Summary
Background
Sparse data on the safety of pyronaridine-artesunate after repeated treatment of malaria episodes restrict its clinical use. We therefore compared the safety of pyronaridine-artesunate after treatment of the first episode of malaria versus re-treatment in a substudy analysis.
Methods
This planned substudy analysis of the randomised, open-label West African Network for Clinical Trials of Antimalarial Drugs (WANECAM) phase 3b/4 trial was done at six health facilities in Mali, Burkina Faso, and Guinea in patients (aged ≥6 months and bodyweight ≥5 kg) with uncomplicated microscopically confirmed Plasmodium spp malaria (parasite density <200 000 per μL blood) and fever or history of fever. The primary safety endpoint was incidence of hepatotoxicity: alanine aminotransferase of greater than five times the upper limit of normal (ULN) or Hy's criteria (alanine aminotransferase or aspartate aminotransferase greater than three times the ULN and total bilirubin more than twice the ULN) after treatment of the first episode of malaria and re-treatment (≥28 days after first treatment) with pyronaridine-artesunate. Pyronaridine-artesunate efficacy was compared with artemether-lumefantrine with the adequate clinical and parasitological response (ACPR) in an intention-to-treat analysis. WANECAM is registered with PACTR.org, number PACTR201105000286876.
Findings
Following first treatment, 13 (1%) of 996 patients had hepatotoxicity (including one [<1%] possible Hy's law case) versus two (1%) of 311 patients on re-treatment (neither a Hy's law case). No evidence was found that pyronaridine-artesunate re-treatment increased safety risk based on laboratory values, reported adverse event frequencies, or electrocardiograph findings. For all first treatment or re-treatment episodes, pyronaridine-artesunate (n=673) day 28 crude ACPR was 92·7% (95% CI 91·0–94·3) versus 80·4% (77·8–83·0) for artemether-lumefantrine (n=671). After exclusion of patients with PCR-confirmed new infections, ACPR was similar on treatment and re-treatment and greater than 95% at day 28 and greater than 91% at day 42 in both treatment groups.
Interpretation
The findings that pyronaridine-artesunate safety and efficacy were similar on first malaria treatment versus re-treatment of subsequent episodes lend support for the wider access to pyronaridine-artesunate as an alternative artemisinin-based combination treatment for malaria in sub-Saharan Africa.
Funding
European and Developing Countries Clinical Trial Partnership, Medicines for Malaria Venture (Geneva, Switzerland), UK Medical Research Council, Swedish International Development Cooperation Agency, German Ministry for Education and Research, University Claude Bernard (Lyon, France), Malaria Research and Training Centre (Bamako, Mali), Centre National de Recherche et de Formation sur le Paludisme (Burkina Faso), Institut de Recherche en Sciences de la Santé (Bobo-Dioulasso, Burkina Faso), and Centre National de Formation et de Recherche en Santé Rurale (Republic of Guinea).
Introduction
Malaria remains a major public health issue across sub-Saharan Africa. The continued availability of affordable, acceptably safe, and effective artemisinin-based combination therapies (ACTs) is necessary to maintain and improve recent reductions in malaria morbidity and mortality rates.
Pyronaridine-artesunate is an oral ACT for Plasmodium falciparum and Plasmodium vivax malaria,1 and the first antimalarial to receive a positive scientific opinion from the European Medicines Agency under Article 58.2 Pyronaridine-artesunate safety and efficacy were shown in four phase 3 randomised clinical trials in adults and children in Africa and Asia.3, 4, 5, 6 In these studies, transient increases in liver enzymes were noted with pyronaridine-artesunate, typically peaking by day 7 and normalising by day 28.3, 4, 5, 6, 7, 8 Although mostly mild, potential Hy's law events (alanine aminotransferase or aspartate aminotransferase greater than three times the upper limit of normal [ULN] plus peak total bilirubin of more than twice the ULN)9 were noted in three (<1%) of 1204 patients treated with pyronaridine-artesunate versus one (<1%) of 603 patients receiving artemether-lumefantrine in two phase 3 studies of P falciparum malaria in Africa.3, 6 Also, two (<1%) of 1076 patients had potential Hy's law events with pyronaridine-artesunate in the two other phase 3 trials (P vivax malaria in Asia and P falciparum in Asia and Africa) versus none of 651 patients in the comparator groups (chloroquine or mefloquine-artesunate).4, 5 No patient had clinical signs of liver injury, and a review by an independent data safety monitoring board of potential Hy's law events concluded that the risk of progressive liver injury with pyronaridine-artesunate was not a public health concern. However, because only a few patients in the phase 3 studies were re-treated with pyronaridine-artesunate, it was unclear whether treatment of repeated malaria episodes would exacerbate increases in hepatic enzymes or lead to liver injury. Consequently, pyronaridine-artesunate use was restricted to a single treatment course in any patient. To permit wider access to this potentially valuable antimalarial drug, more data are needed on pyronaridine-artesunate safety and efficacy in patients re-treated for two or more malaria episodes.
Research in context.
Evidence before this study
The lack of data for the safety and efficacy of re-treatment of malaria with newly available artemisinin-based combination therapies (ACTs) has restricted their use. In phase 2/3 trials of pyronaridine-artesunate, transient increases in transaminases were noted with pyronaridine-artesunate in some patients after a single course of treatment. Whether treatment of repeated malaria episodes would exacerbate increases in hepatic enzymes or lead to liver injury was not clear. Thus, further data are needed on pyronaridine-artesunate safety and efficacy in patients re-treated for more than one malaria episode.
Added value of this study
The results from this substudy suggest that pyronaridine-artesunate re-treatment is well tolerated and that the safety and efficacy of the first malaria treatment episode is maintained with re-treatment of patients who have subsequent episodes of malaria. Particularly, the risk of a hepatotoxicity event was not greater after pyronaridine-artesunate re-treatment versus first treatment (0·19% vs 0·54%).
