Abstract
Background
In the past years many inflammatory markers have been studied in association with clinically manifest cardiovascular disease (CVD) and carotid intima-media thickness (CIMT) in HIV-infected patients, to obtain insights in the increased cardiovascular risk observed in HIV infection. This systematic review provides an oversight of the current knowledge.
Methods
A search was performed in PubMed, Embase and Cochrane in July 2014, identifying all articles from 1996 onwards addressing the relation between inflammatory markers and CVD or CIMT in HIV-positive adults. Two authors, using predefined criteria, independently conducted the selection of articles, critical appraisal and extraction of the data. Analysis was focused on the immune markers that were most frequently assessed. The review protocol was registered in the PROSPERO database at 11 July 2014 (registration number CRD42014010516). This review was performed according to the PRISMA guideline.
Findings
Forty articles were selected; eight addressing cardiovascular disease (CVD) and thirty-two addressing CIMT. C-reactive protein (CRP), interleukin-6 (IL-6) and d-dimer were assessed most frequently in relation to the occurrence of CVD; in four out of eight studies. All three markers were positively related to CVD in three out of four studies. Studies addressing CIMT were too heterogeneous with respect to patient populations, inflammatory markers, CIMT measurement protocols and statistical methods to allow for a formal meta-analysis to obtain summary statistics. CRP, IL-6 and soluble vascular cell adhesion molecule (sVCAM-1) were the most studied markers in relation to CIMT. None of the inflammatory markers showed an association with CIMT.
Interpretation
This review showed a relation between some inflammatory markers and CVD, however, no consistent relation is observed for CIMT. Statistical approaches that yields effect estimates and standardized CIMT protocols should be chosen. Further research should focus on prospective studies and a selected set of inflammatory markers.
Introduction
When the human immunodeficiency virus (HIV) was discovered in the 1980’s, the infection was believed to be immunosuppressive. This view changed in the 1990’s, when evidence became available supporting the presence of chronic inflammation rather than primary immunodeficiency.[1]
With the initiation of antiretroviral therapy, mortality patterns in HIV patients changed from AIDS related opportunistic infections and malignancies to cancers not related to AIDS and cardiovascular disease (CVD).[2] Nearly ten years after the introduction of highly active antiretroviral therapy (HAART) non-AIDS defining illnesses were considered to be responsible for almost 50% of deaths in HIV-positive cohorts in North America; seven to 19% of all deaths were attributed to CVD.[3–5]
Chronic immune activation has a pivotal role in the pathogenesis of atherosclerosis in non-HIV infected patients.[6,7] Moreover, a range of studies has reported an association between immune activation and accelerated atherosclerosis in patients who are HIV-infected.[8–10]
The role of immune markers in relation to CVD risk in HIV-positive patients has not been clarified. Evaluating available data concerning the relation between pro-inflammatory parameters and CVD remains difficult if only because of differences in study design and the availability of various immune markers. Moreover, outcome measures vary from clinical relevant outcomes, like the occurrence of myocardial infarction or cardiac death, to surrogate markers of CVD: notably carotid intima-media thickness (CIMT) and markers of arterial stiffness.
The aim of the current review is to summarize the data on the association of pro-inflammatory markers with CVD, including their prognostic value, in HIV-infected patients.
Methods
Search strategy
The review protocol was registered in the PROSPERO database at 11 July 2014 (registration number CRD42014010516). A systematic literature search was conducted in PubMed, EMBASE and Cochrane library (Table 1). Words and synonyms related to the domain, determinant and outcome were used. The domain were HIV-infected adults. As determinant, plasma or serum immune markers were included. We excluded cellular blood components (i.e. lymphocyte subsets) and genetic markers. Symptomatical cardiovascular disease or surrogate markers for cardiovascular disease (i.e. CIMT, ankle brachial index) were considered as outcomes (S1 Table). Search terms were limited to title and abstract.
Table 1. Search strategy.
| Search terms | Pubmed (Medline) [title/abstract] | EMBASE [title/abstract] | Cochrane [title/abstract] | ||
|---|---|---|---|---|---|
| #1 domain | HIV positive patients | HIV | |||
| human immunodeficiency virus | |||||
| human immuno deficiency virus | |||||
| human immunedeficiency virus | |||||
| human immune deficiency virus | |||||
| aids | |||||
| acquired immunodeficiency syndrome | |||||
| acquired immuno deficiency syndrome | |||||
| acquired immunedeficiency syndrome | |||||
| acquired immune deficiency syndrome | |||||
| AND | 309067 | 358649 | 16040 | ||
| #2 determinant | Pro-inflammatory markers | Inflammatory | |||
| Inflammation | |||||
| Inflamm* | |||||
| Biomarker | |||||
| Biomarkers | |||||
| Immune* | |||||
| AND | 985859 | 1262812 | 34151 | ||
| #3 Outcome | Cardiovascular disease or surrogate markers of cardiovascular disease. | cardiovascular | |||
| CVD | |||||
| Myocardial infarction | |||||
| Mi | |||||
| Coronary heart disease | |||||
| CHD | |||||
| Stroke | |||||
| Carotid intima-media thickness | |||||
| CIMT | |||||
| Arterial stiffness | |||||
| Flow mediated dilation | |||||
| FMD | |||||
| PWV | |||||
| Pulse Wave Velocity | |||||
| Coronoary artery calci* | |||||
| CAC | |||||
| Ankle brachial index | |||||
| ABI | |||||
| 570481 | 476332 | 57183 | |||
| Final number of studies by combining #1 AND #2 AND #3 | 821 | 246 | 70 | ||
| Search date for all databases July 2, 2014 | EMBASE (AND [embase]/lim NOT [medline]/lim) | 1 cochrane review, 68 trials, 1 methods study | |||
Duplicates were removed by using reference management software, and further checked manually. The review was conducted in accordance to the PRISMA and STROME-ID guidelines.[11,12]
Study selection
Study selection was done in three steps (Fig 1). First, all identified records were screened based on titles and abstracts by one author (AV). Second, full text reports of all abstracts were independently read to assess eligibility by two authors (AV, NI), using preset inclusion criteria. Third, references and citations of the selected articles were screened for additional articles. Discrepancies were discussed in a consensus meeting by two authors (AV, NI).
