Abstract
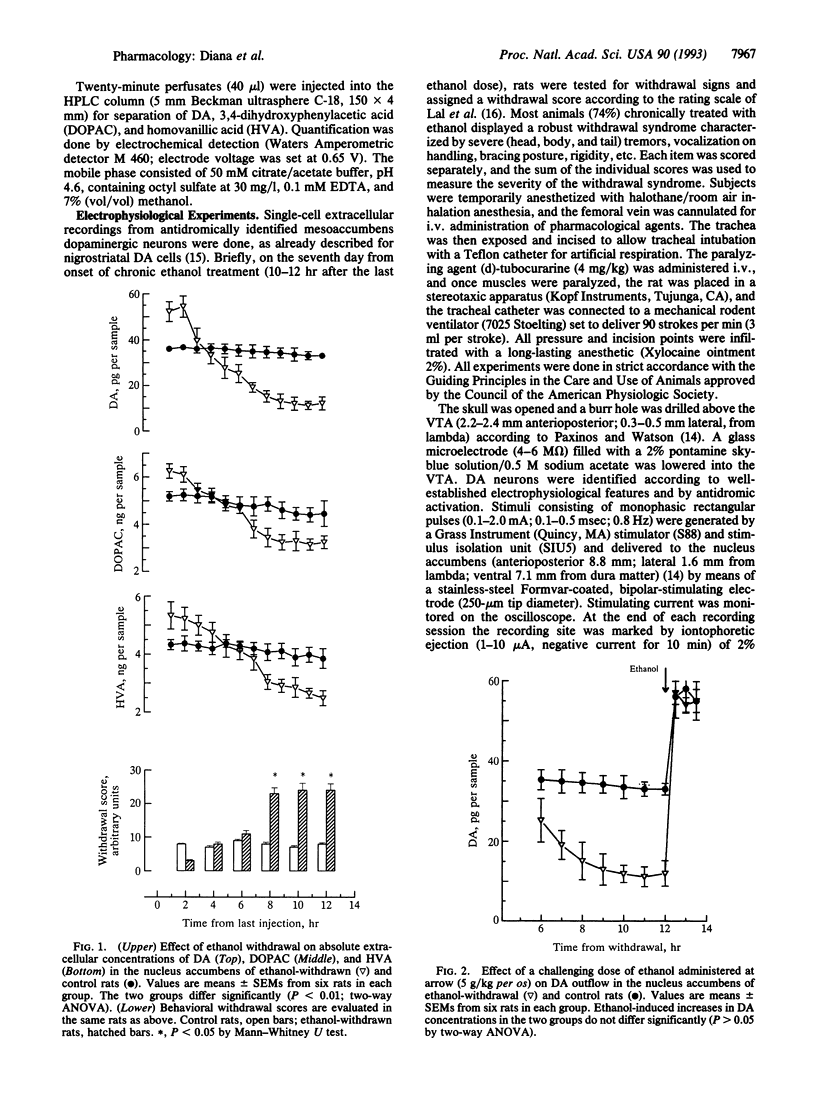
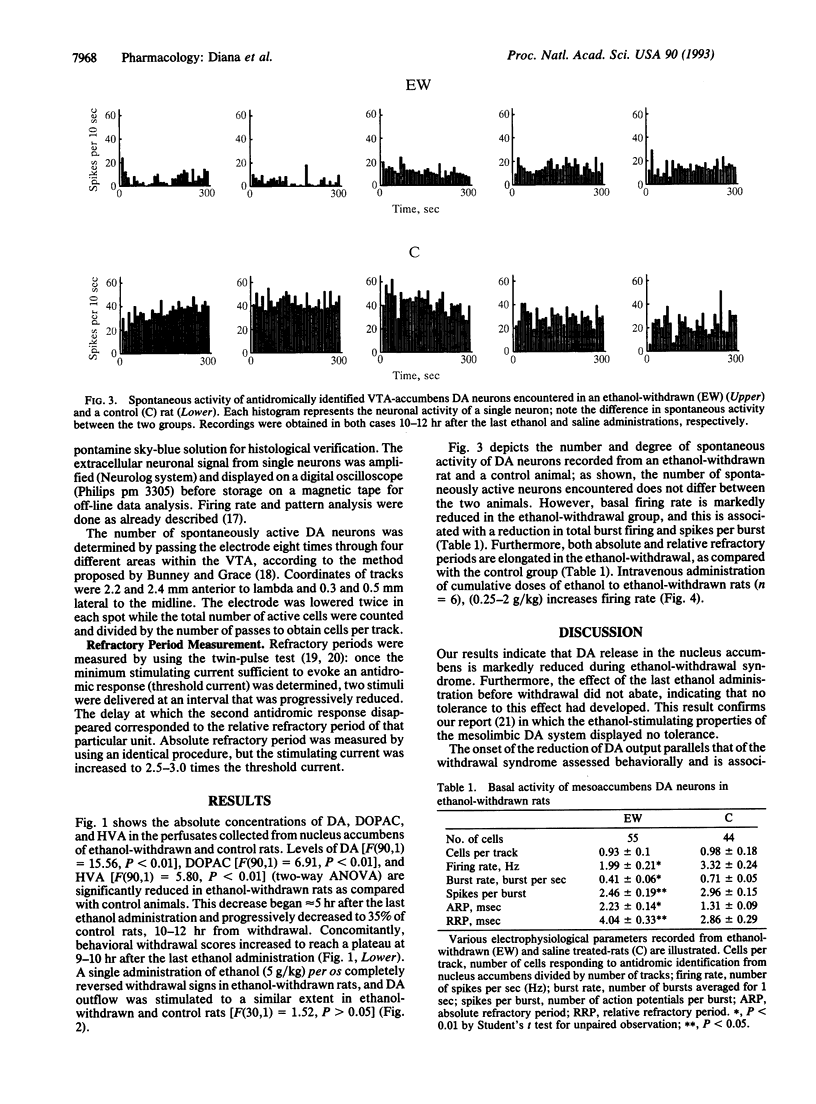
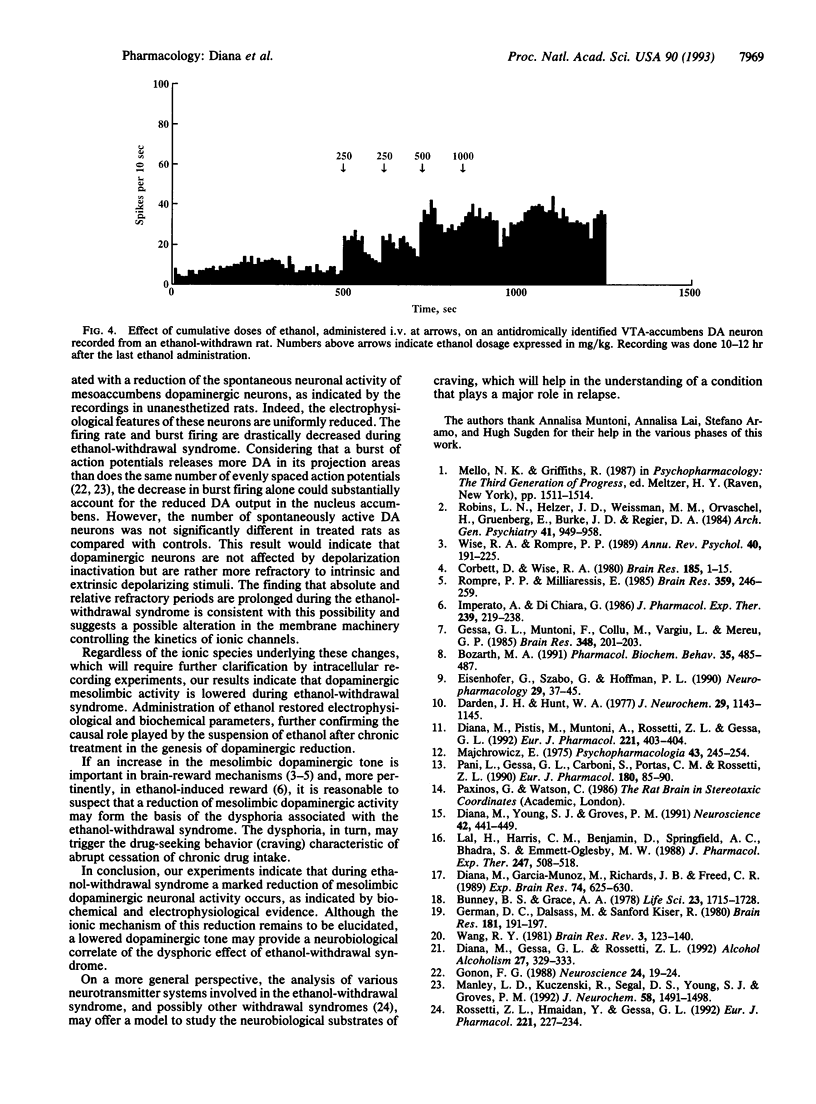
Activity of the mesolimbic dopaminergic system was investigated in rats withdrawn from chronic ethanol administration by single-cell extracellular recordings from dopaminergic neurons of the ventrotegmental area, coupled with antidromic identification from the nucleus accumbens, and by microdialysis-technique experiments in the nucleus accumbens. Spontaneous firing rates, spikes per burst, and absolute burst firing but not the number of spontaneously active neurons were found drastically reduced; whereas absolute and relative refractory periods increased in rats withdrawn from chronic ethanol treatment as compared with chronic saline-treated controls. Consistently, dopamine outflow in the nucleus accumbens and its acid metabolites were reduced after abruptly stopping chronic ethanol administration. All these changes, as well as ethanol-withdrawal behavioral signs, were reversed by ethanol administration. This reversal suggests that the abrupt cessation of chronic ethanol administration plays a causal role in the reduction of mesolimbic dopaminergic activity seen in the ethanol-withdrawal syndrome. Results indicate that during the ethanol-withdrawal syndrome the mesolimbic dopaminergic system is tonically reduced in activity, as indexed by electrophysiological and biochemical criteria. Considering the role of the mesolimbic dopaminergic system in the reinforcing properties of ethanol, the depressed activity of this system during the ethanol-withdrawal syndrome may be relevant to the dysphoric state associated with ethanol withdrawal in humans.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozarth M. A. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacol Biochem Behav. 1990 Feb;35(2):485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- Bunney B. S., Grace A. A. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978 Oct 23;23(16):1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Corbett D., Wise R. A. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: a moveable electrode mapping study. Brain Res. 1980 Mar 3;185(1):1–15. doi: 10.1016/0006-8993(80)90666-6. [DOI] [PubMed] [Google Scholar]