Implications of all the available evidence
These findings support the wider access to pyronaridine-artesunate as an alternative ACT for malaria treatment in sub-Saharan Africa.
The West African Network for Clinical Trials of Antimalarial Drugs (WANECAM) study is a phase 3b/4 randomised, multicentre, open-label trial of the incidence of Plasmodium spp malaria and ACT safety after repeated treatment, with each patient receiving the same ACT for all malaria episodes over a 2-year follow-up. Pyronaridine-artesunate and dihydroartemisinin-piperaquine, which have not been assessed for the re-treatment of two or more malaria episodes, are being compared with the first-line ACTs artesunate-amodiaquine or artemether-lumefantrine, for which results from previous studies support malaria re-treatment.10, 11, 12 The trial is scheduled to be completed in December, 2015.
Here we report the results of a planned analysis of the WANECAM dataset at a prespecified timepoint. The primary objective was to ascertain if the risk of hepatotoxicity with pyronaridine-artesunate was increased on malaria re-treatment versus first treatment. For consistency with the phase 3 studies of pyronaridine-artesunate in children and adults in Africa,3, 6 artemether-lumefantrine was used as a comparator to verify pyronaridine-artesunate efficacy and safety.
Methods
Study design and participants
The WANECAM study protocol, including details of ten protocol amendments, and the substudy statistical analysis plan are available from the corresponding author. The substudy analysis included patients enrolled between Oct 24, 2011, and Oct 31, 2013, at six health facilities in Mali (Sotuba, Kolle, and Bougoula-Hameau), Burkina Faso (Niankoloko and Bobo-Dioulasso), and Guinea (Maferenya).
Male or female patients (aged ≥6 months and bodyweight ≥5 kg) were eligible if they had acute uncomplicated microscopically confirmed P falciparum, Plasmodium malariae, or Plasmodium ovale malaria (<200 000 parasites per μL blood) and fever or a history of fever. In the pyronaridine-artesunate group, eligibility criteria for age and bodyweight were modified in accordance with the planned reviews by the data safety monitoring board: at the start of the study, criteria for inclusion were age of at least 15 years and bodyweight of at least 24 kg; after 20 re-treatments, these criteria were revised to at least 2 years and at least 15 kg; after 40 re-treatments, these criteria were at least 6 months and at least 5 kg. For patients randomly allocated to receive artemether-lumefantrine, age (≥6 months) and bodyweight (≥5 kg) criteria remained unchanged.
Key exclusion criteria were complicated or severe malaria; severe vomiting or diarrhoea; history of clinically significant disease or disorders, including known viral hepatitis, known HIV infection, or alcohol abuse; hepatic or renal impairment; alanine aminotransferase concentration of more than twice the ULN; serum creatinine concentration of more than 1·5 times the ULN; anaemia (haemoglobin <70 g/L); or known hypersensitivity to the study drug. Women of childbearing potential had to have a negative pregnancy test, not be lactating, and not planning pregnancy for 42 days after treatment.
The WANECAM trial is being done in accordance with the good clinical practice guidelines and the Declaration of Helsinki. Each participating centre's ethics committee or institutional review board approved the study protocol. Participants or their parent or guardian provided written informed consent plus assent from children able to understand the study.
Patients were re-treated with their allocated ACT if they presented with malaria at least 28 days after previous treatment. An alternative appropriate antimalarial drug (usually quinine) was used for any re-treatment if the patient had severe malaria; had parasitaemia recurrence before 28 days; had Plasmodium spp parasites of more than 200 000 per μL; had severe vomiting or diarrhoea; had alanine aminotransferase of more than twice the ULN; had serum creatinine of more than 1·5 times the ULN; had active hepatitis A, B, or C; had an ongoing severe adverse event; had arrhythmia or QTc prolongation (>450 ms) during previous treatment or at representation; was pregnant or breastfeeding; or had other antimalarial treatment (except rescue treatment or for severe malaria).
Criteria for treatment discontinuation and study withdrawal were a severe drug-related adverse event, hypersensitivity to the study drug, drug-related QTc prolongation (>450 ms); drug-related alanine aminotransferase of more than five times the ULN or Hy's law; active chronic hepatitis B or C, or HIV infection; travel outside the vicinity of the study for longer than 3 months; any other medical disorder compromising the patient's safety; and withdrawal of consent.
Randomisation and masking
The WANECAM study was open label. The microscopists assessing the parasite outcomes were masked to treatment allocation. At first visit, eligible patients were assigned the lowest number on the randomisation list for allocation to one of three groups in a 1:1:1 ratio: pyronaridine-artesunate, dihydroartemisinin-piperaquine, or first-line ACT comparator (artemether-lumefantrine or artesunate-amodiaquine). Randomisation lists were produced using a validated automated system by the sponsor. Only patients randomly allocated receive to pyronaridine-artesunate or artemether-lumefantrine were analysed in this substudy.
Procedures
Pyronaridine-artesunate tablets (180 mg: 60 mg) or granule sachets (60 mg: 20 mg), donated by Shin Poong Pharmaceutical Company (Ansan, South Korea) and artemether-lumefantrine dispersible tablets (20 mg: 120 mg, Novartis, Basel, Switzerland) and tablets (20 mg: 120 mg, Novartis) were dosed according to a patient's bodyweight (appendix). Pyronaridine-artesunate was administered once a day for 3 days and artemether-lumefantrine twice a day for 3 days. Both treatments were given with water; administration of all doses was supervised.
For each episode of malaria, patients were admitted to hospital if needed for days 0–3 and actively followed up as outpatients on days 7, 14, 21, 28, 35, and 42. Patients were also passively followed up for 2-years between active follow-up. Study teams were available at health centres for 24 h a day to provide health care, including treatment for malaria, free of charge to all study participants. Monthly home visits ensured that study participants were still present in the study area and in good health.
