Abstract
The aim of the present study was to evaluate the association between the levels of lipids and B-type natriuretic peptide (BNP) in systemic lupus erythematosus (SLE) patients with heart failure (HF). A total of 46 patients with active SLE and 40 healthy, age-matched control subjects were studied. BNP was measured by an immunofluorescence assay in fresh plasma. Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, apolipoprotein (Apo) B, ApoA-I and lipoprotein(a) were assessed. Compared with the control subjects, HDL-C and ApoA-I levels were considerably decreased and TG level increased markedly from SLE patients. The average concentration of HDL-C and ApoA-I in the SLE group with HF was significantly reduced compared to those patients without HF. The results showed that the levels of HDL-C and ApoA-I in SLE patients were negatively correlated with BNP. Disease activity was associated with the TC and TG levels. The present data indicated the presence of a cardiovascular (CV) risk in active SLE with high disease activity, which was demonstrated by the high frequency of dyslipidemia and higher BNP concentrations. Therefore, dyslipoproteinemia may underlie some of the increased risk for CV disease and HF in patients with SLE.
Keywords: dyslipidemia, B-type natriuretic peptide, systemic lupus erythematosus
Introduction
Various autoimmune rheumatic diseases (ARDs), including rheumatoid arthritis, spondyloarthritis, vasculitis and systemic lupus erythematosus (SLE), are associated with premature atherosclerosis (1). Cardiovascular disease (CVD) in ARDs is caused by traditional and non-traditional risk factors (1). This association is the result of the complex interaction between classic risk factors, chronic inflammation and the production of autoantibodies. SLE is an autoimmune inflammatory disease in which accelerated atherosclerosis CVD and its sequelae are recognized as one of the most frequent causes of morbidity and mortality (2).
B-type natriuretic peptide (BNP) is the gold standard biomarker in determining the diagnosis and prognosis of heart failure (HF) and studies on natriuretic peptide-guided HF management appear to be promising (3). BNP may be of use in excluding myocardial infarction and to assist in determining prognosis in acute coronary syndrome (3). Patients with HF often present with signs and symptoms that are non-specific and with a wide differential diagnosis, making diagnosis by clinical presentation alone challenging (4).
Although lipids are a major risk factor for CVD and are routinely measured for CVD risk stratification, the association between dyslipidemia and BNP in SLE patients remains unclear. Establishing the association between levels of lipids and BNP in SLE patients is critical for understanding the role of lipids in the CVD risk among SLE patients. No previous study has investigated the correlation between BNP and lipid profiles in active SLE patients with HF. Accordingly, the present study was designed to evaluate the presence of dyslipidemia and the plasma concentrations of BNP in active SLE patients.
Patients and methods
Study population
A total of 46 patients of Northern Han Chinese descent with active SLE, diagnosed according to the criteria of the American College of Rheumatology (5), were enrolled. Patients for the study were selected from individuals attending the in-patient Department of Internal Medicine at Qingdao Municipal Hospital (Qingdao, China). At the same time, 40 healthy controls were recruited, who were ethnically, gender- and age-matched with the patients. All the patients enrolled in the present study were non-smokers, non-alcoholics and had no association with any other autoimmune disease. In the SLE patients, 26 cases were diagnosed with HF and 20 without HF. All the blood samples from the patients and healthy controls were used with informed consent and approval from the Ethics Committee of Qingdao Municipal Hospital.
Patient characteristics and clinical assessment
Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) is a global score reflecting all aspects of disease activity and a validated model for the assessment of disease activity in SLE (6). Disease activity was determined using the SLEDAI score. For all the patients and controls, blood pressure was assessed.
BNP level was defined as a plasma level ≤100 pg/ml. Hypercholesterolemia was defined as a total cholesterol (TC) level of >6.5 mmol/l or low-density lipoprotein cholesterol (LDL-C) level of >3.3 mmol/l. Hypertriglyceridemia was defined as a triglyceride (TG) level of >1.8 mmol/l and hypoalphalipoproteinemia was defined as a high-density lipoprotein cholesterol (HDL-C) level of <0.78 mmol/l. The reference interval was obtained according to the National Guide to Clinical Laboratory Procedures of China (3rd edition) (7).
