Abstract
The aim of the present study was to compare the effects of melatonin and genistein on radiation-induced nephrotoxicity (RIN). A total of 70 Swiss Albino mice were divided into 7 groups. Five control groups were defined, which were sham irradiation (C, G1), radiation therapy only (RT, G2), melatonin (M, G3), genistein (G, G4) and polyethylene glycol-400 (G5), respectively. The co-treatment groups were the RT plus melatonin (RT+M, G6) and RT plus genistein (RT+G, G7) groups. Irradiation was applied using a cobalt-60 teletherapy machine (80-cm fixed source-to-surface distance, 2.5-cm depth). Melatonin was administered (100 mg/kg, intraperitoneal injection) 30 min before the single dose of irradiation, whereas genistein was administered (200 mg/kg, subcutaneous injection) 1 day before the single dose of irradiation. All the mice were sacrificed 6 months after irradiation. As an end point, the extent of renal tubular atrophy for each mouse was quantified with image analysis of histological sections of the kidney. Tissue malondialdehyde (MDA) levels were also measured in each animal. In the histopathological examination of the mouse kidneys, there was a statistically significant reduction (P<0.05) in the presence of tubular atrophy between the RT+M and RT+G groups and the RT group. There was a statistically significant increase in MDA levels in the irradiated versus sham groups (RT vs. C; P<0.05); however, MDA levels were significantly decreased by co-treatment with melatonin or genistein vs. RT alone (RT+M and RT+G vs. RT; P<0.05). In conclusion, the present experimental study showed that melatonin and genistein supplementation prior to irradiation-protected mice against RIN, which may have therapeutic implications for radiation-induced injuries.
Keywords: genistein, nephrotoxicity, melatonin, mice, radiation, radioprotection
Introduction
Radiotherapy is one of the most common and important techniques for cancer treatment that is performed with the intent to cure, or for palliation (1,2). The kidneys are radiosensitive organs. In patients with abdominal malignancies, such as gastric, pancreatic, lymphomas or any other abdominal neoplasms, irradiation of the kidneys is inevitable (3,4). The radiation dose and irradiated volume are the limiting factors in abdominal radiotherapy and should be taken into consideration for the prevention of kidney injuries (4,5). Radiation nephropathy includes increased vascular permeability, perfusion disturbance, inflammatory reactions and fibrosis (3,4).
Melatonin (N-acetyl-5-methoxytryptamine), an endogenous compound synthesized by the pineal gland in the human brain, was discovered ~40 years ago and reported to participate in the regulation of a number of physiological and pathological processes (6). Melatonin has a lipophilic nature, which allows the hormone to enter all the cells and subcellular compartments and establish high concentrations; melatonin also has the ability to cross morphophysiological barriers (7–9). Melatonin has been shown in several experimental and clinical conditions to have antioxidant and prophylactic properties against oxidative stress (10–15).
Genistein (4′,5,7-trihydroxyisoflavone) has antioxidant and anti-inflammatory properties, low toxicity and is commonly used as a dietary supplement (16–18). The compound inhibits tyrosine kinase, possesses phytoestrogen activities, and protects against cerebral ischemia and skin injury by ultraviolet light (19,20). Genistein has been reported to reduce acute lung injury from inflammation following lipopolysaccharide treatment (21); additionally, following whole-body irradiation, the administration of genistein in doses ≤400 mg/kg significantly increases survival without any toxicity (22). Of particular relevance, genistein is radioprotective for normal cells, while radiosensitizing toward a variety of cancer cells. With regards to the antioxidant, anti-inflammatory and anticancer properties of genistein, this compound has potential as a clinical therapeutic agent (20–23). However, little is known regarding the radioprotective role of genistein with respect to radiation-induced kidney injury.
Lipid peroxidation is an important cause of cell membrane destruction and damage, which is a likely contributing factor in the development of radiation-induced tissue damage (24,25). An increase in malondialdehyde (MDA) levels is used as a marker of lipid peroxidation (26). During radiotherapy, melatonin pretreatment significantly reduces the level of MDA and increases the levels of enzymatic antioxidants in the ovaries and in plasma (27–30).
The present study was carried out to evaluate whether melatonin or genistein administration prior to irradiation would have a protective effect on radiation-induced nephrotoxicity (RIN) in an experimental mouse model.