Patients had physical examinations and their vital signs were recorded at screening and all subsequent assessments. Adverse events were recorded at all assessments. 12-lead electrocardiographs were done before treatment, on days 2, 3, and 42, and when clinically indicated. Blood samples for biochemistry (creatinine, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, total bilirubin, and conjugated bilirubin) and haematology tests were obtained before treatment, on days 3, 7, and 28, and when clinically indicated. In the event of potential post-treatment hepatotoxicity, antihepatitis A IgM, antihepatitis B core IgM, hepatitis B surface antigen, hepatitis C RNA, hepatitis E IgM antibody, cytomegalovirus PCR testing, pp65 antigen or cytomegalovirus IgM antibody, and Epstein-Barr virus viral capsid antigen IgM antibody tests were done and serum creatinine phosphokinase and lactase dehydrogenase were measured.
Parasite species were identified, and parasitaemia was defined in accordance with WHO's standards.13 Duplicate Giemsa-stained thick and thin blood smears were prepared before treatment and every 12 h (samples could be taken 2 h either before or after the 12-h point) for up to 72 h (or two consecutive negative smears) after the first dose of treatment, at all follow-up visits, and at treatment failure. At each blood smear, triplicate blood samples were gathered on 3MM filter paper (Whatman Clifton, NJ, USA). P falciparum PCR genotyping was done and recrudescence was defined as at least one matching allelic band for P falciparum marker genes between baseline samples and samples from recurrences after day 7 to day 42.14, 15, 16
Outcomes
The primary safety outcome was post-treatment hepatotoxicity events in the pyronaridine-artesunate group, defined as alanine aminotransferase concentration greater than five times the ULN or potential case of Hy's law (alanine aminotransferase or aspartate aminotransferase greater than three times the ULN and total bilirubin greater than twice the ULN, confirmed by the data safety monitoring board).9
Other safety outcomes were adverse events, serious adverse events (coded using the Medical Dictionary for Regulatory Activities [MedDRA], version 16.1), biochemical and haematological values outside the normal range, and abnormal QTc from electrocardiographs.
Efficacy was assessed with adequate clinical and parasitological response (ACPR) as per WHO's guidelines (ie, absence of parasitaemia on day 28 or 42, irrespective of axillary temperature, without previously meeting any of the criteria of early treatment failure, late clinical failure, or late parasitological failure).13 Parasite clearance time for each malaria episode was defined as the time from treatment start until aparasitaemia, maintained for at least 48 h.
Statistical analysis
Pyronaridine-artesunate safety was compared between the safety population (all patients who were given at least one dose for a first malaria episode) and the safety re-treatment population (all patients who were given at least one dose for any subsequent episode).
The primary safety endpoint was assessed in patients having any post-day 0 liver function test for the safety re-treatment population versus the safety population, excluding patients with missing post-day 0 liver function test data. Non-inferiority of re-treatment to first treatment represented no increased risk of hepatotoxicity events.9 Simulations showed that 190 re-treated patients would have 91·4% power to show non-inferiority of pyronaridine-artesunate re-treatment to first treatment with a non-inferiority margin of 5%, with the actual primary endpoint event rate of 2·7% after first treatment and re-treatment.
The primary safety endpoint was used as the binary dependent variable in a generalised estimating equation (GEE) model, dosing (any re-treatment vs first treatment) as the fixed effect, and patient as the random effect. A 95% one-sided upper confidence limit (95% CI) was computed from the GEE model for the difference between the hepatotoxicity event rate in patients receiving pyronaridine-artesunate re-treatment minus the hepatotoxicity event rate after first treatment. Re-treatment was non-inferior to first treatment if the upper confidence limit was less than 5%, the predetermined non-inferiority margin.
Efficacy was assessed in patients with P falciparum malaria receiving at least one dose of study medication on first treatment (efficacy population) or re-treatment (efficacy re-treatment population). In the primary efficacy analysis for ACPR, done on an intention-to-treat basis, at day 28 or 42, any failure or missing data equalled treatment failure. With a GEE model, overall efficacy across all malaria episodes was estimated: ACPR (success or failure) was the binary dependent variable, treatment group (pyronaridine-artesunate or artemether-lumefantrine) was the fixed effect, and patient was random effect. 95% CIs were calculated for ACPR from the GEE model.
A post-hoc PCR-adjusted ACPR efficacy evaluable analysis was done with the exclusion of all patients with a PCR-confirmed new P falciparum infection before the assessment day; missing data equalled treatment failure. Parasite clearance time was estimated with Kaplan-Meier analysis. Statistical analyses were done with SAS (version 9.3).
WANECAM is registered with PACTR.org, number PACTR201105000286876.
Role of the funding source
Principal site investigators and authors from Medicines for Malaria Venture developed the protocol, oversaw the study, interpreted the data, and developed the report. All authors had access to primary data, accept responsibility for the accuracy and completeness of data reporting, and had final responsibility for the the decision to submit for publication.
