Abstract
Crimean-Congo hemorrhagic fever (CCHF) is a vector-borne viral disease, widely distributed in different regions of the world. The fever is caused by the CCHF virus (CCHFV), which belongs to the Nairovirus genus and Bunyaviridae family. The virus is clustered in seven genotypes, which are Africa-1, Africa-2, Africa-3, Europe-1, Europe-2, Asia-1 and Asia-2. The virus is highly pathogenic in nature, easily transmissible and has a high case fatality rate of 10–40%. The reservoir and vector of CCHFV are the ticks of the Hyalomma genus. Therefore, the circulation of this virus depends upon the distribution of the ticks. The virus can be transmitted from tick to animal, animal to human and human to human. The major symptoms include headache, high fever, abdominal pain, myalgia, hypotension and flushed face. As the disease progresses, severe symptoms start appearing, which include petechiae, ecchymosis, epistaxis, bleeding gums and emesis. Enzyme-linked immunosorbent assay, quantitative polymerase chain reaction, antigen detection, serum neutralization and isolation of the virus by cell culture are the diagnostic techniques used for this viral infection. There is no specific antiviral therapy available thus far. However, ribavirin has been approved by the World Health Organization for the treatment of CCHFV infection. Awareness campaigns regarding the risk factors and control measures can aid in reducing the spread of this disease to a greater extent, particularly in developing countries.
Keywords: Crimean-Congo hemorrhagic fever, Crimean-Congo hemorrhagic fever virus, Pakistan, risk factors, control measures
1. Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a lethal viral infection of medical significance. It is widespread throughout the world and is most common among tick-borne viral diseases. Ticks of the genus Hyalomma are transmission agents of the CCHF virus (CCHFV) in humans. The virus is maintained in tick species through horizontal and vertical transmission and spreads to domestic animals, which further carry the disease to humans. Therefore, it is a zoonotic disease (1–4). CCHFV evolved 3,100–3,500 years ago (5). In 1944, the disease was first reported in Crimea, and was therefore assigned as Crimean hemorrhagic fever. In 1969, the same disease was reported in the Congo region, resulting in its current name: ‘Crimean-Congo hemorrhagic fever.’ Previous studies have observed that the disease is widely distributed in different regions of the world, including Africa; Asia; central southern Europe; eastern Europe, particularly in the former Soviet Union; throughout the Mediterranean; in north-western China; the Middle East; and the Indian subcontinent (2,6). Since 2002, the virus has also shown its emergence in several countries of the Balkans, leading to the concern that CCHF is expanding in its current geographical distribution (7). The virus is clustered among seven genotypes; Africa-1, Africa-2, Africa-3, Europe-1, Europe-2, Asia-1 and Asia-2. These seven genotypes are characterized on the basis of genetic variation in small segments of RNA (8,9). The CCHF is severe as it causes serious medical problems and also results in fatalities when not treated. The disease can be described mainly as the presence of blood in sputum, gums, rectum and urine (10). Another cause for concern is that CCHFV is highly pathogenic in nature, easily transmissible and has a high case-fatality rate of 10–40%. Due to the highly pathogenic nature of CCHFV, the culture of the virus is only permitted in biosafety level four (BSL-4) and in maximum secured laboratories; there is a possible risk of this virus being used as an agent of bioterrorism or as biological warfare (7,11–14).
As the virus has a widespread geographical distribution, it must be recognized as a global health threat. Pakistan has also been experiencing this epidemic disease, covering almost all four of the Punjab, Baluchistan, Khyber Pakhtunkhwan (KPK) and Sindh provinces. Administering preventive measures is urgently required to eradicate the virus from the country, as subsequent to poliovirus, CCHFV may become a serious challenge for the country (15).
In the present review, virology, vector, transmission pathway of the virus, risk factors and control measures are discussed with regards to the current literature to minimize the impact of infection in Pakistan. In additional, the clinical symptoms, diagnostic tests and treatment for this epidemic disease are briefly described.
2. Virology
CCHFV belongs to the genus Nairovirus and family Bunyaviridae (7). The genus Nairovirus contains ~34 tick-borne viruses and these are categorized into seven serogroups (16). The circulation of CCHFV is dependent upon the distribution of ticks, mainly of the Hyalomma genus (1,17). The virus is spherical in shape with a diameter of 80–100 nm, the lipid envelope is 5–7 nm thick and glycoprotein spikes are 8–10 nm in length (18). The genome consists of single-stranded RNA with negative polarity, divided into three segments: Small, medium and large segments. These three segments form a complex with nucleocapsid proteins to become a ribo-nucleocapsid. The virion contains three structural proteins: i) A nucleocapsid protein, ii) glycoproteins (Gn and Gc) and iii) a large polypeptide protein, which is a virion-associated RNA-dependent RNA polymerase with a size of 200 kDa (13,19). The structural features of the virus are shown in Fig. 1.
Figure 1.