Laboratory assessment
Peripheral blood was sampled from the controls and all the patients. Fasting blood samples were obtained from an antecubital vein after subjects had been seated for 30 min. A tourniquet was used but was released prior to withdrawal of blood into the vacuum tubes (Weihai Hongyu Medical Devices Co., Ltd, Weihai, China). Blood samples were divided into 3 parts: One part of the blood was anticoagulated with ethylene diamine tetraacetic acid for the BNP assay, the second part was used to prepare serum by centrifugation at 3,000 × g for 15 min for other laboratory assessments, and the remaining part was anticoagulated with sodium citrate and collected for erythrocyte sedimentation rate (ESR). BNP was measured by an immunofluorescence assay in fresh plasma (Triage® MeterPlus Analyzer; Biosite Incorp., San Diego, CA, USA) and the lipid level was measured using serum. TC and TG were measured by a colorimetric method (NingBo RuiYuan Biotechnology Co., Ltd., Zhejiang, China). HDL-C and LDL-C were quantified by the GPO-PAP method (Beckman Coulter, Miami, FL, USA). Apolipoprotein (Apo) B and ApoA-I were measured by an enzymatic method (Beckman Coulter). Lipoprotein(a) was analyzed by the latex-enhanced immunoturbidimetric method (NingBo RuiYuan Biotechnology Co., Ltd.).
All the biochemical parameters were immediately analyzed using an automatic biochemistry analyzer (Beckman Coulter). The hematological indexes of the blood, including red blood cells (RBC), white blood cells and platelets (PLT), were analyzed by an automatic hematological analyzer (Sysmex XS-800i; Sysmex Corporation, Kobe, Japan). The immunological parameters, including immunoglobulin G (IgG), IgM, IgA, IgE, C3, C4 and high-sensitivity C-reactive protein (CRP), were quantified by immunoturbidimetry using an automatic nephelometric immunoassay analyzer (BN ProSpec; Siemens, Munich, Germany). Autoantibodies, such as antinuclear antibody (ANA), anti-dsDNA and anti-Sm antibodies, were detected using immunoblotting according to the manufacturer's instructions (Euroimmun AG, Lübeck, Germany). For all the subjects, ESR was analyzed using an automatic analyzer (Monitor-J+ analyzer; Electa-Lab s.r.l., Forli, Italy). Accuracy, precision and quality control in the laboratory were under internal performance verification, internal quality control and external quality assessment for laboratory medicine by the National Center for Clinical Laboratories (Beijing, China).
Statistical analysis
Statistical analysis was performed using the SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation. The difference between subject groups was analyzed using the Student's t-test independently. Correlation analysis was performed using Spearman's Rho rank test. P<0.05 was considered to indicate a statistically significant difference. All the figures were generated with the GraphPad Prism software, version 5.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
The demographic characteristics, clinical features, laboratory measurements and physiological characteristics of the patients with SLE and the controls are shown in Table I. Of note, the positive results of ANA, anti-dsDNA and anti-Sm autoantibodies in the SLE patients were found in 46, 28 and 11 patients, respectively. The mean value of ESR for the patients was 56 mm/h with the range from 13 to 140 mm/h. The mean value of CRP for the patients was 21.2 mg/l with the range from 3 to 64 mg/l. The mean value of SLEDAI was 15.4 with the range from 11 to 30. Compared with the control subjects, the median level of ESR in patients with SLE was 56 (range, 13–140), the median RBC counting was 3.1 (range, 1.8–5.1) and the median PLT counting was 151.5 (range, 51–250).
Table I.
Demographic characteristics, clinical features, physiological characteristics and laboratory measurements of the studied subjects.