Results
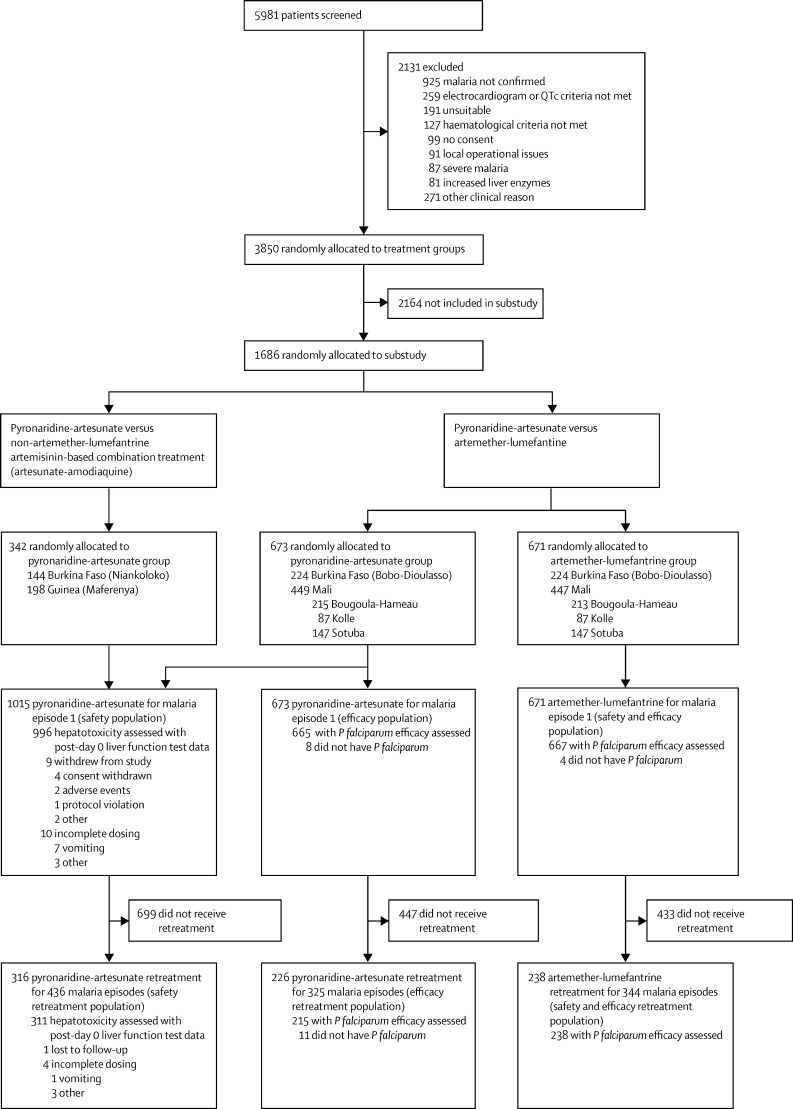
Figure 1 shows the trial profile and table 1 shows the baseline characteristics of the patients. There were no notable differences between patients treated with pyronaridine-artesunate for a first malaria episode versus those who received re-treatment. Also, patients who received pyronaridine-artesunate had similar baseline characteristics to those who received artemether-lumefantrine. Of 1015 patients treated with pyronaridine-artesunate, 316 (31%) were re-treated for 436 malaria episodes (re-treatment population; figure 1): 232 patients were re-treated once, 56 twice, 24 three times, three four times, and one patient eight times. Of 671 patients treated with artemether-lumefantrine, 238 (35%) were re-treated for 344 malaria episodes (figure 1): 157 patients were re-treated once, 61 twice, 16 three times, three four times, and one patient five times. P falciparum was detected in 2406 (99%) of 2439 episodes and 41 (2%) episodes were mixed parasite infections.
Figure 1.
Trial profile
Table 1.
Baseline characteristics of patients
|
Pyronaridine-artesunate safety analysis |
Comparative efficacy analysis |
||||
|---|---|---|---|---|---|
| Safety population* (n=1015) | Safety re-treatment population† (n=316) | Pyronaridine-artesunate (n=673) | Artemether-lumefantrine (n=671) | ||
| Sex, female | 509 (50%) | 149 (47%) | 328 (49%) | 319 (48%) | |
| Age (years) | |||||
| Mean (SD, range) | 10·1 (8·6, 0–62) | 10·3 (8·5, 0–56) | 11·8 (9·4, 1–62) | 11·7 (9·7, 0–69) | |
| <5 | 236 (23%) | 78 (25%) | 116 (17%) | 130 (19%) | |
| ≥5 to <18 | 666 (66%) | 201 (64%) | 453 (67%) | 443 (66%) | |
| ≥18 years | 113 (11%) | 37 (12%) | 104 (15%) | 98 (15%) | |
| Bodyweight (kg) | |||||
| Mean (SD, range) | 28·7 (16·6, 6·7–84·2) | 29·5 (17·7, 7·0–82·0) | 32·0 (17·7, 8·3–84·2) | 31·8 (18·2, 7·8–100·6) | |
| <20 | 393 (39%) | 128 (41%) | 213 (32%) | 233 (35%) | |
| ≥20 | 622 (61%) | 188 (59%) | 460 (68%) | 438 (65%) | |
| Geometric mean parasite count (first episode) per μL (IQR) | 36 092 (1650–51 220) | 38 905 (3010–52 080) | 38 865 (4500–53 040) | 40 918 (4740–56 160) | |
Data are number (%), unless otherwise indicated.
Included all patients who received at least one dose of pyronaridine-artesunate for treatment of a first malaria episode.
Included all patients who received at least one dose of pyronaridine-artesunate for the first malaria episode plus at least one dose of pyronaridine-artesunate for a subsequent malaria episode.
Liver function was assessed primarily through measurements of alanine aminotransferase, aspartate aminotransferase, and total bilirubin blood concentrations. Post-day 0 data for liver function tests were missing for 19 (2%) of 1015 patients in the pyronaridine-artesunate safety population and five (2%) of 316 patients in the safety re-treatment population (figure 1).
13 (1%) of 996 patients had hepatotoxicity events after first treatment with pyronaridine-artesunate versus two (1%) of 311 after re-treatment (table 2). One potential case of Hy's law occurred after first treatment compared with none after re-treatment (table 2).
Table 2.
Incidence of hepatotoxicity events at any post-dosing timepoint after treatment with pyronaridine-artesunate for a first malaria episode (episode 1) versus re-treatment (episode 2+)
|
All patients |
Age ≥6 months to <5 years |
Age ≥5years to <18 years |
Age ≥18 years |
|||||
|---|---|---|---|---|---|---|---|---|
| Episode 1 | Episode 2+ | Episode 1 | Episode 2+ | Episode 1 | Episode 2+ | Episode 1 | Episode 2+ | |
| Patients dosed | 1015 | 316 | 236 | 78 | 666 | 201 | 113 | 37 |
| Total number of post-day 0 liver function tests | 2894 | 1215 | 652 | 276 | 1922 | 789 | 320 | 150 |
| Number of hepatotoxicity events* | 16 (1%) | 2 (<1%) | 5 (1%) | 0 (0%) | 6 (<1%) | 1 (<1%) | 5 (2%) | 1 (1%) |
| Patients with any post day 0 liver function test | 996 | 311 | 224 | 78 | 660 | 197 | 112 | 36 |
| Patients with hepatotoxicity events | 13 (1%) | 2 (1%) | 3 (1%) | 0 | 6 (<1%) | 1 (1%) | 4 (4%) | 1 (3) |
| Potential Hy's law event† | 1 (<1%) | 0 (0%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Data are number or number (%).