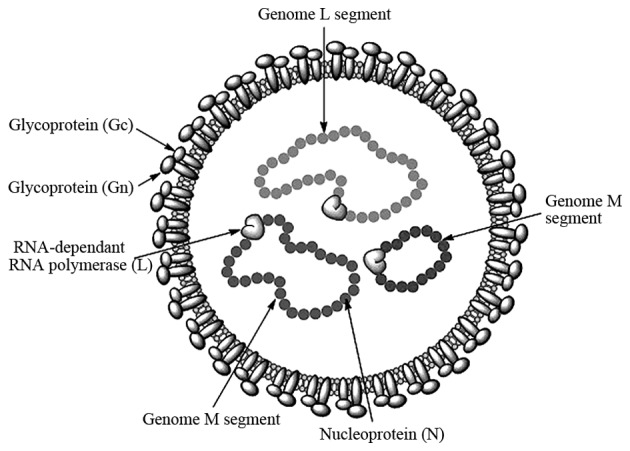
Crimean-Congo hemorrhagic fever virus structure.
3. Vector
CCHFV is mainly tick borne and is also found in one biting midge species (Culicoides spp.). The virus has been isolated from two different families of tick: Argasidae (soft ticks) and Ixodidae (hard ticks) (20). The transmission cycle runs between tick to vertebrate and again to tick. Vertical and horizontal transmissions involve tick and domestic/wild-live stocks causing them to become viremic without any disease symptoms. Migrating birds can easily carry infected ticks and act as a source of virus dispersal (21). CCHFV occurrence has been reported in >30 species of ticks belonging to different genera. Ticks of Hyalomma genus are considered as the major vector for human infection; however, in Kazakhstan, the Dermatocentor niveus ticks are also considered as vectors (22).
4. Transmission
Transmission can be from person to person, through contact with infectious body fluids of the infected person and contact with animal blood or products. The life cycle of ticks and the transmission pathway of CCHFV is shown in Fig. 2. The Hyalomma genus ticks are the reservoirs and vectors of the CCHFV. The larvae and nymphs of two-host ticks of this genus feed on hares and small birds feeding on the ground, while at the adult stage they obtain their nutritional requirements from cattle, sheep and certain large mammals. There are certain other Hyalomma species, which are three-host ticks as they drop off their host following each molt (3). Another factor responsible for the transmission of CCHF is the migration of infected livestock populations from infected areas to new areas (23).
Figure 2.
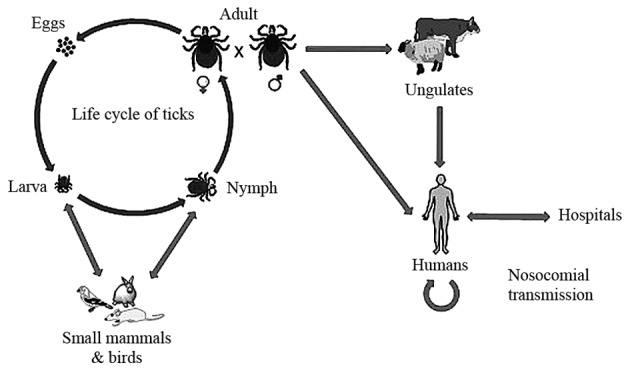
Life cycle of the tick and transmission pathway of Crimean-Congo hemorrhagic fever virus (CCHFV). Subsequent to hatching, larvae find a small animal as its host for its blood meal. Following engorgement, molting of the nymph into an adult occurs and they drop off from their host. Following this, these adult ticks find a large animal for feeding, and mate while attached to their host. CCHFV is transmitted from infected ticks and animals to humans, and from humans to humans in hospitals (nosocomial transmission).
Vertical transmission
While shifting from larva to the adult stage during metamorphosis, tick vectors support the replication of the virus present inside their body tissues. Following this, the virus is transmitted to eggs and adult females from adult females and adult males, respectively (24–27). In the mid-gut lining of the tick, the virus replicates and finally disseminates to different body tissues, for example, reproductive organs and salivary glands (28). Therefore, through transovarian transmission, thousands of infected eggs are being produced, which are sufficient enough to maintain a large population of infected ticks (29).
Horizontal transmission
During the summer and spring months (June-October), the spread of CCHFV between ticks and animals is higher when larvae and nymphs develop into the adult form by taking the blood meal for their growth. A bite of the infected tick to their host, i.e., small vertebrates, results in transmission of the virus from the tick to their host, and subsequently, healthy ticks feeding on the same host followed by virus replication in host tissue and its circulation in the bloodstream. Previous studies have revealed that all the mammals are not susceptible to infection by CCHFV (27,30).
Non-viremic transmission
This is another type of viral transmission that does not require an animal to be viremic, but directly transfer from the infected to healthy ticks feeding together. During co-feeding, viral substances present in the saliva of ticks accelerate the viral transmission (31–33).
Transmission to birds
Birds are commonly resistant to becoming viremic. No specific antibodies are detected in 37 different species of birds infected with the virus, as CCHFV do not rely on birds as a host for its replication (34).