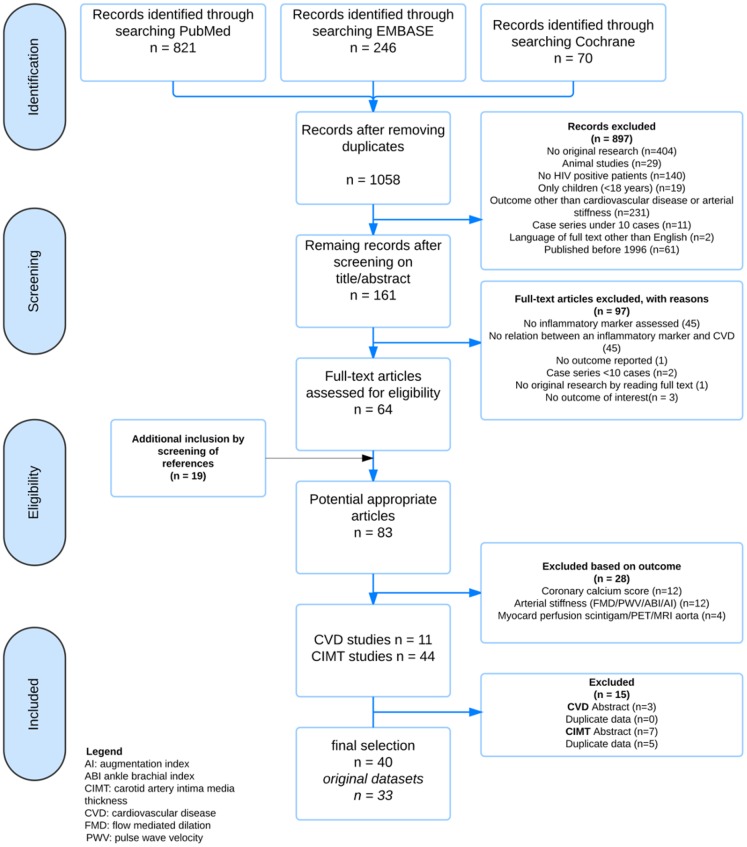
Fig 1. Flowchart inclusion.
AI: augmentation index, ABI: ankle brachial index, CIMT: carotid intima media thickness, CVD: cardiovascular disease, FMD: flow mediated dilation, PWV: pulse wave velocity.
Agreement could be reached for all but one article as there were different opinions on the question whether there was a relation between the immune marker and outcome, or not. After consulting of a third reviewer (KK), the article was excluded. If the same group of patients was described in more than one article the most detailed report was included. If the reports were complementary both were included and data were combined. For studies describing a group of HIV-positive and HIV-negative individuals, only findings of HIV-positive participants were used.
Validity and data extraction
The following data were extracted: year of publication, study design, follow-up duration, number of patients, country, setting, age, sex, years since HIV diagnosis, CD4 level, viral load, ART use and duration, classic cardiovascular risk factors, inflammatory parameters measured, outcomes and outcome measurement methods. ‘In case a database was described in more than one study, baseline characteristics of the most comprehensive article were used.’
Selected studies were critically appraised, particularly for the risk of selection-, detection-, and attrition bias. Bias risk was assigned as likely, unlikely, or unknown. The first author (AV) conducted the data extraction and critical appraisal using a set format. The second author (NI) independently checked all extracted data.
Analysis
As studies were expected to be very heterogeneous, results are descriptive, grouped by outcome and, inflammatory marker. When possible, percentages of common baseline characteristics were calculated. Due to heterogeneity of the data it was impossible to present effect estimates in a clear overview. The only common estimate per study was a p-value; therefore p-values were presented in a figure, stratified by method of analysis and accompanied by the sample size. All p-values of 0.25 or higher were considered to express minimal association. When only ‘no significant’ was reported, the p-value in the figure was also set at 0.25, when a p-value of <0.05 was reported, a value of 0.03 was displayed in the figure. Outcome data did not allow calculations of summary statistics or prognostic value. A correlation was considered relevant if the Rho value was 0.4 or higher. Relevant correlations were depicted with a circle in the figure. The three most commonly studied inflammatory markers were analyzed separately. Besides the top-three-studied immune markers, findings of the remaining markers assessed at least thrice were summarized in a table. Differences in CIMT measurement protocols were not taken into account. In this review C-reactive protein (CRP) refers to both the regular CRP measurement as to the high-sensitive CRP assays.
Results
1058 studies were identified, 64 articles remained after screening (Fig 1). Screening of references yielded another 19 articles, which did not mention immune marker measurement (mainly CRP) in title or abstract. Agreement for inclusion of articles by the two authors (AV, NI) was over 99%.
Due to incomplete information abstracts were excluded (CVD 3 abstracts, CIMT 7 abstracts), in deviation of the initial review protocol. Two studies addressing CVD used the SMART cohort data; both were included in the final analysis since they presented additional information.[9,13] Six populations studied for CIMT were described in more than one article.[14–29] Studies containing additional information remained in the final analysis,[17,19–25,29,30], studies presenting duplicate data were excluded.[14,16,27,28,31] Finally 40 articles remained (8 assessing CVD, 32 assessing CIMT) [9,10,13,15,17–25,29,30,32–56], including 33 original datasets, describing 48 immune markers.