Denominator for the calculation of the percentages was total number of post-day 0 liver function tests.
Denominator for the calculation of the percentages was the number of patients dosed.
After the first treatment with pyronaridine-artesunate, the incidence of hepatotoxicity events was higher in patients aged 18 years and older (four [4%] of 112) versus those younger than 18 years (nine [1%] of 884; table 2) and in patients with bodyweights of at least 20 kg (ten [2%] of 617) versus those with a bodyweight of less than 20 kg (three [1%] of 379). Three patients with hepatotoxicity events after first treatment were re-treated by mistake with pyronaridine-artesunate without recurrence of any hepatotoxicity event.
On re-treatment with pyronaridine-artesunate, two patients aged 10 years and 45 years with bodyweights of at least 20 kg had hepatotoxicity events after the second episode of malaria. One of these patients was re-treated with pyronaridine-artesunate by mistake without recurrence of any hepatotoxicity event.
Analysis of all available laboratory values with the GEE model provided an estimated treatment difference for the hepatotoxicity event rate of −0·36% (one-sided 95% CI 0·03) between re-treatment (0·19%) and first treatment (0·54%). Based on the post-treatment worse case per episode, the GEE estimate for the treatment difference in hepatotoxicity event rate was −0·84% (one-sided 95% CI 0·03) between re-treatment (0·47%) and first treatment (1·31%). In both analyses, confirmation of non-inferiority between re-treatment and first treatment suggested no increase in hepatotoxicity event rate on re-treatment with pyronaridine-artesunate versus first treatment.
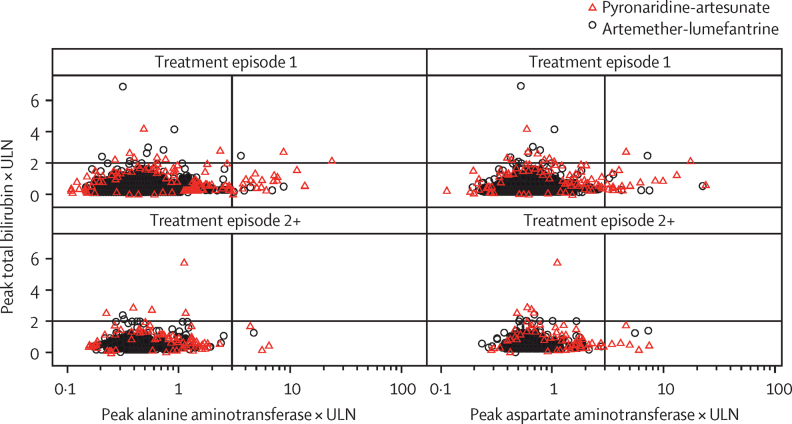
Scatter plots of peak concentrations of total bilirubin versus peak concentrations of alanine aminotransferase or aspartate aminotransferase confirmed that there were no greater increases in concentrations of liver enzymes on re-treatment with pyronaridine-artesunate versus first treatment (figure 2). All hepatotoxicity events resolved by day 28 without clinical sequelae (one patient withdrew consent before day 28).
Figure 2.
Peak bilirubin versus peak alanine aminotransferase or aspartate aminotransferase on or after day 3 following first treatment (episode 1) or re-treatment (episode 2+) with pyronaridine-artesunate or artemether-lumefantrine
ULN=upper limit of normal.
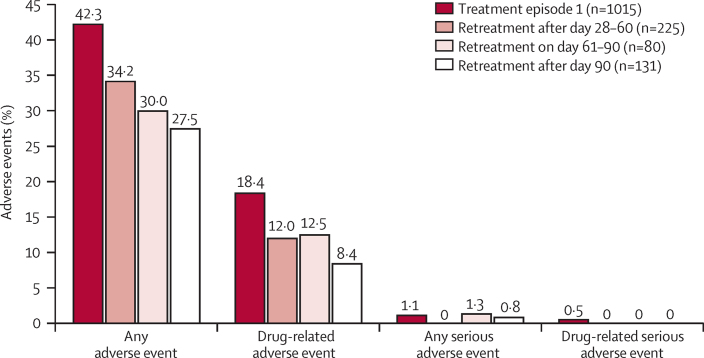
After the first treatment with pyronaridine-artesunate, adverse events from any cause occurred in 429 (42%) of 1015 patients versus 118 (37%) of 316 patients after re-treatment. 225 (52%) of 436 episodes were re-treated with pyronaridine-artesunate within 28–60 days after the first treatment, 80 (18%) of 436 were re-treated after 60–90 days, and 131 (30%) of 436 after 90 days (median 64 days, range 28–452). Within 28–60 days after first treatment, the incidence of adverse events tended to decrease on re-treatment (figure 3).
Figure 3.
Incidence of adverse events after pyronaridine-artesunate for first malaria episode and re-treatment of subsequent malaria episodes by time from first treatment to re-treatment
No trends were noted in the type or incidence of adverse events on first treatment with pyronaridine-artesunate versus re-treatment (table 3). 3% of 1015 patients had increases in aspartate aminotransferase concentrations and 3% had increases in alanine aminotransferase concentrations after first treatment, and 3% and 2% of 316, respectively, had increases after re-treatment (table 3). 187 (18%) of 1015 patients had drug-related adverse events on first treatment with pyronaridine-artesunate versus 45 (14%) of 316 after re-treatment.
Table 3.