| Characteristics | SLE patients, n=46 | Healthy controls, n=40 | P-value |
|---|---|---|---|
| Demographic characteristics | |||
| Female | 40 (87) | 33 (83) | NS |
| Male | 6 (13) | 7 (18) | NS |
| Age, years | 41.7 (24–66) | 40.6 (22–65) | NS |
| SLEDAI | 15.5 (10–30) | – | – |
| Laboratory measurements | |||
| C3, g/l | 0.63 (0.15–1.24) | – | – |
| C4, g/l | 0.21 (0.06–0.6) | – | – |
| IgG, g/l | 12.34 (3.9–20.3) | – | – |
| IgA, g/l | 2.6 (0.3–6.2) | – | – |
| IgM, g/l | 1.37 (0.4–8.3) | – | – |
| IgE, g/l | 391.7 (17–3,280) | – | – |
| ANA | 46 (100) | – | – |
| Anti-dsDNA | 28 (61) | – | – |
| Anti-Sm | 11 (24) | – | – |
| CRP, mg/l | 21.2 (3–64) | – | – |
| ESR, mm/h | 56 (13–140) | 9 (4–20) | <0.01a |
| TC, mmol/l | 5.40±0.40 | 4.30±0.52 | 0.615 |
| LDL-C, mmol/l | 2.82±0.26 | 2.31±0.13 | 0.973 |
| HDL-C, mmol/l | 1.07±0.07 | 1.28±0.22 | <0.001b |
| TG, mmol/l | 4.39±0.55 | 1.65±0.42 | <0.001b |
| ApoA-I, g/l | 1.04±0.08 | 1.52±0.25 | <0.001b |
| ApoB, g/l | 1.1±0.15 | 0.93±0.13 | 0.526 |
| Lp(a), mg/dl | 21.05±0.07 | 19.85±0.11 | 0.573 |
P<0.01,
P<0.001 compared with the control. Values are median (range), n (%) or medians of value ± standard error of medians. SLE, systemic lupus erythematosus; NS, not significant; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; -, not applicable; Ig, immunoglobulin; ANA, antinuclear antibody; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; Apo, apolipoprotein; Lp(a), lipoprotein(a).
Changes in the lipid profile
As shown in Table I, HDL-C and ApoA-I levels were decreased in SLE patients (HDL-C, 1.07±0.07 mmol/l; ApoA-I, 1.04±0.08 mmol/l) compared to the healthy controls (HDL-C, 1.28±0.22 mmol/l; ApoA-I, 1.52±0.25 mmol/l) (P<0.001). The TG level was increased markedly in SLE patients (4.39±1.34 mmol/l) when matched with the healthy controls (1.65±0.42 mmol/l) (P<0.001).
In the analysis of the studied variables, when patients were subclassified according to HF, those with HF showed a markedly increased BNP level (1,112.6±170.4 pg/ml) than those without HF (33.5±4.80 pg/ml) (P<0.0001). The average concentration of HDL-C in the SLE group with HF was 0.95±0.05 mmol/l (range, 0.72–1.69), which significantly declined compared to those patients without HF (1.32±0.05; range, 1.10–1.56) (P<0.0001) (Table II). The level of ApoA-I markedly decreased (0.96±0.10; range, 0.73–1.54) (P<0.0001) in SLE patients with HF compared to those without HF (1.20±0.04; range, 1.00–1.42) (Table II). No statistical significances were found between other lipid parameters in SLE patients with or without HF.
Table II.
Lipid profile and BNP of SLE patients with or without HF.
| Variables | SLE patients with HF | SLE patients without HF | P-value |
|---|---|---|---|
| TC, mmol/l | 5.26±0.51 | 5.68±0.67 | 0.632 |
| LDL-C, mmol/l | 2.83±0.38 | 2.82±0.30 | 0.980 |
| HDL-C, mmol/l | 0.95±0.05 | 1.32±0.05 | <0.0001a |
| TG, mmol/l | 4.97±1.93 | 3.23±1.26 | 0.558 |
| ApoA-I, g/l | 0.96±0.10 | 1.20±0.04 | <0.0001a |
| ApoB, g/l | 1.17±0.21 | 0.95±0.15 | 0.509 |
| Lp(a), mg/dl | 19.43±5.94 | 24.02±4.29 | 0.601 |
| BNP, pg/ml | 1,112.6±170.4 | 33.50±4.80 | – |
Values are medians of value ± standard error of medians.
P<0.0001, compared to SLE patients with HF. BNP, B-type natriuretic peptide; SLE, systemic lupus erythematosus; HF, heart failure; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; Apo, apolipoprotein; Lp(a), lipoprotein(a); -, not applicable.