Baseline characteristics
Almost all studies addressing CVD had a case-control design (S1 Table). The number of cases ranged from 35 to 487 cases [51,56] The majority of patients were men, aged around 47 years. The most frequently assessed markers were CRP, IL-6 and d-dimer; all were assessed in five out of eight studies.
The vast majority of studies addressing CIMT were cross-sectional. Only six out of 32 CIMT studies had a prospective design. The average number of HIV-positive patients per study was 155 (median 129), 80% of which was male. The median age was 46 years and median duration since HIV diagnosis was 9.3 years (interquartile range (IQR) 6·3–13·0). 12 studies had ART coverage of 100%[18,33,37–40,43–45,48–50,57] and three datasets described only ART naïve patients.[19,20,36,47] Average ART coverage in the other studies was 71%, and ART duration was five years (mean and median). Twelve studies were performed in the USA, nine in Europe and one in Africa (Uganda). Nearly 45% of all HIV patients were current smokers and mean body-mass index was 25kg/m2. About 39% of studies specified that plasma was used, mostly frozen, for immune marker measurement. Protocols for CIMT measurements varied from two-point unilateral measurements to a comprehensive protocol with 12 measurements in each carotid artery.
Critical appraisal
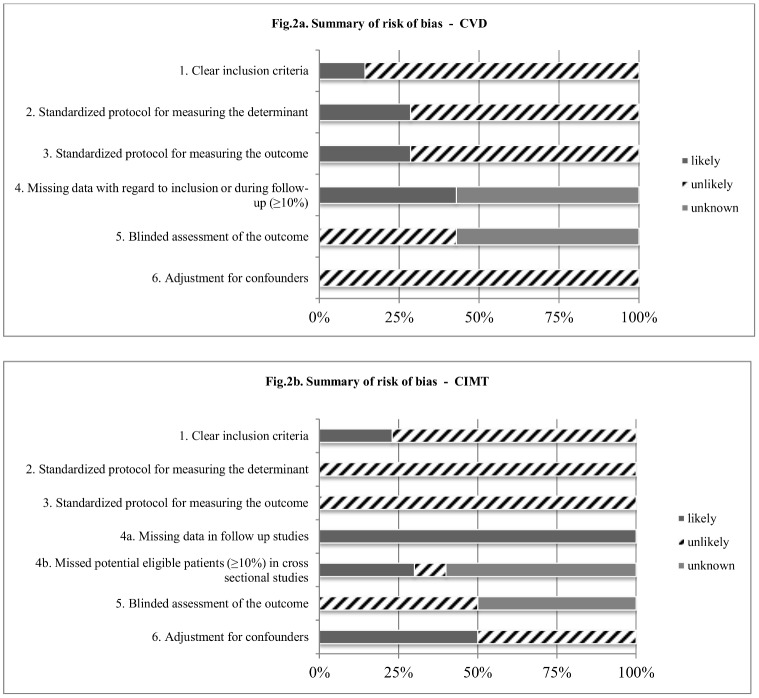
All studies were appraised for eight items (Fig 2, S2 Table). The criteria ‘homogeneous moment of inclusion’ and ‘CIMT protocols’ are not incorporated in the figure, since they could not be categorized as ‘yes’ or ‘no’ due to the different aspects that were covered. S2 Table shows marked heterogeneity with regard to the patient populations included for CIMT studies. Although all studies had a standardized procedure for measuring immune markers and outcome, these procedures were different between studies.
Fig 2. Summary of risk of bias.
Cardiovascular disease
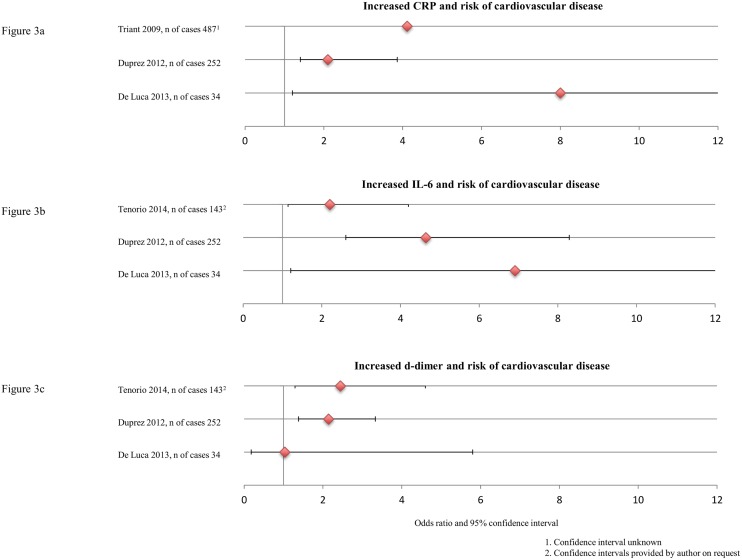
Most frequently assessed markers across eight studies were CRP (five times), IL-6 (five times), d-dimer (five times) and sCD14 (three times). CRP, IL-6 and d-dimer were assessed four times in relation to the occurrence of CVD [9,51, 52,54,56], and one time in relation to fatal versus non-fatal CVD [13]. These markers were found to be significantly associated with the occurrence of CVD in three out of four studies (Fig 3). [9,51,52,54,56] One article[52] could not be included in the figure since no odds ratios were presented. The authors did not find a relation between CRP, IL-6 and CVD, but they found an association between d-dimer and CVD; it was increased at both 4 months and 2 years prior to events.
Fig 3. Increased CRP, IL-6 and d-dimer and risk of cardiovascular disease.