Adverse events of interest from any cause after pyronaridine-artesunate treatment of a first malaria episode (episode 1) versus re-treatment (episode 2+)
|
Pyronaridine-artesunate group |
Artemether-lumefantrine group |
|||
|---|---|---|---|---|
| Episode 1 (n=1015) | Episode 2+ (n=316) | Episode 1 (n=671) | Episode 2+ (n=238) | |
| Any events | 429 (42%) | 118 (37%) | 303 (45%) | 96 (40%) |
| Aspartate aminotransferase increased | 29 (3%) | 10 (3%) | 10 (1%) | 1 (<1%) |
| Alanine aminotransferase increased | 28 (3%) | 5 (2%) | 7 (1%) | 2 (1%) |
| Hypercreatinaemia | 16 (2%) | 7 (2%) | 16 (2%) | 8 (3%) |
| Hyperbilirubinaemia | 2 (<1%) | 0 (0%) | 2 (<1%) | 2 (1%) |
| Drug-induced liver injury | 1 (<1%) | 0 (0%) | 1 (<1%) | 0 (0%) |
| Electrocardiogram QT prolonged | 28 (3%) | 10 (3%) | 43 (6%) | 7 (3%) |
| Abdominal pain | 34 (3%) | 3 (1%) | 16 (2%) | 0 (0%) |
| Vomiting | 33 (3%) | 4 (1%) | 10 (1%) | 3 (1%) |
| Diarrhoea | 10 (1%) | 0 (0%) | 2 (<1%) | 0 (0%) |
| Neutropenia | 34 (3%) | 7 (2%) | 25 (4%) | 12 (5%) |
| Anaemia | 22 (2%) | 3 (1%) | 24 (4%) | 2 (1%) |
| Monocytosis | 13 (1%) | 3 (1%) | 12 (2%) | 8 (3%) |
| Thrombocytopenia | 8 (1%) | 4 (1%) | 5 (1%) | 0 (0%) |
Data are number (%). Data for artemether-lumefantrine are shown for reference.
After the first treatment with pyronaridine-artesunate, 11 (1%) of 1015 patients had at least one serious adverse event versus two (1%) of 316 on re-treatment. With the exception of an unrelated death from trauma, all adverse events resolved without further sequelae. Five patients had serious adverse events, all on first treatment, that were thought to be drug related: anaemia and malaria in a 3-year-old girl; alanine aminotransferase 852 IU/L and aspartate aminotransferase 298 IU/L at day 7 in a 2-year-old girl; alanine aminotransferase 339 IU/L and aspartate aminotransferase 417 IU/L at day 35 in a 3-year-old boy; aspartate aminotransferase 196 IU/L, alanine aminotransferase 379 IU/L, and bilirubin 38 μmol/L, which the data safety monitoring board discounted as a Hy's law case because of the high baseline bilirubin concentration (46 μmol/L), in a 31-year old woman; and peak alanine aminotransferase 1229 IU/L, aspartate aminotransferase 890 IU/L, and bilirubin 41 μmol/L on day 7, alkaline phosphatase 571 IU/L on day 7, which peaked at 901 IU/L on day 14, in a 2-year-old girl. The data safety monitoring board concluded that this event was an acute hepatocellular liver injury (ie, potential Hy's law case), possibly exacerbated by concomitant metamizole administration, and was followed by substantial improvement.
The safety profile of artemether-lumefantrine was as expected (table 3). After first treatment, 45% of 671 patients had adverse events (table 3), and 127 (19%) were thought to be drug related. Five (1%) of 671 patients had serious adverse events and one adverse event was thought to be drug related (toxic epidermal necrolysis). One patient had a serious adverse event (liver injury: alanine aminotransferase 298 IU/L, aspartate aminotransferase 195 IU/L, and bilirubin 48 μmol/L—ie, potential Hy's law event), but was not thought to be drug related because it occurred on day 56.
Two patients died during the study, but neither death was thought to be drug related: road traffic accident (in the pyronaridine-artesunate group), and pneumopathy and immunosuppression caused by HIV infection (in the artemether-lumefantrine group).
Risk of Fridericia-corrected QTc (QTcF) prolongation was not increased with repeated pyronaridine-artesunate treatment (table 4). Four patients had QTcF prolongation of at least 30 ms for two treatment episodes (two consecutive and two non-consecutive). These male patients were aged 16–21 years with QTcF prolonged by 35–86 ms versus baseline, but values did not exceed 450 ms (396–437).
Table 4.
Categorical QT values corrected with Fridericia's formula after pyronaridine-artesunate treatment of a first malaria episode (episode 1) versus re-treatment (episode 2+)
|
Pyronaridine-artesunate |
Artemether-lumefantrine |
|||
|---|---|---|---|---|
| Episode 1 (n=396) | Episode 2+ (n=126) | Episode 1 (n=192) | Episode 2+ (n=71) | |
| Highest post-dose increase versus day 0 | ||||
| ≤0 ms | 129 (33%) | 30 (24%) | 30 (16%) | 10 (14%) |
| >0–<30 ms | 174 (44%) | 65 (52%) | 90 (47%) | 30 (42%) |
| 30–60 ms | 45 (11%) | 17 (13%) | 43 (22%) | 17 (24%) |
| >60 ms | 5 (1%) | 2 (2%) | 3 (2%) | 3 (4%) |
| Data missing | 43 (11%) | 12 (10%) | 26 (14%) | 11 (15%) |
| Any post-dose value | ||||
| >450 ms | 0 (0%) | 2 (2%) | 0 (0%) | 2 (3%) |
| >480 ms | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| >500 ms | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Data are number (%). Numbers represent patients who were assessable. Data for artemether-lumefantrine are shown for reference.
Risk of QTcF on repeated treatment with artemether-lumefantrine was not increased versus first treatment (table 4). Four patients had QTcF prolongation of at least 30 ms for two treatment episodes (all consecutive episodes 1 and 2). Two patients were boys, aged 9 years and 15 years, and two were girls aged 2 years and 10 years, with QTcF prolonged by 33–70 ms versus baseline, but values did not exceed 450 ms (390–446) .