Materials and methods
Study design
Swiss Albino mice (10–12-week-old, weighing 25±2 g) were purchased from the Center for Laboratory Animals at the Karadeniz Technical University (Trabzon, Turkey). All the mice were acclimatized upon arrival, and representative animals were screened for evidence of disease. The Institutional Animal Care and Use Committee at Karadeniz Technical University approved the protocol used in the present study.
Animals were housed 4 per cage in a controlled animal holding room with a 12/12-h light/dark cycle; temperature and relative humidity were continually monitored to provide standard laboratory conditions. Food and water were provided ad libitum. Mice were divided into 7 groups composed of 10 animals. C was defined as the control group, and mice in this group were sham irradiated. RT was the radiation therapy only group. The M, G and PEG groups represented the melatonin, genistein and polyethylene glycol-400 (PEG-400) control groups, respectively. RT+M and RT+G represented the RT plus melatonin and RT plus genistein groups, respectively (Table I). Melatonin was administered 30 min before the RT, and genistein was administered 24 h before the RT. The two co-treatments were continued until the animals were sacrificed 24 weeks later. As an end point, the extent of renal tubular atrophy for each mouse was quantified with image analysis of histological sections of the kidney. Tissue MDA concentrations were also measured in each animal.
Table I.
Abbreviations used for the study groups.
| Abbreviations | Study groups |
|---|---|
| C | Sham-irradiated control |
| RT | 6-Gy |
| M | Melatonin control |
| G | Genistein control |
| PEG | PEG-400 control |
| RT+M | 6 Gy + melatonin |
| RT+G | Gy + genistein |
PEG-400, polyethylene glycol-400; RT, radiation therapy.
Irradiation protocol
Prior to whole-body irradiation, the animals were anesthetized with intraperitoneal (i.p.) injections of 90 mg/kg ketamine and 10 mg/kg xylazine. Subsequently, the animals were placed on a straphore in the prone position by taping their extremities. Correct positioning of the fields was controlled for each mouse via a therapy simulator. A 6-Gy single dose γ-radiation was selected according to previous studies (31,32). Mice in the RT, RT+M and RT+G groups were irradiated with a Co60 teletherapy machine from a source-to-surface distance of 80 cm. A single dose of 6 Gy γ-radiation was delivered to the whole-body area at a dose rate of 47.50 Gy/min. The dose was calculated for the central axis at a depth of 2.5 cm.
Melatonin and genistein protocols
For the mice in M and RT+M, melatonin (Melatonin Crystalline; Sigma-Aldrich, St. Louis, MO, USA) was prepared at a 1% concentration by dissolving in ethanol and diluting in 0.9% sodium chloride, and was administered at a dose of 100 mg/kg i.p. 30 min prior to exposure to radiation. The selection of a 30-min interval between the melatonin administration and exposure to radiation was based on 2 previous studies in animals (33,34) and human volunteers (35).
Genistein and PEG, of molecular weight 400, were obtained from Sigma-Aldrich. Genistein was solubilized in PEG-400 on the day of the experiment using 20 sec of sonication (Heat Systems-Ultrasonics Inc., Plainview, NY, USA). Genistein was administered at a dose of 100 mg/kg subcutaneously (s.c.) 24 h prior to being exposed to radiation. 0.9% sodium chloride was prepared at an equal volume with melatonin, and the remaining procedure was applied identically for G and RT+G mice. PEG-400 was prepared at an equal volume with genistein, and the rest of the procedure was applied identically for group PEG mice. The selection of a 24-h interval between genistein administration and exposure to radiation was based on one earlier study in animals (20).
Determination of MDA activity
Kidney tissues were weighed and homogenized in ice-cold 1.15% KCl (2 and 10% w/v, respectively). The homogenate was centrifuged at 2,000 × g for 10 min. MDA levels in tissue samples were determined by the method of Mihara and Uchiyama (36). Tetramethoxypropane was used as a standard, and tissue MDA levels were calculated as nmol/g wet tissue.
Morphological study and light microscopy
The animals were anesthetized and sacrificed by cervical dislocation 24 weeks after the start of irradiation. The kidneys were excised and fixed in a 10% formaldehyde solution and embedded in paraffin for light microscopic examination. One transverse section of each kidney was taken using vertical sections. The slices obtained were stained with hematoxylin and eosin to evaluate the fibrosis in the kidney. Tissues were also processed using a sirius red stain to examine for tubular atrophy. Kidney damage was scored based on the presence of tubular atrophy as none (0), light (1), moderate (2) or severe (3) damage.