1. Confidence interval unknown. 2. Confidence interval provided by author on request.
Nordell and colleagues[13] used fatal versus non-fatal CVD as outcome. CRP showed no relation, but an increase in IL-6 or d-dimer increased the risk of a fatal CVD, odds ratio and 95% confidence interval for highest versus lowest tertile at baseline were 2.62 (1.26–6.46) and 2.70 (1.27–5.75) respectively. sCD14 was not associated with CVD in any of the three studies.[52,54,58] All other markers (n = 32) were assessed less than three times.
Carotid intima-media thickness
In studies using CIMT as the endpoint, the most frequently studied inflammation markers were CRP (23 times), interleukin-6 (IL-6) (13 times) and soluble vascular cell adhesion molecule-1 (sVCAM-1) (10 times).
C-reactive protein
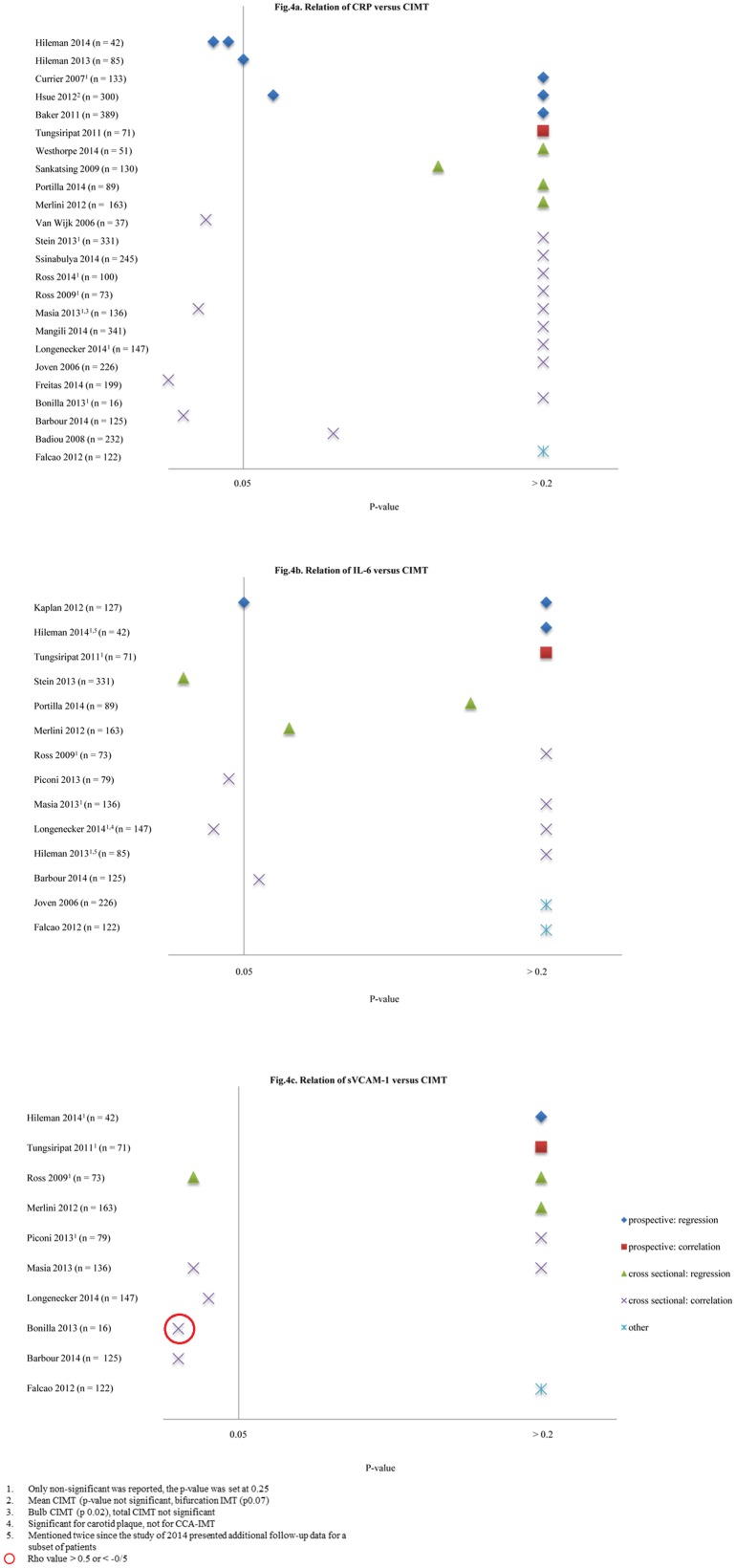
Fig 4a shows the results of all studies addressing the relation between CRP and CIMT. Four out of seven significant results were calculated using correlation coefficients.[37,39,48,50] The correlations, however, were weak; the highest Rho value was 0.33,[37,48] and were not confirmed in a regression analysis in in two out of four studies.[48,50]
Fig 4. Relation of CRP, IL-6 and sVCAM-1 versus CIMT.
1. Only non-significant was reported, the p-value was set at 0.25, 2. Mean cIMT (p-value not significant, bifurcation IMT p0.07), 3. Bulb CIMT (p0.02), total CIMT not significant, 4. Significant for carotid plaque, not for CCA-IMT, 5. Mentioned twice since the study of 2014 presented additional follow-up data for a subset of patients, Rho value >0.5 or < -0.5.
Six studies, describing five patient populations, were prospective with a follow-up duration ranging from 48 to 144 weeks.[19,20,23,30,32,33] The methods that were used to assess the relation between CRP and CIMT differed, varying from a change in CRP versus CIMT progression in a follow-up period of 48 weeks[34], to the association of the baseline level of CRP and CIMT progression in a follow-up period of 48 to 96 weeks[19,20] to the association of the increase of CRP at baseline (in units or doubling of the normal value) versus CIMT increase in millimeters per year[30,59].