Associations of lipid profiles and BNP with SLE patients
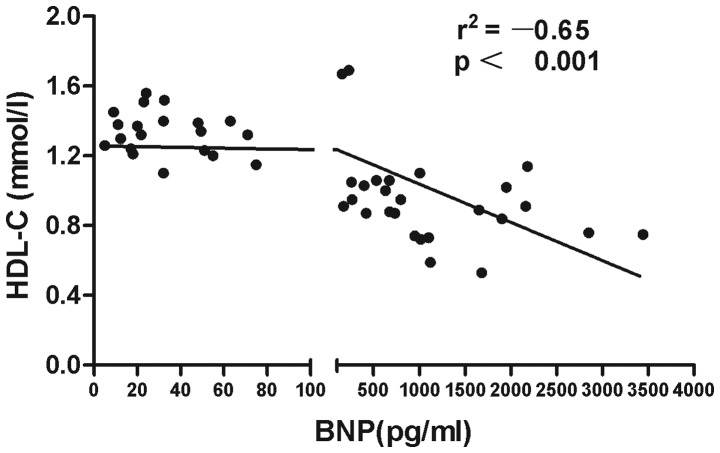
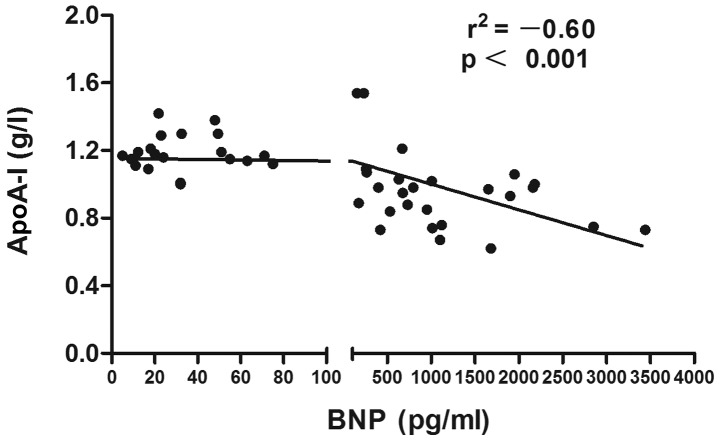
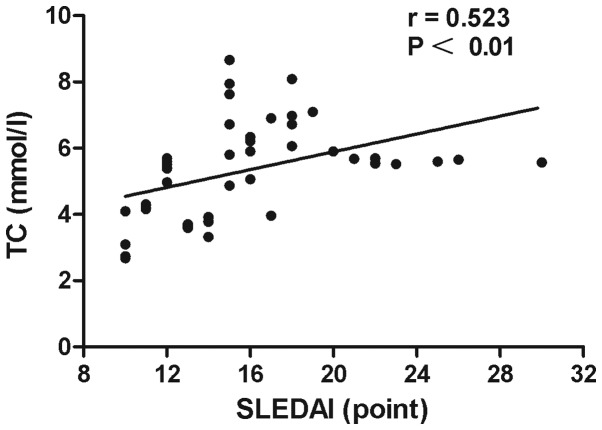
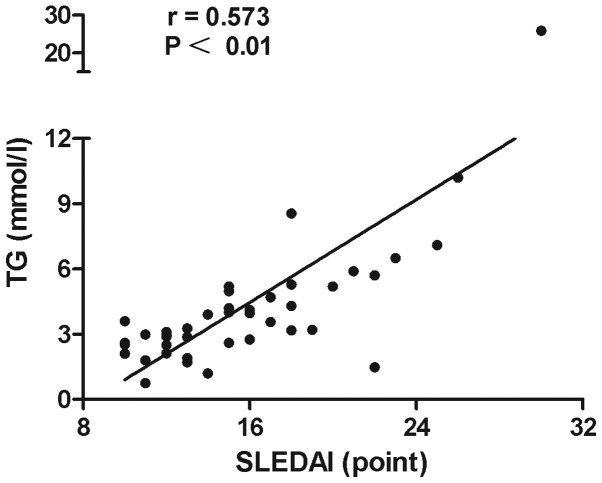
The associations of lipid profiles, SLEDAI with BNP and SLEDAI with lipid profiles in SLE patients were detected by Spearman's correlation analysis. The results showed that the level of HDL-C in SLE patients was negatively correlated with BNP (r=–0.65, P<0.0001) (Fig. 1). A significant inverse association was observed between ApoA-I and BNP level (r=–0.60, P<0.0001) (Fig. 2). Disease activity was positively associated with the TC (r=0.523, P<0.01) (Fig. 3) and TG levels (r=0.573, P<0.01) (Fig. 4). No statistically significant associations were identified between the BNP level and other characteristics, clinical manifestations or laboratory parameters in the patients with SLE.
Figure 1.