Statistical analysis
Compatibility of the variables to normal distribution was investigated using visual (histogram and probability graphs) and analytical methods (one simple Kolmogorov-Smirnov test). The data are reported as mean ± standard error and were analysed using one-way analysis of variance followed by a post hoc test for multiple comparisons. Type 1 errors of <5% were accepted as statistically significant. All the statistical analyses were performed using SPSS version 13 (SPSS, Inc., Chicago, IL, USA).
Results
Histopathological examination of the mice kidneys
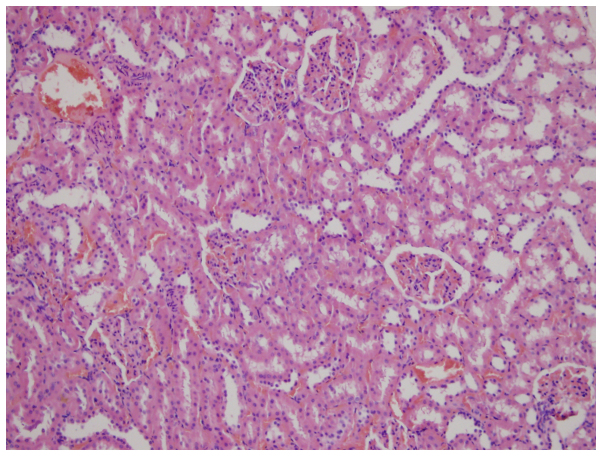
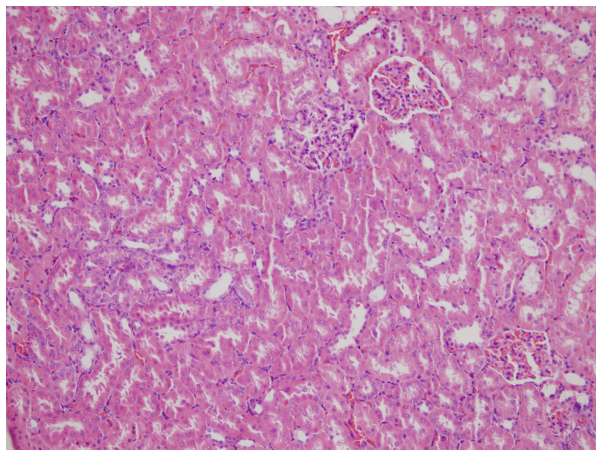
Glomerular and tubular structures were histopathologically normal in the C group (Fig. 1). Widespread kidney tubular atrophy and diffuse intertubular fibrosis were present in the RT group (Fig. 2). The rates, as assessed using slit-lamp biomicroscopy of grade 1, 2 and 3 tubular atrophy, respectively, in the C (0, 0 and 0%), PEG (33.3, 50 and 16.7%,), M (50, 0 and 0%,), G (80, 0 and 0%), RT (40, 20 and 40%), RT+M (75, 0 and 0%) and RT+G groups (83.3, 16.7 and 0%) are presented in Table II.
Figure 1.
In the control group, glomerular and tubular structures were observed to be histopathologically normal (hematoxylin and eosin; magnification, ×50).
Figure 2.
Renal tubular atrophy and diffuse intertubular fibrosis in the radiation therapy group (hematoxylin and eosin; magnification, ×50).
Table II.
Tubular atrophy in the mice kidneys for each group of mice as examined by slit-lamp microscopy.
| Tubular atrophy grade | |||||
|---|---|---|---|---|---|
| Groups | Absent | Grade 1 | Grade 2 | Grade 3 | Total |
| Sham-irradiated control group (C) | 8 | 0 | 0 | 0 | 8 |
| 6 Gy group (RT) | 0 | 4 | 2 | 4 | 10 |
| Melatonin control group (M) | 4 | 4 | 0 | 0 | 8 |
| Genistein control group (G) | 2 | 8 | 0 | 0 | 10 |
| PEG-400 control group (PEG-400) | 0 | 2 | 3 | 1 | 6 |
| 6 Gy+melatonin group (RT+M) | 2 | 6 | 0 | 0 | 8 |
| 6 Gy+genistein group (RT+G) | 0 | 5 | 1 | 0 | 6 |
| Total | 16 | 29 | 6 | 5 | 56 |
PEG-400, polyethylene glycol-400; RT, radiation therapy.