The cohort described by Hsue and colleagues[30] showed a significant association between a two-fold increase in CRP at baseline and IMT in univariate analysis, but this association disappeared in multivariable analysis (data not shown).[24,25]
When comparing outcomes from studies including only ART-treated patients (n = 10) [18,33,37,40,43–45,48–50] and studies including only ART-naïve patients (n = 4)[19,20,36,47], no differences were present.
Only one out of eight studies including patients with a suppressed viral load[33,37,40,42–45,49] found a positive correlation[37].
Interleukin-6
IL-6 was assessed in 13 studies [10,15,18–20,33,34,39–43,47,50] (Fig 4b), six studies only mentioned that the association was non-significant.[18–20,33,39,43]
Of the prospective studies only Kaplan and colleagues[10] reported a positive association of IL-6 with CIMT in a subset of 81 out of 127 patients. However, the association was very modest (3.1 micrometers CIMT difference per 10% increase in biomarker, 95% CI -0.1–6.3, p 0.05) and only seen following ART initiation.
In a cross-sectional analysis on ART-naïve HIV infected adults, Stein and colleagues[47] found a significant association between IL-6 and carotid lesions (OR 2.1, 95% CI 1.2–3.4), but not for other CIMT segments. Two cross-sectional studies reported a statistically significant but very weak correlation (maximum Rho value 0.22).[18,41]
Soluble Vascular Cellular Adhesion Molecule
Ten studies addressed the relation between sVCAM-1 and CIMT (Fig 4c).[18,20,33,34,36,39–41,43,50] In the two prospective studies, no relation was found.[20,33] Although four positive associations were reported in cross-sectional studies [18,36,39,50] only the study of Bonilla and colleagues[36] showed a relevant association for bulb CIMT (Rho-value 0.66). The other correlations were weak, ranging from 0.22 to 0.28 across different CIMT segments.
Other markers
Of the remaining markers, twelve were assessed three times or more and 16 markers were only studied once or twice (Table 2). As shown in the table, the majority of these markers did not appear to be significantly associated with CIMT.
Table 2. Relation between immune markers and CIMT.
| Positive association | Negative association | No association | |
|---|---|---|---|
| Inflammation | |||
| TNF- α | 2 | 1 | 7 |
| sTNFR-1 | 1 | 1 | 5 |
| sTNFR-2 | 0 | 6 | |
| sCD14 | 11 | 8 | |
| sCD163 | 12 | 3 | |
| MCP-1 | 2 | 6 | |
| MPO | 1 | 3 | |
| LPS | 1 | 3 | |
| Endothelial activation | |||
| sICAM-1 | 0 | 7 | |
| Coagulation | |||
| d-dimer | 1 | 6 | |
| fibrinogen | 1 | 7 | |
| tPAI-1 | 0 | 3 | |
| Other markers assessed less than 3 times | |||
| CX3CL1 | Interleukin-1β | Interleukin-8 | Interleukin-10 |
| soluble Interleukin-2 receptor | Mean malonyldialdehyde (MDA) | Matrix metallopeptidase 9 (MMP-9) | Neopterin |
| Osteoprotegerin (OPG) | Serum amyloid A (SAA) | serum amyloid P component (SAP) | sE-selectin |
| soluble receptor for advanced glycation end products (sRAGE) | Receptor activator of nuclear factor kappa-B ligand (RANKL) | vascular endothelial growth factor (VEGF) | Von Willebrand Factor (vWF) |
1. Positive for yearly rate of change in CIMT versus baseline sCD14, cross-sectionally no association,
2. Positive correlation for total CIMT, not for bulb CIMT. CIMT carotid intima media thickness
Discussion
We identified forty articles describing 33 original datasets, that addressed the relation between immune markers and CVD or CIMT in HIV-infected individuals. Increased levels of CRP, IL-6 and d-dimer were associated with an increased risk of CVD. Data did not allow calculation of the average effect size or prognostic value for any of the markers. No clear conclusion can currently be drawn for any of the markers assessed in relation to CIMT. This reflects, among other reasons, the heterogeneity in patient populations, cross-sectional nature of most studies and the variability in methods of data analysis.
The finding that levels of CRP, IL-6 and d-dimer are related to CVD is in line with findings in the general population and in populations with other chronic inflammatory conditions like psoriasis and rheumatoid arthritis.[60–66]
Given this evidence, one would expect a positive association between inflammatory markers and CIMT as well. In a recent meta-analysis of individual patient data in the general population [67], a significant relation between CRP and fibrinogen and CIMT at baseline was indeed found. However, none of these markers were longitudinally associated with CIMT or CIMT progression after adjustment for classic cardiovascular risk factors, perhaps reflecting the relative healthy population and a short follow-up (mean of 3.9 years).
Baldassarre and colleagues[68] conducted a systematic review on the relation of immune makers to CIMT in the general population. They reported a significant association between CRP and fibrinogen in relation to CIMT based on a Fisher exact test since it was not possible to perform a formal meta-analysis due to the heterogeneity in ultrasound methodologies and statistical approaches. A Fisher exact test can be used to assess whether or not the number of studies reporting a relation between two determinants is larger than expected under the null hypothesis of no association. When using the Fisher exact test, we similarly found an association between CRP and CIMT (p 0.03), but the use of this test can be questioned. First, as results are simply categorized as ‘positive’ or ‘negative’, depending on the p-value, no between-study differences were taken into account. Second, most positive associations were found by correlation analysis. A positive association, however, does not mean that there is indeed a real association given that a very low correlation coefficient can be statistically significant if numbers are large enough.