In a conservative intention-to-treat efficacy analysis of patients with P falciparum infection using a GEE model, across all treatment and re-treatment episodes, day-28 crude ACPR was 92·7% (95% CI 91·0–94·3) with pyronaridine-artesunate and 80·4% (77·8–83·0) with artemether-lumefantrine (treatment difference 12·3%, 95% CI 9·1–15·4; p<0·0001). Day 42 ACPR was 77·3% (74·5–80·1) versus 65·4% (62·2–68·5), respectively (12·0%, 7·7–16·2; p<0·0001; appendix). After exclusion of patients with PCR-confirmed new infections, ACPR was similar between pyronaridine-artesunate and artemether-lumefantrine treatments and greater than 95% at day 28 and greater than 91% at day 42 in both treatment groups (appendix).
Median parasite clearance time with pyronaridine-artesunate was shorter for the first treatment episode (24·8 h, 95%CI 24·3–33·9) than with artemether-lumefantrine (34·5 h, 34·2–35·2), but similar for subsequent episodes (appendix).
Discussion
The results of the primary safety analysis for this substudy confirmed that the risk of a hepatotoxicity event was not greater after pyronaridine-artesunate re-treatment versus first treatment (0·19% vs 0·54%). The safety profiles of pyronaridine-artesunate and artemether-lumefantrine in this study were consistent with those in previous comparative phase 2 and 3 trials.3, 4, 5, 6, 7, 8 In previous trials, transient increases in transaminases were noted after one course of pyronaridine-artesunate in some patients.
In our study, laboratory defined hepatotoxicity events occurred in some patients, but without clinical manifestations and the outcomes were favourable. There was no indication of any increased risk of hepatoxicity on re-treatment of patients with bodyweights of less than 20 kg versus those 20 kg or greater or for those younger than 18 years versus older patients. Although hepatotoxicity events were more common in patients older than 18 years, three of four hepatic-related serious drug-related adverse events were in children up to 3 years of age.
Pyronaridine-artesunate re-treatment did not increase overall safety risk based on adverse event frequencies, laboratory values, or electrocardiograph findings. The half-life of pyronaridine is about 14 days so the period between exposures could be affected by residual blood concentrations (five half-lives equal to 60 days). However, there was no evidence of increased risk according to time between treatments. Thus, because more than 50% of patients were re-treated with pyronaridine-artesunate within 28–60 days of the first treatment, the safety findings lend support for re-treatment of patients at least 28 days after previous exposure.
Efficacy was assessed in an intention-to-treat analysis and outcomes were consistent with this conservative approach. For crude ACPR, overall efficacy was higher with pyronaridine-artesunate than with artemether-lumefantrine treatment. However, after exclusion of patients with new infections, the results of PCR-adjusted ACPR were consistent with the findings from two previous phase 3 trials, showing similar efficacy for the two agents.3, 6 Thus, the difference in crude ACPR resulted from a higher re-infection rate in the artemether-lumefantrine group than in the pyronaridine-artesunate group and might be explained by the longer elimination half-life of pyronaridine (14 days) than of lumefantrine (3–4 days).17 Notably, both ACTs met the WHO criteria of greater than 95% PCR-adjusted ACPR at day 28.18
As with most clinical trials, patients were excluded on the basis of criteria that were applicable to real-life settings—ie, pre-existing increased liver function tests or hepatitis. Thus, additional studies might be needed to obtain comprehensive safety information in these populations. Also, patients who had hepatotoxicity events on first treatment with pyronaridine-artesunate were to be excluded from subsequent treatment. Four patients who had a hepatotoxicity event after pyronaridine-artesunate were re-treated with the same drug by mistake and none had another hepatotoxicity event or any hepatic related adverse event. These errors occurred because of absence of any clinical symptoms and delay in drawing attention to the abnormal laboratory values. In the 15 patients who had hepatotoxicity events with pyronaridine-artesunate, although a panel of hepatic assessments should have been initiated, hepatitis could only be ruled out as a possible cause of increased liver enzymes in five patients, and two of these had evidence of previous Epstein-Barr virus infection (data not shown).
Children are at highest risk of severe malaria. Although 236 (23%) of 1015 patients treated with pyronaridine-artesunate were younger than 5 years and more than a third were less than 20 kg in bodyweight, only 13 children were younger than 1 year. Further data are therefore needed about the safety of pyronaridine-artesunate in infants. Many more infants were unlikely to have been recruited into the WANECAM trial after the cutoff date for inclusion in this substudy, and further studies might be warranted.
This substudy was not designed to assess efficacy and the final results from the WANECAM trial should be awaited before drawing conclusions about the relative performance of the two comparator treatments. Based on local practices, artemether-lumefantrine was administered without specifically providing fatty intake and data on food consumption before dosing was not gathered. However, only a small amount of dietary fat is necessary to ensure optimum efficacy of artemether-lumefantrine and the fat content of breast milk or standard meals in sub-Saharan Africa is adequate.19
The similar safety and efficacy of pyronaridine-artesunate on first treatment of malaria versus re-treatment of subsequent malaria episodes lend support to wider access to pyronaridine-artesunate as an alternative ACT for the treatment of malaria in sub-Saharan Africa.
Acknowledgments
Acknowledgments
The WANECAM study is mainly funded by the European and Developing Countries Clinical Trial Partnership, and co-funded by Medicines for Malaria Venture (MMV; Geneva, Switzerland), UK Medical Research Council, Swedish International Development Cooperation Agency, German Ministry for Education and Research, University Claude Bernard (Lyon, France), Malaria Research and Training Centre (Bamako, Mali), Centre National de Recherche et de Formation sur le Paludisme (Burkina Faso), Institut de Recherche en Sciences de la Santé (Bobo-Dioulasso, Burkina Faso), and Centre National de Formation et de Recherche en Santé Rurale (Guinea). Pyronaridine-artesunate is being developed in a public–private partnership between Shin Poong Pharmaceutical Company (Seoul, South Korea) and MMV. Medicines for Malaria Venture provided funding to Naomi Richardson of Magenta Communications (Abingdon, UK) to develop a draft paper from statistical outputs under author guidance and to coordinate author contributions. We thank Sarah Arbe-Barnes (Aptiv Solutions, Stevenage, UK), Ghiorghis Belai (FHI 360, Nairobi, Kenya), Iwona Oborska (consultant to MMV; Horsham, UK), and Martina Wibberg (DATAMAP, Freiburg, Germany); and the patients and their parents or legal guardians, the field study teams, and health-care authorities.
