [PubMed] [Google Scholar]
- 3.Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, et al. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 2011;6: e17154 10.1371/journal.pone.0017154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visvikis-Siest S, Marteau JB, Samara A, Berrahmoune H, Marie B, Pfister M. Peripheral blood mononuclear cells (PBMCs): a possible model for studying cardiovascular biology systems. Clin Chem Lab Med. 2007;45: 1154–1168. [DOI] [PubMed] [Google Scholar]
- 5.Oliver P, Reynés B, Caimari A, Palou A. Peripheral blood mononuclear cells: a potential source of homeostatic imbalance markers associated with obesity development. Pflugers Arch. 2013;465: 459–468. 10.1007/s00424-013-1246-8 [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 2000;102: 113–124. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. 2010;24: 525–528. 10.1016/j.bbi.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levite M. Dopamine in the immune system: dopamine receptors in immune cells, potent effects, endogenous production and involvement in immune and neuropsychiatric diseases In Levite M, editors. Nerve-driven-immunity–Neurotransmitters and neuropeptides in the immune system. Springer-Verlag, Wien; 2012. pp 1–45. [Google Scholar]
- 9.Cosentino M, Marino F. Adrenergic and dopaminergic modulation of immunity in multiple sclerosis: teaching old drugs new tricks? J Neuroimmune Pharmacol. 2013;8: 163–179. 10.1007/s11481-012-9410-z [DOI] [PubMed] [Google Scholar]
- 10.Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci U S A. 1994;91: 12912–12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosentino M, Marino F, Bombelli R, Ferrari M, Lecchini S, Frigo G. Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci. 1999;64: 975–981. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, et al. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109: 632–642. [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu JM, Gainetdinov RR The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63: 182–217. 10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- 14.Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014;5: 919 10.3389/fpsyg.2014.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenny PJ, Voren G, Johnson PM. Dopamine D2 receptors and striatopallidal transmission in addiction and obesity. Curr Opin Neurobiol. 2013;23: 535–538. 10.1016/j.conb.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baik JH. Dopamine signaling in food addiction: role of dopamine D2 receptors. BMB Rep. 2013;46: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist Circumference and Cardiometabolic Risk: a Consensus Statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring). 2007;15: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 19.Lima M, Almeida J, Montero AG, Teixeira Mdos A, Queirós ML, Santos AH, et al. Clinicobiological, Immunophenotypic, and Molecular Characteristics of Monoclonal CD56−/+dim Chronic Natural Killer Cell Large Granular Lymphocytosis. Am J Pathol. 2004;165: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaffaroni M, Marino F, Bombelli R, Rasini E, Monti M, Ferrari M, et al. Therapy with interferon-beta modulates endogenous catecholamines in lymphocytes of patients with multiple sclerosis. Exp Neurol. 2008;214: 315–321. 10.1016/j.expneurol.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 21.Rubí B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance. Endocrinology. 2010;151: 5570–5581. 10.1210/en.2010-0745 [DOI] [PubMed] [Google Scholar]
- 22.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357: 354–357. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Cornago A, Ramírez MJ, Zulet MÁ, Martinez JA. Effect of dietary restriction on peripheral monoamines and anxiety symptoms in obese subjects with metabolic syndrome. Psychoneuroendocrinology. 2014;47: 98–106. 10.1016/j.psyneuen.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 24.Carlin J, Hill-Smith TE, Lucki I, Reyes TM. Reversal of dopamine system dysfunction in response to high-fat diet. Obesity (Silver Spring). 2013;21: 2513–2521. 10.1002/oby.20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaczmarczyk MM, Machaj AS, Chiu GS, Lawson MA, Gainey SJ, York JM, et al. Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology. 2013;38:1553–1564. 10.1016/j.psyneuen.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280: 36824–36832. [DOI] [PubMed] [Google Scholar]
- 27.Borcherding DC, Hugo ER, Idelman G, De Silva A, Richtand NW, Loftus J, Ben-Jonathan N. Dopamine receptors in human adipocytes: expression and functions. PLoS One. 2011;6:e25537 10.1371/journal.pone.0025537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magro F, Cunha E, Araujo F, Meireles E, Pereira P, Dinis-Ribeiro M, Veloso FT, et al. Dopamine D2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig Dis Sci. 2006;51: 2039–2044. [DOI] [PubMed] [Google Scholar]
- 29.Jafari M, Ahangari G, Saberi M, Samangoui S, Torabi R, Zouali M. Distorted expression of dopamine receptor genes in systemic lupus erythematosus. Immunobiology. 2013;218: 979–983. 10.1016/j.imbio.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 30.McMurray RW. Bromocriptine in rheumatic and autoimmune diseases. Semin Arthritis Rheum. 2001;31: 21–32. [DOI] [PubMed] [Google Scholar]
- 31.Mobini M, Kashi Z, Mohammad Pour AR, Adibi E. The effect of cabergoline on clinical and laboratory findings in active rheumatoid arthritis. Iran Red Crescent Med J. 2011;13: 749–750. [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang X, Konkalmatt P, Yang Y, Gildea J, Jones JE, Cuevas S, et al. Single-nucleotide polymorphisms of the dopamine D2 receptor increase inflammation and fibrosis in human renal proximal tubule cells. Hypertension. 2014;63: e74–80. 10.1161/HYPERTENSIONAHA.113.02569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNF alpha or both. J Neuroimmunol. 2005;169: 161–171. [DOI] [PubMed] [Google Scholar]
- 34.Giorelli M, Livrea P, Trojano M. Dopamine fails to regulate activation of peripheral blood lymphocytes from multiple sclerosis patients: effects of IFN-beta. J Interferon Cytokine Res. 2005;25: 395–406. [DOI] [PubMed] [Google Scholar]
- 35.Cosentino M, Zaffaroni M, Marino F. Levels of mRNA for dopaminergic receptor D(5) in circulating lymphocytes may be associated with subsequent response to interferon-beta in patients with multiple sclerosis. J Neuroimmunol. 2014;277: 193–196. 10.1016/j.jneuroim.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 36.Yu C, Wang Z, Han Y, Liu Y, Wang WE, Chen C, et al. Dopamine D(4) receptors inhibit proliferation and migration of vascular smooth muscle cells induced by insulin via down-regulation of insulin receptor expression. Cardiovasc Diabetol. 2014;13: 97 10.1186/1475-2840-13-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am. 2013;42: 545–563. 10.1016/j.ecl.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 38.Pearse AG. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem 1969;17:303–313. [DOI] [PubMed] [Google Scholar]
- 39.Eisenhofer G, Aneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, et al. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82: 3864–3871. [DOI] [PubMed] [Google Scholar]
- 40.Vincent S, Bieck PR, Garland EM, Loghin C, Bymaster FP, Black BK, et al. Clinical assessment of norepinephrine transporter blockade through biochemical and pharmacological profiles. Circulation. 2004;109: 3202–3207. [DOI] [PubMed] [Google Scholar]
- 41.Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 2007;1156: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.