CRP is lower in individuals with chronic HCV infection.[69] As chronic HCV infection is common among HIV-infected individuals, this might be a confounding variable, explaining why no relation between CRP and CIMT was observed.
For other, less frequently investigated, markers, conclusions on the association with CIMT are even more difficult. We did show a relation between immune activation and CVD, therefore a similar relation for CIMT was expected. The inconclusive results for CIMT are likely due to the already mentioned between-study heterogeneity and the scarcity of prospective data. Besides, only a few markers are analysed in depth as a result of the enormous variety in marker choice, not allowing for firm statements with regard to the majority of markers. From a pragmatic point of view and with an eye on the costs of marker measurement (approximately £5.50/sample), future research should first explore the value of well-established biomarkers, before embarking on a fishing expedition to find any immune-marker ‘associated’ with CIMT.
Strengths and limitations
To our knowledge this is the first review that provides a full overview of immune-markers in relation to CVD and CIMT in HIV-infected patients. We used a systematic approach covering all available evidence from 1996 onwards, after the initiation of HAART, to July 2014. Since this review directly focuses on the role of immune-markers, it provided a clear, global overview of the current knowledge.
To appreciate the results some limitations need to be mentioned. First, across studies there was a marked heterogeneity in study population, design and methodology of data analysis, limiting the possibilities for a clear summary of outcome data. Second, the vast majority of studies were cross-sectional rather than prospective. Third, the only common measure of association in studies assessing CIMT was a p-value. For reasons of comparability we decided to present the p-value, although we recognize the dependence on the sample size and the lack of parameter estimates. Forth, co-infection with hepatitis C was not taken into account, which may have led to an underestimation of our results. Finally, we did not take into account the differences in protocols for the assessment of CIMT nor the relation of markers for the diverse CIMT segments (common, bulb, internal). By regarding all segments as being the same we might have overlooked a specific association.
Recommendations
To obtain reliable information on the prognostic value of inflammatory markers in relation to CVD in HIV-infected-patients research addressing hard CVD outcomes in follow-up studies is needed. Currently some large prospective studies are undertaken, like the REPRIEVE trial[70] and the PURE study [71] that will provide data addressing the relation between inflammation, cardiovascular diseases and HIV infection.
As long as these data are not available CIMT could be used as a surrogate, preferably prospectively and with extended follow-up, and choice of immune-markers should focus on a selective set of markers. Furthermore, studies should be optimized with regard to definition of patient population, data-analysis and reporting. Finally, for reasons of comparability, it would be advisable to standardize the CIMT protocols and the definitions of outcomes.
Conclusion
This review gives an overview of available evidence regarding the role of inflammation in relation to CVD and CIMT in HIV-infection. Although an association between three immune-markers (CRP, IL-6 and d-dimer) and CVD was observed, no consistent relation with CIMT could be detected for any of the immune-makers. This might reflect the heterogeneity of the CIMT-studies and the lack of adequate prospective data. In view of the costs and interpretability, the search for immune markers ‘associated’ with CIMT in cross-sectional studies should be reconsidered. Future research should aim to be of prospective design, utilizing standardized approaches for the selection of participants, immune markers and assessment of the outcome.
Supporting Information
(DOC)
(PDF)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Miedema F, Hazenberg MD, Tesselaar K, van Baarle D, de Boer RJ, Borghans JA. Immune activation and collateral damage in AIDS pathogenesis. Front Immunol 2013. September 26;4:298 10.3389/fimmu.2013.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The "Mortalite 2000 and 2005" surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr 2008. August 15;48(5):590–598. 10.1097/QAI.0b013e31817efb54 [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006. September;43(1):27–34. [DOI] [PubMed] [Google Scholar]
- 4.Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group, Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010. June 19;24(10):1537–1548. 10.1097/QAD.0b013e32833a0918 [DOI] [PubMed] [Google Scholar]
- 5.Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013. March 27;27(6):973–979. 10.1097/QAD.0b013e32835cae9c [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009. December 1;54(23):2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011. November 7;17(11):1410–1422. 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 8.Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis 2012. June;205 Suppl 3:S355–61. 10.1093/infdis/jis195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012;7(9):e44454 10.1371/journal.pone.0044454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Pmc3400505; Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr 2012. August 1;60:359–368. 10.1097/QAI.0b013e31825b03be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009. July 21;339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field N, Cohen T, Struelens MJ, Palm D, Cookson B, Glynn JR, et al. Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): an extension of the STROBE statement. Lancet Infect Dis 2014. April;14(4):341–352. 10.1016/S1473-3099(13)70324-4 [DOI] [PubMed] [Google Scholar]
- 13.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of Cardiovascular Disease Outcomes Among Patients With HIV Is Related to Markers of Inflammation and Coagulation. J Am Heart Assoc 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso-Villaverde C, Coll B, Parra S, Montero M, Calvo N, Tous M, et al. Atherosclerosis in patients infected with HIV is influenced by a mutant monocyte chemoattractant protein-1 allele. Circulation 2004. October 12;110(15):2204–2209. [DOI] [PubMed] [Google Scholar]