Contributors
ISa, AHB, IZ, ISo, FN, AD, J-BO, and SBS made substantial contributions to the concept and design of the study and oversaw data acquisition in the field. BF, DC, AFS, ASC, OBT, ND, MJTK, YDC, MMS, and MSD were involved in the acquisition of data. IT was involved with data management. IB-F, JPG, SB, SD, RMM, OKD, JS, and AB made substantial contributions to the concept and design of the study. AAD made substantial contributions to the concept and design of the study, oversaw data acquisition in the field, coordinated the writing of the manuscript, and submitted the final draft. All authors critically reviewed the paper and approved the final version of the paper for submission.
Declaration of interests
ISa reports grants and non-financial support from MMV and European and Developing Countries Clinical Trial Partnership (EDCTP), and funding from Université des Sciences, des Techniques et des Technologies de Bamako during the conduct of the trial. AHB, IZ, ISo, BF, DC, AFS, ASC, OBT, ND, MJTK, IT, YDC, MMS, FN, MSD, AD, OKD, AB, J-BO, SBS declare grants for the study from MMV and EDCTP. IB-F and SD are employees of MMV. RMM reports fees from Shin Poong Pharmaceutical Company during the conduct of the study and has represented the safety interest for the company as their qualified person for pharmacovigilance, and has been involved with all aspects of the safety interpretation of the data for pyronaridine-artesunate used as the fixed combination. JS reports personal fees from Shin Poong Pharmaceutical Company outside the submitted work. AAD declares grants from the study funded by MMV and EDCTP and personal fees from Novartis outside the submitted work. The other authors declare no competing interests.
Supplementary Material
References
- 1.Croft SL, Duparc S, Arbe-Barnes SJ. Review of pyronaridine anti-malarial properties and product characteristics. Malar J. 2012;11:270. doi: 10.1186/1475-2875-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee for Medicinal Products for Human Use (CHMP) Summary of opinion: Pyramax (pyronaridine tetraphosphate/artesunate) 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/02/WC500122945.pdf (accessed Sept 8, 2014).
- 3.Kayentao K, Doumbo OK, Penali LK. Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malar J. 2012;11:364. doi: 10.1186/1475-2875-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poravuth Y, Socheat D, Rueangweerayut R. Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial. PLoS One. 2011;6:e14501. doi: 10.1371/journal.pone.0014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rueangweerayut R, Phyo AP, Uthaisin C. Pyronaridine-artesunate versus mefloquine plus artesunate for malaria. N Engl J Med. 2012;366:1298–1309. doi: 10.1056/NEJMoa1007125. [DOI] [PubMed] [Google Scholar]
- 6.Tshefu AK, Gaye O, Kayentao K, the Pyronaridine-artesunate Study Team Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet. 2010;375:1457–1467. doi: 10.1016/S0140-6736(10)60322-4. [DOI] [PubMed] [Google Scholar]
- 7.Duparc S, Borghini-Fuhrer I, Craft CJ. Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malar J. 2013;12:70. doi: 10.1186/1475-2875-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramharter M, Kurth F, Schreier AC. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J Infect Dis. 2008;198:911–919. doi: 10.1086/591096. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Center for biologics Evaluation and Research (CBER) Guidance for Industry. Drug-induced Liver Injury: premarketing Clinical Evaluation. 2009. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/Guidances/UCM174090.pdf (accessed April 5, 2014).
- 10.Yeka A, Lameyre V, Afizi K. Efficacy and safety of fixed-dose artesunate-amodiaquine vs. artemether-lumefantrine for repeated treatment of uncomplicated malaria in Ugandan children. PLoS One. 2014;9:e113311. doi: 10.1371/journal.pone.0113311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagara I, Fofana B, Gaudart J. Repeated artemisinin-based combination therapies in a malaria hyperendemic area of Mali: efficacy, safety, and public health impact. Am J Trop Med Hyg. 2012;87:50–56. doi: 10.4269/ajtmh.2012.11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndiaye JL, Faye B, Gueye A. Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial. Malar J. 2011;10:237. doi: 10.1186/1475-2875-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. 2003. http://www.who.int/malaria/publications/atoz/whohtmrbm200350/en/ (accessed March 10, 2014).
- 14.Ranford-Cartwright LC, Taylor J, Umasunthar T. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans R Soc Trop Med Hyg. 1997;91:719–724. doi: 10.1016/s0035-9203(97)90539-3. [DOI] [PubMed] [Google Scholar]
- 15.Sagara I, Dicko A, Djimde A. A randomized trial of artesunate-sulfamethoxypyrazine-pyrimethamine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg. 2006;75:630–636. [PubMed] [Google Scholar]
- 16.Snounou G, Viriyakosol S, Zhu XP. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 17.Djimde A, Lefevre G. Understanding the pharmacokinetics of Coartem. Malar J. 2009;8(suppl 1):S4. doi: 10.1186/1475-2875-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Methods of surveillance of antimalarial drug efficacy. 2009. http://www.who.int/malaria/publications/atoz/9789241597531/en/ (accessed April 24, 2015).
- 19.Premji ZG, Abdulla S, Ogutu B. The content of African diets is adequate to achieve optimal efficacy with fixed-dose artemether-lumefantrine: a review of the evidence. Malar J. 2008;7:244. doi: 10.1186/1475-2875-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.