In the histopathological examination of the mice kidneys, there was a statistically significant elevation of tubular atrophy induced by γ-irradiation (C vs. RT; P<0.05; Table III). A significant elevation in all the investigated histopathological parameters was identified in the PEG and RT+G groups versus the C group (P<0.05) but not between the M, G and RT+M groups versus the C group (P>0.05). In addition, a significant elevation was observed in all the investigated histopathological parameters in the RT group compared to the M, G, RT+M and RT+G groups (P<0.05) but not the PEG group (P>0.05), indicating reduced injury in the irradiation plus co-treatment groups. However, there was no statistically significant difference between the RT+M and the RT+G groups (P>0.05). At the end of the histological examination, all the mice in each group had a certain degree of tubular atrophy.
Table III.
Mean value of tubular atrophy for each group of mice.
| Groups | Mean tubular atrophy value |
|---|---|
| Sham-irradiated control group (C) | 0.12±0.35b,e,g |
| 6-Gy group (RT) | 2.00±0.94a,c,d,f,g |
| Melatonin control group (M) | 0.50±0.53b,e |
| Genistein control group (G) | 0.80±0.42b,e |
| PEG-400 control group (PEG-400) | 1.83±0.75a,c,d,f,g |
| 6 Gy+melatonin group (RT+M) | 0.75±0.46b,e |
| 6 Gy+genistein group (RT+G) | 1.16±0.40a,b,e |
PEG-400, polyethylene glycol-400; RT, radiation therapy. Values are presented as mean ± standard error for 7 mice in each group. P<0.05 compared to
C;
RT;
M;
G;
PEG;
RT+M;
RT+G.
Changes in MDA level following irradiation
Whole-body irradiation by 6 Gy of γ-irradiation, as a single dose, significantly increased the MDA level (P<0.05) in the mice kidneys when compared to the untreated controls (RT vs. C; Table IV). Melatonin and genistein supplementation in conjunction with body irradiation significantly decreased the MDA level in the kidney (RT vs. RT+M and RT vs. RT+G; P<0.05), but there was no statistically significant difference between the co-treatment groups (RT+M vs. RT+G; P>0.05).
Table IV.
Level of MDA in the mice kidneys.
| Groups | Mean MDA value, nmol/mg protein |
|---|---|
| Sham-irradiated control group (C) | 37.3±1.11b, |
| 6-Gy group (RT) | 45.6±0.90a,c,d,f,g |
| Melatonin control group (M) | 40.4±1.00b |
| Genistein control group (G) | 35.2±0.94b |
| PEG-400 control group (PEG-400) | 38.5±1.28b |
| 6 Gy+melatonin group (RT+M) | 37.5±1.17b, |
| 6 Gy+genistein group (RT+G) | 39.1±0.75b |
MDA, malondialdehyde; PEG-400, polyethylene glycol-400; RT, radiation therapy. Values are presented as mean ± standard error for 7 mice in each group. P<0.05 compared to
C;
RT;
M;
G;
PEG;
RT+M;
RT+G.
Discussion
In numerous clinical and experimental studies, kidneys have been shown to be highly sensitive to radiation injuries (1). Radiation nephropathy presents itself in 20% of patients following irradiation; the clinical presentations include acute radiation nephritis, chronic radiation nephritis, malignant hypertension and benign hypertension (37). Clinical signs of radiation damage that develop after a period of 4–12 months are of particular concern (3,38–40). In the kidney, irradiation leads to a progressive reduction in function associated with concomitant glomerulosclerosis and/or tubulointerstitial fibrosis, which largely depends on the total radiation dose, dose per fraction, irradiated volume and age at the time of irradiation (3).
Melatonin is a highly efficient free radical scavenger and general antioxidant that protects DNA, lipids and proteins (12–15). The radioprotective effect of melatonin was confirmed in in vitro (41,42) and in vivo studies (4,36), as well as when administered to humans (42,43), mainly by assessing the induction of chromosomal aberration and micronucleus in cultured lymphocytes. Several studies have demonstrated that melatonin appears to ameliorate irradiation-induced injury in various organs including the spleen (44,45), liver (29), lung, colon, ileum (46), kidney (37), lens (47), spinal cord (48) and brain (49). Doses of melatonin in mice, 10–250 mg/kg, have been tested in in vivo investigations (4). In the present study, 100 mg/kg melatonin was administered by i.p. injection in accordance with the literature.