- 15.Joven J, Coll B, Tous M, Ferre N, Alonso-Villaverde C, Parra S, et al. The influence of HIV infection on the correlation between plasma concentrations of monocyte chemoattractant protein-1 and carotid atherosclerosis. Clin Chim Acta 2006. June;368:114–119. [DOI] [PubMed] [Google Scholar]
- 16.Coll B, Parra S, Alonso-Villaverde C, de Groot E, Aragones G, Montero M, et al. HIV-infected patients with lipodystrophy have higher rates of carotid atherosclerosis: the role of monocyte chemoattractant protein-1. Cytokine 2006. April;34:51–55. [DOI] [PubMed] [Google Scholar]
- 17.Parra S, Coll B, Aragones G, Marsillach J, Beltran R, Rull A, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med 2010. April;11:225–231. 10.1111/j.1468-1293.2009.00766.x [DOI] [PubMed] [Google Scholar]
- 18.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014. April 24;28:969–977. 10.1097/QAD.0000000000000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hileman CO, Carman TL, Longenecker CT, Labbato DE, Storer NJ, White CA, et al. Rate and predictors of carotid artery intima media thickness progression in antiretroviral-naive HIV-infected and uninfected adults: a 48-week matched prospective cohort study. Antivir Ther (Lond) 2013;18:921–929. [DOI] [PubMed] [Google Scholar]
- 20.Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. J Acquir Immune Defic Syndr 2014. March 1;65:340–344. 10.1097/QAI.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 21.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Pmc3475633; Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012. November 15;206:1558–1567. 10.1093/infdis/jis545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelesidis T, Kendall MA, Yang OO, Hodis H, Currier JS. Pmc3653400; Perturbations of circulating levels of RANKL-osteoprotegerin axis in relation to lipids and progression of atherosclerosis in HIV-infected and -uninfected adults: ACTG NWCS 332/A5078 Study. AIDS Res Hum Retroviruses 2013. June;29:938–948. 10.1089/AID.2012.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS 2007. May 31;21(9):1137–1145. [DOI] [PubMed] [Google Scholar]
- 24.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006. November 28;20:2275–2283. [DOI] [PubMed] [Google Scholar]
- 25.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Pmc2691772; Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009. June 1;23:1059–1067. 10.1097/QAD.0b013e32832b514b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsue PY, Deeks SG, Hunt PW. Pmc3349295; Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012. June;205 Suppl 3:S375–82. 10.1093/infdis/jis200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangili A, Gerrior J, Tang AM, O'Leary DH, Polak JK, Schaefer EJ, et al. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis 2006. December 1;43(11):1482–1489. [DOI] [PubMed] [Google Scholar]
- 28.Mangili A, Jacobson DL, Gerrior J, Polak JF, Gorbach SL, Wanke CA. Metabolic syndrome and subclinical atherosclerosis in patients infected with HIV. Clin Infect Dis 2007. May 15;44(10):1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangili A, Ahmad R, Wolfert RL, Kuvin J, Polak JF, Karas RH, et al. Pmc3935500; Lipoprotein-associated phospholipase A2, a novel cardiovascular inflammatory marker, in HIV-infected patients. Clin Infect Dis 2014. March;58:893–900. 10.1093/cid/cit815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, et al. Pmc3487373; Carotid Intima-Media Thickness Progression in HIV-Infected Adults Occurs Preferentially at the Carotid Bifurcation and Is Predicted by Inflammation. J Am Heart Assoc 2012. April;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longenecker C, Funderburg N, Jiang Y, Debanne S, Storer N, Labbato D, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV medicine 2013;14:385–390. 10.1111/hiv.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis 2011. October;53(8):826–835. 10.1093/cid/cir497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tungsiripat M, El-Bejjani D, Rizk N, Dogra V, O'Riordan M, Ross A, et al. Carotid intima media thickness, inflammatory markers, and endothelial activation markers in HIV Patients with lipoatrophy increased at 48 weeks regardless of use of rosiglitazone or placebo. AIDS Res Hum Retroviruses 2011;27:295–302. 10.1089/aid.2010.0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falcao Mda C, Zirpoli JC, Albuquerque VM, Markman Filho B, Araujo NA, Falcao CA, et al. Association of biomarkers with atherosclerosis and risk for coronary artery disease in patients with HIV. Arq Bras Cardiol 2012. November;99:971–978. [DOI] [PubMed] [Google Scholar]
- 35.Badiou S, Thiebaut R, Aurillac-Lavignolle V, Dabis F, Laporte F, Cristol JP, et al. Association of non-HDL cholesterol with subclinical atherosclerosis in HIV-positive patients. J Infect 2008. July;57:47–54. 10.1016/j.jinf.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 36.Bonilla H, McShannic J, Goldberg E, Chua D, Conner R, Fiorentino M, et al. Impact of human immunodeficiency virus infection on measures of cardiovascular disease in long-term nonprogressors. Infectious Diseases in Clinical Practice 2013;21:177–180. [Google Scholar]
- 37.Freitas P, Carvalho D, Santos AC, Madureira AJ, Martinez E, Pereira J, et al. Carotid intima media thickness is associated with body fat abnormalities in HIV-infected patients. BMC Infect Dis 2014. June 23;14:348 10.1186/1471-2334-14-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong SJ, Kim CO, Song YG, Baek JH, Kim SB, Jin SJ, et al. Low plasma levels of the soluble receptor for advanced glycation end products in HIV-infected patients with subclinical carotid atherosclerosis receiving combined antiretroviral therapy. Atherosclerosis 2011. December;219:778–783. 10.1016/j.atherosclerosis.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 39.Masia M, Robledano C, Ortiz de lT, Antequera P, Lopez N, Gutierrez F. Pmc3662719; Increased carotid intima-media thickness associated with antibody responses to varicella-zoster virus and cytomegalovirus in HIV-infected patients. PLoS One 2013;8:e64327 10.1371/journal.pone.0064327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, et al. Pmc3459872; T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One 2012;7:e46073 10.1371/journal.pone.0046073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piconi S, Parisotto S, Rizzardini G, Passerini S, Meraviglia P, Schiavini M, et al. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS 2013. January 28;27:381–389. 10.1097/QAD.0b013e32835abcc9 [DOI] [PubMed] [Google Scholar]