Transmission to humans
Humans are considered as the dead-end host of CCHFV. CCHFV infection is most common in rural areas where exposure to ticks is high and people become infected when bitten by infected ticks. Physical contact with infected bodily fluids or blood can transmit the virus from person to person within 7–10 days of illness. Transmission can also occur by contact with infected animal blood. This type of transmission is extremely common in butchers' shops (3).
5. Clinical symptoms
There are mainly four different phases that are involved in the infection of the CCHFV: Incubation period (non-symptomatic phase), pre-hemorrhagic, hemorrhagic and convalescent (symptomatic phases). The incubation period lasts from 3–7 days of infection. The disease starts with the pre-hemorrhagic period for 4–5 days. The major symptoms include headache, high fever, abdominal pain, myalgia, hypotension and flushed face (10). As the disease progresses, severe symptoms starts appearing including petechiae (red spots on skin), ecchymosis (extravasation of blood), epistaxis (nose bleeding), gum bleeding and emesis (35–37). Nausea, diarrhea, emesis, neuropsychiatric and cardiovascular changes can be additional symptoms (20). When the disease is not treated, patients may succumb due to multiorgan failure. The convalescent period begins in survivors after 10–20 days of illness (16). Full recovery can take a complete year in survivors of CCHF (1).
6. Scenario of CCHF in Pakistan
Due to its name, there is confusion surrounding the prevalance of the virus outside of the Congo; however, it has been reported in Pakistan and the ‘Congo virus is a reality in the country’, as stated by Dr Muhammad Najeeb Khan Durrani, a Senior Surveillance Coordinator of Communicable Diseases in Islamabad (38). In Pakistan, the virus was first isolated from the Hyalomma tick species in 1960 (39). Since then, sporadic cases and repeated outbreaks have been observed mainly in people who deal with livestock (40). The most prevalent genotype of CCHFV in Pakistan is Asia-1; however, in Baluchistan the Asia-1 and Asia-2 genotypes have been reported (8). In 1976, at a general hospital of Rawalpindi, a person suffering from abdominal pain and hematemesis (blood vomiting) was reported to be the first case of CCHF in Pakistan (41,42). According to published studies of CCHF between 1976 and 2000, there were 23 cases of CCHF in Pakistan with a case fatality rate of 39% (12,43). From 2000, a significant increase in CCHF cases was observed, with 50–60 cases reported annually. In general, there is usually a biannual surge of cases in the country appearing in June and October, at its peak in association with the life cycle of the tick (44). The country experienced an outbreak of CCHF in 2012 when there were 61 suspected cases with 17 fatalities and a 27.8% case-fatality rate. The disease was mainly prevalent in the province of Baluchistan; however, cases were also reported in Sindh, KPK and Punjab (45). Another outbreak of the virus was reported on September 7, 2013 in Haripur when four butchers succumbed due to working with the meat of an infected sheep (38). In 2014, confirmation of the virus in Baluchistan triggered a further requirement to improve the management of the virus (46). The annual suspected cases and the case fatality rate are shown in Figs. 3 and 4, respectively.
Figure 3.
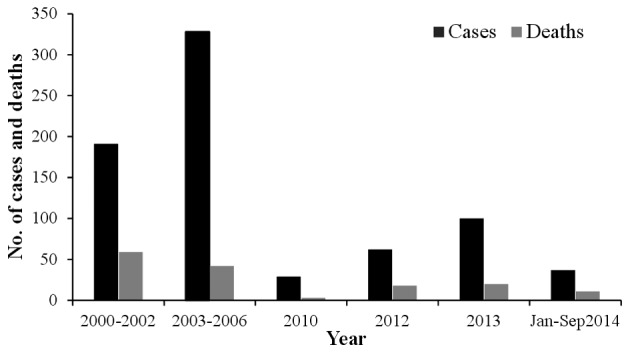
Annually reported cases of Crimean-Congo hemorrhagic fever and resulting deaths in Pakistan.
Figure 4.
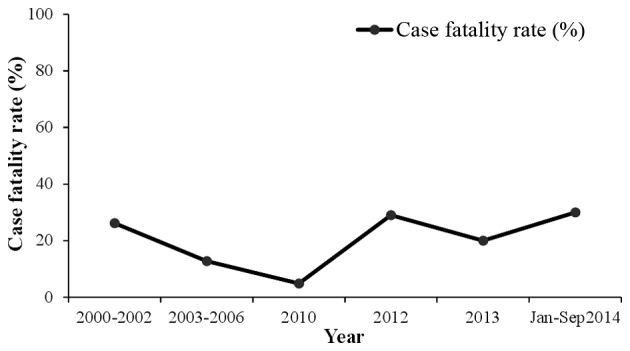
Annual case fatality rate (%) of Crimean-Congo hemorrhagic fever in Pakistan.