Genistein has antioxidant and anti-inflammatory properties, has low toxicity and is commonly used as a dietary supplement (16,50). Wei et al (51) reported that genistein provided protection against non-ionizing ultraviolet-B radiation through either direct quenching of reactive oxygen species (ROS) or indirect anti-inflammatory effects when it was applied to the skin of hairless mice 1 h before exposure. Genistein also reduced the frequency of micronucleated reticulocytes in the peripheral blood of mice receiving a sublethal dose of ionizing radiation (52). Thus, the antioxidant activity of genistein and its ability to protect against radiation-induced cytogenetic damage could contribute to its radioprotective action. Landauer et al (20) demonstrated in a preliminary study that oral administration of pharmacological doses of genistein is radioprotective in adult mice. However, oral administration required a multiple dosing regimen beginning several days prior to irradiation. The beneficial effects of single-dose s.c. administered radioprotectants are also being evaluated in the clinic in conjunction with RT (53). The study by Landauer et al (20) reported the results of experiments designed to assess in vivo radioprotection in whole-body γ-irradiated mice with genistein. Radioprotection was demonstrated without the toxicity or performance-degrading side effects in mice receiving a single s.c. administration of genistein. Therefore, the present study administered genistein at 100 mg/kg s.c. in accordance with the literature.
Tubular interstitial injury is an additional feature of radiation nephropathy. Morphological and physiological studies have identified the renal tubule system as the site of maximum radiation damage (1,38,54,55). The results of the present study indicated that the tubular toxicity induced by 6 Gy irradiation became apparent during the 6-month period after radiation exposure. The differences observed during histopathological evaluation were statistically significant. The degrees of grades 2 and 3 tubular atrophy were 20 and 40%, respectively, for the RT controls. Treatment with RT+M blocked all grade 2 and 3 tubular atrophy, and RT+G treatment blocked all grade 3 and some grade 2 atrophy (16.7%). These results indicate that pretreatment with melatonin and genistein markedly decreased the severity of tubular changes that occurred following irradiation.
The present results with supplemental melatonin are in agreement with the published literature on the antioxidant effects of melatonin. Melatonin administration prior to total body irradiation with a single dose of 6 Gy prevents rat liver damage induced by irradiation, reflecting the antioxidant roles of melatonin against γ-irradiation-induced oxidative damage (29). The liver tissue MDA levels in irradiated rats that were pretreated with melatonin (5 or 10 mg/kg) were significantly decreased, while the superoxide dismutase and glutathione peroxidase activities were significantly increased. Similarly, the levels of mouse kidney MDA in the γ-irradiation-plus 100 mg/kg melatonin (RT+M) group were significantly decreased when compared with the γ-irradiation-only (RT) group.
Genistein has stronger antioxidative properties combined with its capacity to activate the antioxidant systems; the resulting reduction of free radical lipid peroxidation protects and stabilizes the cellular membrane structure (50). Genistein protects against ultraviolet-B radiation either by directly quenching ROS or by indirect anti-inflammatory effects when applied to the skin of hairless mice prior to radiation exposure (51). Kim et al (56) demonstrated that genistein can significantly protect against a radiation-induced increase of ROS in the testis, suggesting that genistein protects against testicular injury from radiation via a protective mechanism that includes antioxidative activity. In agreement with the published literature on the radioprotective and antioxidant effects of genistein in the skin and tests, the levels of kidney MDA in the γ-irradiation-plus 100 mg/kg genistein (RT+G) group were significantly decreased when compared with the γ-irradiation-only (RT) group.
In conclusion, melatonin and genistein have clear antioxidant properties and are likely to be valuable adjuvant drugs for the protection against γ-irradiation and/or use as antioxidants against oxidative stress. Light microscopic examinations and MDA measurement performed at the end of the 6-month follow-up period revealed that the kidneys of the mice in the RT+M and RT+G groups were healthier compared with the radiotherapy only group mice. Based on the present findings, additional studies of the protective effects of melatonin and genistein against RIN are merited.