Negative correlation between BNP levels and HDL-C for all systemic lupus erythematosus patients (n=46). BNP, B-type natriuretic peptide; HDL-C, high-density lipoprotein cholesterol.
Figure 2.
Negative correlation between BNP levels and ApoA-I for all systemic lupus erythematosus patients (n=46). BNP, B-type natriuretic peptide; ApoA-I, apolipoprotein A-I.
Figure 3.
Positive correlation between TC levels and SLEDAI for all systemic lupus erythematosus patients (n=46). TC, total cholesterol; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Figure 4.
Positive correlation between TG levels and SLEDAI for all systemic lupus erythematosus patients (n=46). TG, triglyceride; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Discussion
Myocardial infarction, cerebrovascular events and subclinical atherosclerosis are increasingly recognized as serious complications of SLE (8,9). Patients with SLE have a higher prevalence of subclinical atherosclerosis and a higher risk of CV events compared to the general population (10). The estimated prevalence of CVD in the SLE population is between 6 and 10%, with an annual incidence of 1.3–1.5% (9,11,12).
Systemic inflammation and autoimmune reactions lead to monocyte and lymphocyte recruitment and activation. Increased lipid deposition and augmented inflammation in vascular intima have been suggested to underlie accelerated atherosclerosis in ARDs (13). In particular, the association between dyslipidaemia and CV risk in AIRD appears to be more complex compared to the general population (14).
Dyslipoproteinemia observed in patients with SLE has a multifactorial origin, and elucidating which factors are clearly involved in the pathophysiology of this lipid disorder is complex. The present study reported dyslipidemia in Northern Han Chinese patients with active SLE. The data clearly showed that TG increased and HDL-C and ApoA-I were reduced significantly in active SLE patients.
The natriuretic peptides represent the gold standard for biomarkers in HF and the understanding regarding their biology and their clinical use have grown exponentially (4). BNP production in normal healthy individuals is minimal, with a level of ~10 pg/ml (15). In the conditions of myocardial stretch, the induction of the BNP gene results in the production and secretion. Elevated BNP levels are also predictors of future HF or other CV events in asymptomatic patients without evident HF (4). The present study identified significantly higher concentrations of BNP in the active SLE patients with HF compared with those without HF. The novel findings of the investigation were the significantly reduced level of HDL-C and ApoA-I in active SLE patients with HF. These findings indicated a negative association of the levels of BNP with HDL-C and ApoA-I in the patients with active SLE. Therefore, it is possible that HDL-C and ApoA-I may play an extremely important role for the evaluation of HF in active SLE patients.
The association between disease activity, which was evaluated by the SLEDAI, and changes in the lipid profile has commonly been reported in cross-sectional studies in the literature (16–20). The dyslipoproteinemia (elevated TC, LDL-C and TG) that has been reported in the SLE population has been associated with disease activity (21) and future CV events (22).
Borba et al (21) confirmed that untreated SLE patients have dyslipoproteinemia that is aggravated by disease activity. The biochemical mechanism that may explain the lipoprotein abnormalities in untreated SLE patients includes decreased lipoprotein lipase and Apo C-II activity (23), which could be correlated with autoimmune phenomena (24). In the patients of the present study, who in the majority of cases were on corticosteroid therapy, a significant independent positive association was identified between SLEDAI and TG or TC levels in these active SLE patients.
The results of the present study support the previous finding that dyslipoproteinemia is common in SLE patients, with a pattern that is characterized by an increase in TG and a decrease of HDL-C and ApoA-I. The data indicated the presence of a CV risk in active SLE with high disease activity, which was demonstrated by the high frequency of dyslipidemia and higher BNP concentrations. Therefore, dyslipoproteinemia may underlie some of the increased risk for CVD and HF in patients with SLE.