7. Risk factors
The virus can be transmitted from person to person through contact with animal blood or products, contact with infectious body fluids of an infected person and by handling the infected ticks (47). The areas outside the range of tick distribution are at little or no risk of exposure to ticks. Crushing and rubbing the infected tick on skin or slaughtering the infected animal is also one of the main risk factors towards the exposure of CCHFV. Another well-documented risk factor is nosocomial infection. This is most common among health care workers, particularly during the hemorrhagic period of the disease (48,49). As previously reported, this factor was exemplified in January 1976, when at Central Government Hospital Rawalpindi (Pakistan), a nosocomial incident occurred (10,43), in which the infection was transmitted from a shepherd to a female physician, a surgeon, an assistant surgeon and other health workers. In South Africa at the Tygerberg Hospital, another nosocomial outbreak occurred in which 33% of the health workers developed CCHF by accidental contact with the patient through a needle prick, and 8.7% were infected through contact with the blood or other body fluids of the patient. Droplet-respiratory route of infection is also counted as one of the risk factors of CCHF (13). This is supported by several cases of laboratory-acquired CCHF in Africa. Laboratory personnel dealing with viral samples are also highly likely to develop the disease, as supported by numerous cases of CCHF acquired from a laboratory in Africa (50). For all these reasons, CCHFV has been characterized as a BSL-4 pathogen in the United States by the Center for Disease Control and Prevention (51).
8. Control measures
As the life cycle of the tick remains unnoticed in animals, control of CCHF infection in animals and ticks is difficult. The infection is not usually apparent in animals, and only viremia occurs (20). There is no vaccine available, the only way to reduce the infection is by creating public awareness regarding the risk factors of the disease and possible preventive measures, which aid in reducing the exposure to the virus and controlling the spread of the disease.
The risk of the tick to human transmission can be minimized by avoiding areas with a high prevalence of ticks and undertaking special precautions in the most active season of ticks. People who are in high-risk occupations (such as butchers, veterinarians and shepherds) should undergo every possible measure to avoid exposure to virus-infected ticks or virus-contaminated animal blood and other tissues. For instance, the use of gloves and minimal exposure of naked skin to fresh blood animal and other tissues are effective control measures. Similarly, medical workers caring for suspected patients of CCHF should adopt standard barrier nursing techniques. Unpasteurized milk should not be utilized. Only properly cooked food should be consumed, as this kills the viruses. Treating the livestock with acaricides is effective in decreasing the population of infected ticks. The use of commercially available insect repellents, including diethyl toluamide on naked skin is also protective against tick bites. Clothes should be treated with permethrin spray, as it also shields against tick bites (20).
For reducing the risk of animal to human transmission, quarantine measures should be taken while importing animals and they should be treated with pesticides regularly. Maintenance of hygienic conditions during slaughtering, butchering and culling procedures in slaughterhouses or at home is mandatory. Gloves should be worn during the handling of meat. Following the slaughter of an animal, the utensils and other equipment should be washed prior to reuse (14).
To reduce the risk of human-human transmission, close physical contact with the infected person should be avoided. Hands should be washed properly and regularly subsequent to visiting and caring for ill people (14). In certain developed countries, it is recommended that health care workers must use high efficiency air respirators (52); however, this practice is not feasible in a country such as Pakistan (53). Face shields, safety goggles and surgical masks should be used when coming into contact with the patient from three feet away (54,55). Isolation of the patient and barrier nursing is also recommended.
Disposal of used instruments and equipment, including needles, syringes and employing safe burial practices, should be implemented (56). Disinfectants, including 2% glutaraldehyde and 1% hypochlorite, can inactivate the CCHFV by heating at 56°C for 30 min (16).
9. Diagnostic tests
Diagnosis at an early stage is indispensable to prevent further transmission of the infection. There are different techniques for the infection diagnosis, including enzyme-linked immunosorbent assay (ELISA), quantitative polymerase chain reaction (qPCR), antigen detection, serum neutralization and isolation of the virus by cell culture (14,57). At the Bernhard-Nocht-Institute for Tropical Medicine (Hamburg, Germany), scientists have experimented with certain test systems for the detection of infection with CCHFV. All the tests use ELISA for detecting pathogen-specific immunoglobulins (Ig); human IgM or IgG blood serum antibodies. Tests were based on specific monoclonal antibodies. The hybridoma cell lines were offered for co-development of diagnostic test systems using ELISA or other technology platforms (58). The patients of CCHF are viremic in 7–10 days of disease, and by the end of the first day, weak IgM becomes detectable followed by IgG (3). The viral antigen can also be visualized in formalin-fixed tissues by immunohistochemical staining (59). The screening tests are available in the majority of diagnostic labs in Pakistan, including the Islamabad Diagnostic Center, Chughtais Lahore lab, Centre of Excellence in Molecular Biology, Lahore and Shoukat Khanum Memorial Cancer Hospital. Reliable and sensitive diagnostic tests, including ELISA and qPCR, provide an essential tool for viral detection and are helpful in minimizing the impact of infection.