References
- 1.Robbins ME, Bonsib SM. Radiation nephropathy: A review. Scanning Microsc. 1995;9:535–560. [PubMed] [Google Scholar]
- 2.Bolling T, Schuck A, Rube C, Hesselmann S, Pape H, Dieckmann K, Pollinger B, Kortmann RD, Speiser-Held I, Meyer FM, et al. Therapy-associated late effects after irradiation of malignant diseases in childhood and adolescence. Feasibility analyses of a prospective multicenter register study. Strahlenther Onkol. 2006;182:443–449. doi: 10.1007/s00066-006-1517-9. (In German) [DOI] [PubMed] [Google Scholar]
- 3.Cohen EP, Robbins ME. Radiation nephropathy. Semin Nephrol. 2003;23:486–499. doi: 10.1016/S0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 4.Vijayalaxmi Meltz ML, Reiter RJ, Herman TS, Kumar KS. Melatonin and protection from whole-body irradiation: Survival studies in mice. Mutat Res. 1999;425:21–27. doi: 10.1016/S0027-5107(98)00246-2. [DOI] [PubMed] [Google Scholar]
- 5.El-Missiry MA. Prophylactic effect of melatonin on lead-induced inhibition of heme biosynthesis and deterioration of antioxidant system in male rats. J Biochem Mol Toxicol. 2000;14:57–62. doi: 10.1002/(SICI)1099-0461(2000)14:1<57::AID-JBT8>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.El-Missiry MA, El-Aziz Abd AF. Influence of melatonin on proliferation and antioxidant system in Ehrlich ascites carcinoma cells. Cancer Lett. 2000;151:119–125. doi: 10.1016/S0304-3835(99)00366-3. [DOI] [PubMed] [Google Scholar]
- 7.Shirazi A, Ghobadi G, Ghazi-Khansari M. A radiobiological review on melatonin: A novel radioprotector. J Radiat Res (Tokyo) 2007;48:263–272. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 8.Vijayalaxmi Reiter RJ, Tan DX, Herman TS, Thomas CR., Jr Melatonin as a radioprotective agent: A review. Int J Radiat Oncol Biol Phys. 2004;59:639–653. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Reiter RJ, Tan DX, Cabrera J, D'Arpa D, Sainz RM, Mayo JC, Ramos S. The oxidant/antioxidant network: Role of melatonin. Biol Signals Recept. 1999;8:56–63. doi: 10.1159/000014569. [DOI] [PubMed] [Google Scholar]
- 10.Othman AI, El-Missiry MA, Amer MA. The protective action of melatonin on indomethacin-induced gastric and testicular oxidative stress in rats. Redox Rep. 2001;6:173–177. doi: 10.1179/135100001101536283. [DOI] [PubMed] [Google Scholar]
- 11.Othman AI, al Sharawy S, el-Missiry MA. Role of melatonin in ameliorating lead induced haematotoxicity. Pharmacol Res. 2004;50:301–307. doi: 10.1016/j.phrs.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 13.Reiter RJ, Tan DX, Gitto E, Sainz RM, Mayo JC, Leon J, Manchester LC, Vijayalaxmi Kilic E, Kilic U. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol J Pharmacol. 2004;56:159–170. [PubMed] [Google Scholar]
- 14.Kaya H, Delibas N, Serteser M, Ulukaya E, Ozkaya O. The effect of melatonin on lipid peroxidation during radiotherapy in female rats. Strahlenther Onkol. 1999;175:285–288. doi: 10.1007/BF02743581. [DOI] [PubMed] [Google Scholar]
- 15.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 16.Kruk I, Aboul-Enein HY, Michalska T, Lichszteld K, Kladna A. Scavenging of reactive oxygen species by the plant phenols genistein and oleuropein. Luminescence. 2005;20:81–89. doi: 10.1002/bio.808. [DOI] [PubMed] [Google Scholar]
- 17.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899:44–60. doi: 10.1111/j.1749-6632.2000.tb06175.x. [DOI] [PubMed] [Google Scholar]
- 18.Liang HW, Qiu SF, Shen J, Sun LN, Wang JY, Bruce IC, Xia Q. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neurosci Lett. 2008;438:116–120. doi: 10.1016/j.neulet.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 19.Kang JL, Lee HW, Lee HS, Pack IS, Castranova V, Koh Y. Time course for inhibition of lipopolysaccharide-induced lung injury by genistein: Relationship to alteration in nuclear factor-kappaB activity and inflammatory agents. Crit Care Med. 2003;31:517–524. doi: 10.1097/01.CCM.0000049941.84695.BA. [DOI] [PubMed] [Google Scholar]