- Darden J. H., Hunt W. A. Reduction of striatal dopamine release during an ethanol withdrawal syndrome. J Neurochem. 1977 Dec;29(6):1143–1145. doi: 10.1111/j.1471-4159.1977.tb06522.x. [DOI] [PubMed] [Google Scholar]
- Diana M., Garcia-Munoz M., Richards J., Freed C. R. Electrophysiological analysis of dopamine cells from the substantia nigra pars compacta of circling rats. Exp Brain Res. 1989;74(3):625–630. doi: 10.1007/BF00247365. [DOI] [PubMed] [Google Scholar]
- Diana M., Gessa G. L., Rossetti Z. L. Lack of tolerance to ethanol-induced stimulation of mesolimbic dopamine system. Alcohol Alcohol. 1992 Jul;27(4):329–333. [PubMed] [Google Scholar]
- Diana M., Pistis M., Muntoni A., Rossetti Z. L., Gessa G. Marked decrease of A10 dopamine neuronal firing during ethanol withdrawal syndrome in rats. Eur J Pharmacol. 1992 Oct 20;221(2-3):403–404. doi: 10.1016/0014-2999(92)90734-l. [DOI] [PubMed] [Google Scholar]
- Diana M., Young S. J., Groves P. M. Modulation of dopaminergic terminal excitability by D1 selective agents: further characterization. Neuroscience. 1991;42(2):441–449. doi: 10.1016/0306-4522(91)90387-4. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G., Szabo G., Hoffman P. L. Opposite changes in turnover of noradrenaline and dopamine in the CNS of ethanol-dependent mice. Neuropharmacology. 1990 Jan;29(1):37–45. doi: 10.1016/0028-3908(90)90081-2. [DOI] [PubMed] [Google Scholar]
- German D. C., Dalsass M., Kiser R. S. Electrophysiological examination of the ventral tegmental (A10) area in the rat. Brain Res. 1980 Jan 6;181(1):191–197. doi: 10.1016/0006-8993(80)91269-x. [DOI] [PubMed] [Google Scholar]