10. Treatment
The treatment for CCHF viral infection mainly depends on the severity and symptoms of disease. Currently, there is no antiviral drug against CCHFV that is approved by the Food and Drug Administration (60). However, ribavirin (Virazole) is the only antiviral drug used against CCHFV, which is only effective in certain cases (61). Despite the verified insufficient efficacy of ribavirin for CCHF patients by two systematic reviews and meta-analyses, the World Health Organization has approved antiviral ribavirin for treatment of CCHFV infection based on in vitro data (7,60,62,63). Ribavirin can be taken orally and intravenously. For effective results, ribavirin is used along with supportive therapy, such as interferons (16). In numerous in vitro studies, interferon type-I is shown to have antiviral activity, however, no clinical data is available on interferon use (60,64). A recent study utilized modified vaccinia virus Ankara (attenuated poxvirus vector) to develop a recombinant vaccine that expresses glycoproteins of CCHFV in two mouse strains. A cellular and humoral immune response was confirmed against this vaccine, which protected the recipient model animals from developing the lethal disease (61). Studies were also conducted to determine the role of immunotherapy in the treatment of CCHF. A new immunoglobulin, Venin, which is specific to CCHFV, has been prepared from the plasma pool of boosted donors through ethanol-polyethylene glycol fractionation and an ion-exchange purification step (65). However, in the case of CCHFV, the beneficial effects of immunotherapy are extremely limited (20,66).
11. Conclusion
CCHF is harmful in the sense that it does not have any specific treatment. The only way to avoid this widespread infection is prevention. In a developing country such as Pakistan, the disease poses more serious effects due to inadequate resources. Due to the risks of disease in Pakistan, cross-border surveillance needs to be strengthened. Reinforcing the control measures to prevent the transmission of the disease to new areas is necessary. The animal and health sectors, by taking solid steps, can contribute to reduce the spread of this disease across the country. Awareness campaigns regarding risk factors and control measures can aid in apprising the public of the ill effects of this virus.
Glossary
Abbreviations
- CCHF
Crimean-Congo hemorrhagic fever
- CCHFV
CCHF virus
- BSL
biosafety level
- KPK
Khyber Pakhtunkhwan
- ELISA
enzyme-linked immunosorbent assay
- qPCR
quantitative polymerase chain reaction
- Ig
immunoglobulin
References
- 1.Ergönül O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahzounieh M, Dincer E, Faraji A, Akin H, Akkutay AZ, Ozkul A. Relationship between Crimean-Congo hemorrhagic fever virus strains circulating in Iran and Turkey: Possibilities for transborder transmission. Vector Borne Zoonotic Dis. 2012;12:782–785. doi: 10.1089/vbz.2011.0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Papa A, Sidira P, Larichev V, Gavrilova L, Kuzmina K, Mousavi-Jazi M, Mirazimi A, Ströher U, Nichol S. Crimean-Congo hemorrhagic fever virus, Greece. Emerg Infect Dis. 2014;20:288–290. doi: 10.3201/eid2002.130690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll SA, Bird BH, Rollin PE, Nichol ST. Ancient common ancestry of Crimean-Congo hemorrhagic fever virus. Mol Phylogenet Evol. 2010;55:1103–1110. doi: 10.1016/j.ympev.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Ergönül O. Crimean-Congo hemorrhagic fever virus: New outbreaks, new discoveries. Curr Opin Virol. 2012;2:215–220. doi: 10.1016/j.coviro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Burt FJ, Goedhals D. Crimean-Congo haemorrhagic fever virus, an emerging and re-emerging pathogen. In: Sing A, editor. Zoonoses Infections Affecting Humans and Animals. Netherlands: Springer; 2015. pp. 977–996. [Google Scholar]
- 8.Alam MM, Khurshid A, Sharif S, Shaukat S, Suleman RM, Angez M, Zaidi SS. Crimean-Congo hemorrhagic fever Asia-2 Genotype, Pakistan. Emerging. Infect Dis J. 2013;19:1017–1019. doi: 10.3201/eid1906.120771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mild M, Simon M, Albert J, Mirazimi A. Towards an understanding of the migration of Crimean-Congo hemorrhagic fever virus. J Gen Virol. 2010;91:199–207. doi: 10.1099/vir.0.014878-0. [DOI] [PubMed] [Google Scholar]