- 20.Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23:379–385. doi: 10.1002/jat.904. [DOI] [PubMed] [Google Scholar]
- 21.Davis TA, Mungunsukh O, Zins S, Day RM, Landauer MR. Genistein induces radioprotection by hematopoietic stem cell quiescence. Int J Radiat Biol. 2008;84:713–726. doi: 10.1080/09553000802317778. [DOI] [PubMed] [Google Scholar]
- 22.Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M, Joiner MC, Marples B, Forman JD, Sarkar FH. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3:1271–1279. [PubMed] [Google Scholar]
- 23.Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 26.Kleinman WA, Richie JP., Jr Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol. 2000;60:19–29. doi: 10.1016/S0006-2952(00)00293-8. [DOI] [PubMed] [Google Scholar]
- 27.Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225:9–22. doi: 10.1111/j.1525-1373.2000.22502.x. [DOI] [PubMed] [Google Scholar]
- 28.Undeger U, Giray B, Zorlu AF, Oge K, Bacaran N. Protective effects of melatonin on the ionizing radiation induced DNA damage in the rat brain. Exp Toxicol Pathol. 2004;55:379–384. doi: 10.1078/0940-2993-00332. [DOI] [PubMed] [Google Scholar]
- 29.Taysi S, Koc M, Buyukokuroglu ME, Altinkaynak K, Sahin YN. Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J Pineal Res. 2003;34:173–177. doi: 10.1034/j.1600-079X.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 30.Tahamtan R, Shabestani Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA, Naghizadeh MM, Ghasemi D, Keshavarz M, Haddadi GH. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J. 2015;17:111–120. doi: 10.22074/cellj.2015.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaldir M, Cosar-Alas R, Cermik TF, Yurut-Caloglu V, Saynak M, Altaner S, Caloglu M, Kocak Z, Tokatli F, Ture M, et al. Amifostine use in radiation-induced kidney damage. Preclinical evaluation with scintigraphic and histopathologic parameters. Strahlenther Onkol. 2008;184:370–375. doi: 10.1007/s00066-008-1777-7. [DOI] [PubMed] [Google Scholar]
- 32.Cosar R, Yurut-Caloglu V, Eskiocak S, Ozen A, Altaner S, Ibis K, Turan N, Denizli B, Uzal C, Saynak M, et al. Radiation-induced chronic oxidative renal damage can be reduced by amifostine. Med Oncol. 2012;29:768–775. doi: 10.1007/s12032-011-9870-7. [DOI] [PubMed] [Google Scholar]
- 33.Anwar MM, Moustafa MA. The effect of melatonin on eye lens of rats exposed to ultraviolet radiation. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:57–63. doi: 10.1016/S1532-0456(01)00180-6. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs FP, Vriend J. The half-life of melatonin elimination from rat plasma. Endocrinology. 1981;109:1796–1798. doi: 10.1210/endo-109-5-1796. [DOI] [PubMed] [Google Scholar]
- 35.Wetterberg L, Eriksson O, Friberg Y, Vangbo B. A simplified radioimmunoassay for melatonin and its application to biological fluids. Preliminary observations on the half-life of plasma melatonin in man. Clin Chim Acta. 1978;86:169–177. doi: 10.1016/0009-8981(78)90130-4. [DOI] [PubMed] [Google Scholar]
- 36.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 37.Kucuktulu E, Yavuz AA, Cobanoglu U, Yenilmez E, Eminagaoglu S, Karahan C, Topbas M, Kucuktulu U. Protective effect of melatonin against radiation induced nephrotoxicity in rats. Asian Pac J Cancer Prev. 2012;13:4101–4105. doi: 10.7314/APJCP.2012.13.8.4101. [DOI] [PubMed] [Google Scholar]
- 38.Cassady JR. Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys. 1995;31:1249–1256. doi: 10.1016/0360-3016(94)00428-N. [DOI] [PubMed] [Google Scholar]
- 39.Kim TH, Freeman CR, Webster JH. The significance of unilateral radiation nephropathy. Int J Radiat Oncol Biol Phys. 1980;6:1567–1571. doi: 10.1016/0360-3016(80)90016-4. [DOI] [PubMed] [Google Scholar]