- 42.Portilla J, Moreno-Perez O, Serna-Candel C, Escoin C, Alfayate R, Reus S, et al. Pmc4021989; Vitamin D insufficiency and subclinical atherosclerosis in non-diabetic males living with HIV. J Int AIDS Soc 2014;17:18945 10.7448/IAS.17.1.18945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross AC, Rizk N, O'Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Pmc3895473; Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009. October 1;49:1119–1127. 10.1086/605578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross Eckard A, Longenecker C, Jiang Y, Debanne S, Labbato D, Storer N, et al. Lipoprotein-associated phospholipase A and cardiovascular disease risk in HIV infection. HIV Med 2014. March 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sankatsing RR, Wit FW, Vogel M, de Groot E, Brinkman K, Rockstroh JK, et al. Increased carotid intima-media thickness in HIV patients treated with protease inhibitors as compared to non-nucleoside reverse transcriptase inhibitors. Atherosclerosis 2009. February;202(2):589–595. 10.1016/j.atherosclerosis.2008.05.028 [DOI] [PubMed] [Google Scholar]
- 46.Ssinabulya I, Kayima J, Longenecker C, Luwedde M, Semitala F, Kambugu A, et al. Pmc3938501; Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS One 2014;9:e89537 10.1371/journal.pone.0089537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein J, Brown T, Ribaudo H, Chen Y, Yan M, Lauer-Brodell E, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS 2013;27:929–937. 10.1097/QAD.0b013e32835ce27e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Wijk JP, de Koning EJ, Cabezas MC, Joven J, op't Roodt J, Rabelink TJ, et al. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol 2006. March 21;47:1117–1123. [DOI] [PubMed] [Google Scholar]
- 49.Westhorpe CL, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol 2014. February;92:133–138. 10.1038/icb.2013.84 [DOI] [PubMed] [Google Scholar]
- 50.Barbour JD, Jalbert EC, Chow DC, Gangcuangco LMA, Norris PJ, Keating SM, et al. Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis 2014;232:52–58. 10.1016/j.atherosclerosis.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Luca A, de GD, Colafigli M, Cozzi-Lepri A, De Curtis A, Gori A, et al. Pmc3846422; The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: a nested case-control study. BMC Infect Dis 2013;13:414 10.1186/1471-2334-13-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. Pmc2884071; Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010. June 19;24:1509–1517. 10.1097/QAD.0b013e32833ad914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knudsen A, Moller HJ, Katzenstein TL, Gerstoft J, Obel N, Kronborg G, et al. Pmc3663777; Soluble CD163 does not predict first-time myocardial infarction in patients infected with human immunodeficiency virus: a nested case-control study. BMC Infect Dis 2013;13:230 10.1186/1471-2334-13-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non-AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J Infect Dis 2014. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Pmc3071127; Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011. March 15;203:780–790. 10.1093/infdis/jiq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009. July 1;51(3):268–273. 10.1097/QAI.0b013e3181a9992c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr 2012. August 1;60(4):359–368. 10.1097/QAI.0b013e31825b03be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011. March 15;203(6):780–790. 10.1093/infdis/jiq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker J, Neuhaus J, Duprez D, Cooper D, Hoy J, Kuller L, et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS 2011;25:2133–2142. 10.1097/QAD.0b013e32834be088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pirro M, Stingeni L, Vaudo G, Mannarino MR, Ministrini S, Vonella M, et al. Systemic inflammation and imbalance between endothelial injury and repair in patients with psoriasis are associated with preclinical atherosclerosis. Eur J Prev Cardiol 2014. June 6. [DOI] [PubMed] [Google Scholar]
- 61.Goodson NJ, Symmons DP, Scott DG, Bunn D, Lunt M, Silman AJ. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum 2005. August;52(8):2293–2299. [DOI] [PubMed] [Google Scholar]
- 62.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004. April 1;350(14):1387–1397. [DOI] [PubMed] [Google Scholar]
- 63.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003. January 28;107(3):363–369. [DOI] [PubMed] [Google Scholar]
- 64.Willeit P, Thompson A, Aspelund T, Rumley A, Eiriksdottir G, Lowe G, et al. Hemostatic factors and risk of coronary heart disease in general populations: new prospective study and updated meta-analyses. PLoS One 2013;8(2):e55175 10.1371/journal.pone.0055175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JK, Bettencourt R, Brenner D, Le TA, Barrett-Connor E, Loomba R. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One 2012;7(4):e34218 10.1371/journal.pone.0034218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su D, Li Z, Li X, Chen Y, Zhang Y, Ding D, et al. Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Mediators Inflamm 2013;2013:726178 10.1155/2013/726178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willeit P, Thompson SG, Agewall S, Bergstrom G, Bickel H, Catapano AL, et al. Inflammatory markers and extent and progression of early atherosclerosis: Meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur J Prev Cardiol 2014. November 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baldassarre D, De Jong A, Amato M, Werba JP, Castelnuovo S, Frigerio B, et al. Carotid intima-media thickness and markers of inflammation, endothelial damage and hemostasis. Ann Med 2008;40(1):21–44. [DOI] [PubMed] [Google Scholar]
- 69.Shah S, Ma Y, Scherzer R, Huhn G, French AL, Plankey M, et al. Association of HIV, hepatitis C virus and liver fibrosis severity with interleukin-6 and C-reactive protein levels. AIDS. 2015. July 7;29(11):1325–33. 10.1097/QAD.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.www.reprievetrial.org. Assessed on 10th of November 2015
- 71.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S; PURE Investigators-Writing Group. The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J. 2009. July;158(1):1–7. 10.1016/j.ahj.2009.04.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.