- 10.Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- 11.http://www.nih.org.pk/files/Newsletter/Seasonal%20Awarness%20and%20Alert%20Letter%20%28SAAL%29%2029th%20Issue.pdf. [Oct 08;2014 ];National Institute of Health, World Health Organization Paistan: Seasonal awareness and alert letter (SAAL) for epidemic prone infectious diseases in Pakistan winter season. Accessed. [Google Scholar]
- 12.Smego RA, Jr, Sarwari AR, Siddiqui AR. Crimean-Congo hemorrhagic fever: Prevention and control limitations in a resource-poor country. Clin Infect Dis. 2004;38:1731–1735. doi: 10.1086/421093. [DOI] [PubMed] [Google Scholar]
- 13.Whitehouse C. Risk groups and control measures for Crimean-Congo hemorrhagic fever. In: Ergonul O, Whitehouse C, editors. Crimean-Congo Hemorrhagic Fever. Netherlands: Springer; 2007. pp. 273–280. [DOI] [Google Scholar]
- 14.http://www.who.int/mediacentre/factsheets/fs208/en/ [Mar;2015 ];WHO: Crimean Congo haemorrhagic fever. 2013 Jan; Fact Sheet No. 208. Accessed. [Google Scholar]
- 15.Baloch S. Congo virus a fresh challenge for Balochistan. The Express Tribune. 2014 Aug 23; [Google Scholar]
- 16.Appannanavar SB, Mishra B. An update on Crimean-Congo hemorrhagic fever. J Glob Infect Dis. 2011;3:285–292. doi: 10.4103/0974-777X.83537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogstraal H. Research Report. Washington, DC: 1956. African Ixodoidea. Ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews pf the genera Boophilus, Margaropus, and Hyalomma) p. 1101. NM 005.050.29.27. Department of the Navy, Bureau of Medicine and Surgery. [Google Scholar]
- 18.Marriott AC, Nuttall PA. Molecular biology of nairoviruses. In: Elliott R, editor. The Bunyaviridae. US: Springer; 1996. pp. 91–104. [DOI] [Google Scholar]
- 19.Marriott AC, Nuttall PA. Large RNA segment of Dugbe nairovirus encodes the putative RNA polymerase. J Gen Virol. 1996;77:1775–1780. doi: 10.1099/0022-1317-77-8-1775. [DOI] [PubMed] [Google Scholar]
- 20.Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64:145–160. doi: 10.1016/S0166-3542(04)00163-9. [DOI] [PubMed] [Google Scholar]
- 21.Palomar AM, Portillo A, Santibáñez P, Mazuelas D, Arizaga J, Crespo A, Gutiérrez Ó, Cuadrado JF, Oteo JA. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg Infect Dis. 2013;19:260–263. doi: 10.3201/eid1902.121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onishchenko GG, Tumanova IIU, Vyshemirskiĭ OI, Kuhn J, Seregin SV, Tiunnikov GI, Petrova ID, Tishkova FKH, Ospanov KS, Kazakov SV, et al. Study of virus contamination of Ixodes ticks in the foci of Crimean-Congo hemorrhagic fever in Kazakhstan and Tajikistan. Zh Mikrobiol Epidemiol Immunobiol. 2005;1:27–31. (In Russian) [PubMed] [Google Scholar]
- 23.Alam MM, Khurshid A, Sharif S, Shaukat S, Rana MS, Angez M, Zaidi SS. Genetic analysis and epidemiology of Crimean-Congo hemorrhagic fever viruses in Baluchistan province of Pakistan. BMC Infect Dis. 2013;13:201. doi: 10.1186/1471-2334-13-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dohm DJ, Logan TM, Linthicum KJ, Rossi CA, Turell MJ. Transmission of Crimean-Congo hemorrhagic fever virus by Hyalomma impeltatum (Acari: Ixodidae) after experimental infection. J Med Entomol. 1996;33:848–851. doi: 10.1093/jmedent/33.5.848. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez JP, Camicas JL, Cornet JP, Faye O, Wilson ML. Sexual and transovarian transmission of Crimean-Congo haemorrhagic fever virus in Hyalomma truncatum ticks. Res Virol. 1992;43:23–28. doi: 10.1016/S0923-2516(06)80073-7. [DOI] [PubMed] [Google Scholar]
- 26.Logan TM, Linthicum KJ, Bailey CL, Watts DM, Dohm DJ, Moulton JR. Replication of Crimean-Congo hemorrhagic fever virus in four species of ixodid ticks (Acari) infected experimentally. J Med Entomol. 1990;27:537–542. doi: 10.1093/jmedent/27.4.537. [DOI] [PubMed] [Google Scholar]
- 27.Shepherd AJ, Swanepoel R, Shepherd SP, Leman PA, Mathee O. Viraemic transmission of Crimean-Congo haemorrhagic fever virus to ticks. Epidemiol Infect. 1991;106:373–382. doi: 10.1017/S0950268800048524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson DL, Turell MJ. Replication and tissue tropisms of Crimean-Congo hemorrhagic fever virus in experimentally infected adult Hyalomma truncatum (Acari: Ixodidae) J Med Entomol. 1992;29:767–773. doi: 10.1093/jmedent/29.5.767. [DOI] [PubMed] [Google Scholar]