- 40.Prager W, Merkelbach K. Long-term sequelae in the kidneys following abdominal irradiation of advanced malignant ovarian tumors. Radiobiol Radiother (Berl) 1986;27:341–345. (In German) [PubMed] [Google Scholar]
- 41.Kim BC, Shon BS, Ryoo YW, Kim SP, Lee KS. Melatonin reduces X-ray irradiation-induced oxidative damages in cultured human skin fibroblasts. J Dermatol Sci. 2001;26:194–200. doi: 10.1016/S0923-1811(01)00088-3. [DOI] [PubMed] [Google Scholar]
- 42.Vijayalaxmi Reiter RJ, Herman TS, Meltz ML. Melatonin and radioprotection from genetic damage: In vivo/in vitro studies with human volunteers. Mutat Res. 1996;371:221–228. doi: 10.1016/S0165-1218(96)90110-X. [DOI] [PubMed] [Google Scholar]
- 43.Vijayalaxmi Reiter RJ, Herman TS, Meltz ML. Melatonin reduces gamma radiation-induced primary DNA damage in human blood lymphocytes. Mutat Res. 1998;397:203–208. doi: 10.1016/S0027-5107(97)00211-X. [DOI] [PubMed] [Google Scholar]
- 44.Sharma S, Haldar C, Chaube SK, Laxmi T, Singh SS. Long-term melatonin administration attenuates low-LET gamma-radiation-induced lymphatic tissue injury during the reproductively active and inactive phases of Indian palm squirrels (Funambulus pennanti) Br J Radiol. 2010;83:137–151. doi: 10.1259/bjr/73791461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma S, Haldar C, Chaube SK. Effect of exogenous melatonin on X-ray induced cellular toxicity in lymphatic tissue of Indian tropical male squirrel, Funambulus pennanti. Int J Radiat Biol. 2008;84:363–374. doi: 10.1080/09553000802029894. [DOI] [PubMed] [Google Scholar]
- 46.Sener G, Jahovic N, Tosun O, Atasoy BM, Yeğen BC. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003;74:563–572. doi: 10.1016/j.lfs.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Karslioglu I, Ertekin MV, Taysi S, Koçer I, Sezen O, Gepdiremen A, Koç M, Bakan N. Radioprotective effects of melatonin on radiation-induced cataract. J Radiat Res (Tokyo) 2005;46:277–282. doi: 10.1269/jrr.46.277. [DOI] [PubMed] [Google Scholar]
- 48.Haddadi G, Shirazi A, Sepehrizadeh Z, Mahdavi SR, Haddadi M. Radioprotective effect of melatonin on the cervical spinal cord in irradiated rats. Cell J. 2013;14:246–253. [PMC free article] [PubMed] [Google Scholar]
- 49.Erol FS, Topsakal C, Ozveren MF, Kaplan M, Ilhan N, Ozercan IH, Yildiz OG. Protective effects of melatonin and vitamin E in brain damage due to gamma radiation: An experimental study. Neurosurg Rev. 2004;27:65–69. doi: 10.1007/s10143-003-0291-8. [DOI] [PubMed] [Google Scholar]
- 50.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/S0300-483X(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 51.Wei H, Zhang X, Wang Y, Lebwohl M. Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by isoflavone genistein. Cancer Lett. 2002;185:21–29. doi: 10.1016/S0304-3835(02)00240-9. [DOI] [PubMed] [Google Scholar]
- 52.Shimoi K, Masuda S, Furugori M, Esaki S, Kinae N. Radioprotective effect of antioxidative flavonoids in gamma-ray irradiated mice. Carcinogenesis. 1994;15:2669–2672. doi: 10.1093/carcin/15.11.2669. [DOI] [PubMed] [Google Scholar]
- 53.Anné PR, Curran WJ., Jr A phase II trial of subcutaneous amifostine and radiation therapy in patients with head and neck cancer. Semin Radiat Oncol. 2002;12(Suppl 1):18–19. doi: 10.1053/srao.2002.31358. [DOI] [PubMed] [Google Scholar]
- 54.White DC. The histopathologic basis for functional decrements in late radiation injury in diverse organs. Cancer. 1976;37(Suppl 2):1126–1143. doi: 10.1002/1097-0142(197602)37:2+<1126::AID-CNCR2820370823>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 55.Williams MV, Denekamp J. Sequential functional testing of radiation-induced renal damage in the mouse. Radiat Res. 1983;94:305–317. doi: 10.2307/3575965. [DOI] [PubMed] [Google Scholar]
- 56.Kim JS, Heo K, Yi JM, Gong EJ, Yang K, Moon C, Kim SH. Genistein mitigates radiation-induced testicular injury. Phytother Res. 2012;26:1119–1125. doi: 10.1002/ptr.3689. [DOI] [PubMed] [Google Scholar]