References
- 1.Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M, Shields K, et al. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev. 2013;12:1004–1015. doi: 10.1016/j.autrev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Nikpour M, Urowitz MB, Ibañez D, Gladman DD. Frequency and determinants of flare and persistently active disease in systemic lupus erythematosus. Arthritis Rheum. 2009;61:1152–1158. doi: 10.1002/art.24741. [DOI] [PubMed] [Google Scholar]
- 3.Gaggin HK, Januzzi JL., Jr Natriuretic peptides in heart failure and acute coronary syndrome. Clin Lab Med. 2014;34:43–58. doi: 10.1016/j.cll.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Gaggin HK, Januzzi JL., Jr Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. 2013;1832:2442–2450. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 6.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, Austin A, Bell A, Bloch DA, Corey PN, Decker JL, et al. The Committee on Prognosis Studies in SLE: Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 7.Ye Y, Wang Y, Shen Z. Medical Administration Department of Ministry of Public Health. 3rd. China: 2006. National Guide to Clinical Laboratory Procedures of China. [Google Scholar]
- 8.Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9:166–169. doi: 10.1191/096120300678828208. [DOI] [PubMed] [Google Scholar]
- 9.Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Rairie JE, Tracy RP, Kuller LH. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Ammirati E, Moroni F, Pedrotti P, Scotti I, Magnoni M, Bozzolo EP, Rimoldi OE, Camici PG. Non-invasive imaging of vascular inflammation. Front Immunol. 2014;5:399. doi: 10.3389/fimmu.2014.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce IN, Gladman DD, Urowitz MB. Premature atherosclerosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2000;26:257–278. doi: 10.1016/S0889-857X(05)70138-1. [DOI] [PubMed] [Google Scholar]
- 12.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Côte R, Grover SA, Fortin PR, Clarke AE, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::AID-ART395>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F, Meroni PL. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73:609–615. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 14.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 15.Hunt PJ, Richards AM, Nicholls MG, Yandle TG, Doughty RN, Espiner EA. Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): A new marker of cardiac impairment. Clin Endocrinol (Oxf) 1997;47:287–296. doi: 10.1046/j.1365-2265.1997.2361058.x. [DOI] [PubMed] [Google Scholar]
- 16.Ardoin SP, Schanberg LE, Sandborg C, Yow E, Barnhart HX, Mieszkalski K, Ilowite NT, von Scheven E, Eberhard A, Levy DM, et al. APPLE investigators: Laboratory markers of cardiovascular risk in pediatric SLE: The APPLE baseline cohort. Lupus. 2010;19:1315–1325. doi: 10.1177/0961203310373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soep JB, Mietus-Snyder M, Malloy MJ, Witztum JL, von Scheven E. Assessment of atherosclerotic risk factors and endothelial function in children and young adults with pediatric-onset systemic lupus erythematosus. Arthritis Rheum. 2004;51:451–457. doi: 10.1002/art.20392. [DOI] [PubMed] [Google Scholar]
- 18.Posadas-Romero C, Torres-Tamayo M, Zamora-González J, Aguilar-Herrera BE, Posadas-Sánchez R, Cardoso-Saldaña G, de Guevara Ladrón G, Solis-Vallejo E, El Hafidi M. High insulin levels and increased low-density lipoprotein oxidizability in pediatric patients with systemic lupus erythematosus. Arthritis Rheum. 2004;50:160–165. doi: 10.1002/art.11472. [DOI] [PubMed] [Google Scholar]
- 19.Lilleby V, Haugen M, Mørkrid L, Frey Frøslie K, Holven KB, Førre O. Body composition, lipid and lipoprotein levels in childhood-onset systemic lupus erythematosus. Scand J Rheumatol. 2007;36:40–47. doi: 10.1080/03009740600907881. [DOI] [PubMed] [Google Scholar]
- 20.Tyrrell PN, Beyene J, Benseler SM, Sarkissian T, Silverman ED. Predictors of lipid abnormalities in children with new-onset systemic lupus erythematosus. J Rheumatol. 2007;34:2112–2119. [PubMed] [Google Scholar]
- 21.Borba EF, Bonfá E. Dyslipoproteinemias in systemic lupus erythematosus: Influence of disease, activity, and anticardiolipin antibodies. Lupus. 1997;6:533–539. doi: 10.1177/096120339700600610. [DOI] [PubMed] [Google Scholar]
- 22.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. doi: 10.1001/jama.1996.03540110036029. [DOI] [PubMed] [Google Scholar]
- 23.Ilowite NT, Samuel P, Ginzler E, Jacobson MS. Dyslipoproteinemia in pediatric systemic lupus erythematosus. Arthritis Rheum. 1988;31:859–863. doi: 10.1002/art.1780310706. [DOI] [PubMed] [Google Scholar]
- 24.Beaumont JL, Beaumont V. Autoimmune hyperlipidemia. Atherosclerosis. 1977;26:405–418. doi: 10.1016/0021-9150(77)90111-3. [DOI] [PubMed] [Google Scholar]