- 29.Labuda M, Nuttall PA. Tick-borne viruses. Parasitology. 2004;129:S221–S245. doi: 10.1017/S0031182004005220. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd AJ, Leman PA, Swanepoel R. Viremia and antibody response of small African and laboratory animals to Crimean-Congo hemorrhagic fever virus infection. Am J Trop Med Hyg. 1989;40:541–547. doi: 10.4269/ajtmh.1989.40.541. [DOI] [PubMed] [Google Scholar]
- 31.Nuttall PA, Labuda M. Tick-host interactions: Saliva-activated transmission. Parasitology. 2004;129:S177–S189. doi: 10.1017/S0031182004005633. [DOI] [PubMed] [Google Scholar]
- 32.Nuttall PA, Labuda M. Dynamics of infection in tick vectors and at the tick-host interface. Adv Virus Res. 2003;60:233–272. doi: 10.1016/S0065-3527(03)60007-2. [DOI] [PubMed] [Google Scholar]
- 33.Jones LD, Davies CR, Steele GM, Nuttall PA. A novel mode of arbovirus transmission involving a nonviremic host. Science. 1987;237:775–777. doi: 10.1126/science.3616608. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd AJ, Swanepoel R, Leman PA, Shepherd SP. Field and laboratory investigation of Crimean-Congo haemorrhagic fever virus (Nairovirus, family Bunyaviridae) infection in birds. Trans R Soc Trop Med Hyg. 1987;81:1004–1007. doi: 10.1016/0035-9203(87)90379-8. [DOI] [PubMed] [Google Scholar]
- 35.Ergönül O, Celikbaş A, Dokuzoguz B, Eren S, Baykam N, Esener H. Characteristics of patients with Crimean-Congo hemorrhagic fever in a recent outbreak in Turkey and impact of oral ribavirin therapy. Clin Infect Dis. 2004;39:284–287. doi: 10.1086/422000. [DOI] [PubMed] [Google Scholar]
- 36.Bakir M, Ugurlu M, Dokuzoguz B, Bodur H, Tasyaran MA, Vahaboglu H. Turkish CCHF Study Group: Crimean-Congo haemorrhagic fever outbreak in Middle Anatolia: A multicentre study of clinical features and outcome measures. J Med Microbiol. 2005;54:385–389. doi: 10.1099/jmm.0.45865-0. [DOI] [PubMed] [Google Scholar]
- 37.Ozkurt Z, Kiki I, Erol S, Erdem F, Yilmaz N, Parlak M, Gundogdu M, Tasyaran MA. Crimean-Congo hemorrhagic fever in Eastern Turkey: Clinical features, risk factors and efficacy of ribavirin therapy. J Infect. 2006;52:207–215. doi: 10.1016/j.jinf.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Butt Q. Another outbreak?: Congo virus threatens lives, warn doctors. The Express Tribune. 2013 Oct 07; [Google Scholar]
- 39.Begum F, Wisseman CL, Jr, Casals J. Tick-borne viruses of West Pakistan. IV. Viruses similar to or identical with, Crimean hemorrhagic fever (Congo-Semunya), Wad Medani and Pak Argas 461 isolated from ticks of the Changa Manga Forest, Lahore District, and of Hunza, Gilgit Agency, W. Pakistan. Am J Epidemiol. 1970;92:197–202. doi: 10.1093/oxfordjournals.aje.a121199. [DOI] [PubMed] [Google Scholar]
- 40.Jamil B, Hasan RS, Sarwari AR, Burton J, Hewson R, Clegg C. Crimean-Congo hemorrhagic fever: Experience at a tertiary care hospital in Karachi, Pakistan. Trans R Soc Trop Med Hyg. 2005;99:577–584. doi: 10.1016/j.trstmh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Malik S, Diju IU, Naz F. Crimean Congo hemorrhagic fever in Hazara division. J Ayub Med Coll Abbottabad. 2011;23:90–92. [PubMed] [Google Scholar]
- 42.Athar MN, Baqai HZ, Ahmad M, Khalid MA, Bashir N, Ahmad AM, Balouch AH, Bashir K. Short report: Crimean-Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002. Am J Trop Med Hyg. 2003;69:284–287. [PubMed] [Google Scholar]
- 43.Burney MI, Ghafoor A, Saleen M, Webb PA, Casals J. Nosocomial outbreak of viral hemorrhagic fever caused by Crimean Hemorrhagic fever-Congo virus in Pakistan, January 1976. Am J Trop Med Hyg. 1980;29:941–947. doi: 10.4269/ajtmh.1980.29.941. [DOI] [PubMed] [Google Scholar]
- 44.http://www.nih.org.pk/files/Guidelines/CCHF%20guidelines%20September%202013.pdf. [Mar;2015 ];NIH and WHO: Guidelines for Crimean-Congo hemorrhagic fever (CCHF) 2013 Sep; Accessed. [Google Scholar]
- 45.WHO: Crimean-Congo haemorrhagic fever (CCHF) in Pakistan. http://www.emro.who.int/index.html. [Apr;2015 ];Surveillance, Forecasting and Response. 2013 Jun 14; 2013. Accessed. [Google Scholar]
- 46.Abbas T, Younus M, Muhammad SA. Spatial cluster analysis of human cases of Crimean-Congo hemorrhagic fever reported in Pakistan. Infect Dis Poverty. 2015;4:9. doi: 10.1186/2049-9957-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gozel MG, Bakir M, Oztop AY, Engin A, Dokmetas I, Elaldi N. Investigation of Crimean-Congo hemorrhagic fever virus transmission from patients to relatives: a prospective contact tracing study. Am J Trop Med Hyg. 2014;90:160–162. doi: 10.4269/ajtmh.13-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Wal BW, Joubert JR, van Eeden PJ, King JB. A nosocomial outbreak of Crimean-Congo haemorrhagic fever at Tygerberg Hospital. Part IV. Preventive and prophylactic measures. S Afr Med J. 1985;68:729–732. [PubMed] [Google Scholar]