- Gessa G. L., Muntoni F., Collu M., Vargiu L., Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985 Nov 25;348(1):201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gonon F. G. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988 Jan;24(1):19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Imperato A., Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986 Oct;239(1):219–228. [PubMed] [Google Scholar]
- Lal H., Harris C. M., Benjamin D., Springfield A. C., Bhadra S., Emmett-Oglesby M. W. Characterization of a pentylenetetrazol-like interoceptive stimulus produced by ethanol withdrawal. J Pharmacol Exp Ther. 1988 Nov;247(2):508–518. [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975 Sep 17;43(3):245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Manley L. D., Kuczenski R., Segal D. S., Young S. J., Groves P. M. Effects of frequency and pattern of medial forebrain bundle stimulation on caudate dialysate dopamine and serotonin. J Neurochem. 1992 Apr;58(4):1491–1498. doi: 10.1111/j.1471-4159.1992.tb11369.x. [DOI] [PubMed] [Google Scholar]
- Pani L., Gessa G. L., Carboni S., Portas C. M., Rossetti Z. L. Brain dialysis and dopamine: does the extracellular concentration of dopamine reflect synaptic release? Eur J Pharmacol. 1990 May 3;180(1):85–90. doi: 10.1016/0014-2999(90)90595-w. [DOI] [PubMed] [Google Scholar]
- Robins L. N., Helzer J. E., Weissman M. M., Orvaschel H., Gruenberg E., Burke J. D., Jr, Regier D. A. Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatry. 1984 Oct;41(10):949–958. doi: 10.1001/archpsyc.1984.01790210031005. [DOI] [PubMed] [Google Scholar]
- Rompre P. P., Miliaressis E. Pontine and mesencephalic substrates of self-stimulation. Brain Res. 1985 Dec 16;359(1-2):246–259. doi: 10.1016/0006-8993(85)91435-0. [DOI] [PubMed] [Google Scholar]
- Rossetti Z. L., Hmaidan Y., Gessa G. L. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992 Oct 20;221(2-3):227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Wise R. A., Rompre P. P. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]