- 49.Fisher-Hoch SP, Khan JA, Rehman S, Mirza S, Khurshid M, McCormick JB. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet. 1995;346:472–475. doi: 10.1016/S0140-6736(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 50.Simpson DI, Knight EM, Courtois G, Williams MC, Weinbren MP, Kibukamusoke JW. Congo virus: A hitherto undescribed virus occurring in Africa. I. Human isolations - clinical notes. East Afr Med J. 1967;44:86–92. [PubMed] [Google Scholar]
- 51.Richmond JY, McKinney RW, editors. Biosafety in microbiological and biomedical laboratories. 4th. Washington: U.S. Government Printing Office; Apr, 1999. [Google Scholar]
- 52.Fisher-Hoch SP, Price ME, Craven RB, Price FM, Forthall DN, Sasso DR, Scott SM, McCormick JB. Safe intensive-care management of a severe case of Lassa fever with simple barrier nursing techniques. Lancet. 1985;2:1227–1229. doi: 10.1016/S0140-6736(85)90752-4. [DOI] [PubMed] [Google Scholar]
- 53.Athar MN, Khalid MA, Ahmad AM, Bashir N, Baqai HZ, Ahmad M, Balouch AH, Bashir K. Crimean-Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002: Contact tracing and risk assessment. Am J Trop Med Hyg. 2005;72:471–473. [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC): Update: Management of patients with suspected viral hemorrhagic fever - United States. MMWR Morb Mortal Wkly Rep. 1995;44:475–479. [PubMed] [Google Scholar]
- 55.Leblebicioglu H, Bodur H, Dokuzoguz B, Elaldi N, Guner R, Koksal I, Kurt H, Senturk GC. Case management and supportive treatment for patients with Crimean-Congo hemorrhagic fever. Vector Borne Zoonotic Dis. 2012;12:805–811. doi: 10.1089/vbz.2011.0896. [DOI] [PubMed] [Google Scholar]
- 56.Lloyd E, Perry H. Centers for Disease Control and Prevention. Atlanta: 1998. Infection control for viral haemorrhagic fevers in the African health care setting. Centers for Disease Control and Prevention and World Health Organization, Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting; pp. 1–198. [Google Scholar]
- 57.Vanhomwegen J, Alves MJ, Zupanc TA, Bino S, Chinikar S, Karlberg H, Korukluoğlu G, Korva M, Mardani M, Mirazimi A, et al. Diagnostic assays for Crimean-Congo hemorrhagic fever. Emerg Infect Dis. 2012;18:1958–1965. doi: 10.3201/eid1812.120710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stachelhaus DT. Efficient tools for sensitive and specific detection of infections with Chikungunya virus, Crimean-Congo hemorrhagic fever virus and other viruses causing emerging diseases. Ascenion reference number TO 12–00042 [Google Scholar]
- 59.http://www.cdc.gov/vhf/crimean-congo/diagnosis/index.html. [Feb 21;2015 ];CDC. Accessed. [Google Scholar]
- 60.Oncü S. Crimean-Congo hemorrhagic fever: An overview. Virol Sin. 2013;28:193–201. doi: 10.1007/s12250-013-3327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buttigieg KR, Dowall SD, Findlay-Wilson S, Miloszewska A, Rayner E, Hewson R, Carroll MW. A novel vaccine against Crimean-Congo haemorrhagic fever protects 100% of animals against lethal challenge in a mouse model. PLoS One. 2014;9:e91516. doi: 10.1371/journal.pone.0091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soares-Weiser K, Thomas S, Thomson G, Garner P. Ribavirin for Crimean-Congo hemorrhagic fever: Systematic review and meta-analysis. BMC Infect Dis. 2010;10:207. doi: 10.1186/1471-2334-10-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ascioglu S, Leblebicioglu H, Vahaboglu H, Chan KA. Ribavirin for patients with Crimean-Congo haemorrhagic fever: A systematic review and meta-analysis. J Antimicrob Chemother. 2011;66:1215–1222. doi: 10.1093/jac/dkr136. [DOI] [PubMed] [Google Scholar]
- 64.Ergonul O. Treatment of Crimean-Congo hemorrhagic fever. Antiviral Res. 2008;78:125–131. doi: 10.1016/j.antiviral.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Vassilenko SM, Vassilev TL, Bozadjiev LG, Bineva IL, Kazarov GZ. Specific intravenous immunoglobulin for Crimean-Congo haemorrhagic fever. Lancet. 1990;335:791–792. doi: 10.1016/0140-6736(90)90906-L. [DOI] [PubMed] [Google Scholar]
- 66.Flick R, Whitehouse CA. Crimean-Congo hemorrhagic fever virus. Curr Mol Med. 2005;5:753–760. doi: 10.2174/156652405774962335. [DOI] [PubMed] [Google Scholar]


