Abstract
The substance P (SP; also known as TAC1)/neurokinin-1 receptor (NK1R; also known as TACR1) complex is a critical part in the development of cancer. Therefore, NK1R antagonists, such as the clinical drug aprepitant, are currently under investigation as future anticancer agents. In a previous study, NK1R (TACR1) was identified as a potent anticancer target in hepatoblastoma (HB). However, little is known regarding the exact distribution of this target among HB subsets and whether it correlates with clinical features and prognosis. In the present study, mRNA was isolated from 47 children with HB, and reverse transcription-quantitative polymerase chain reaction was performed on the samples to analyze the expression of full-length-TACR1 (fl-TACR1) and truncated-TACR1 (tr-TACR1). These data were correlated with data obtained from 9 tumor-free controls, as well as with the presence of metastasis, PRETEXT, vascular invasion, histology, age of diagnosis, multifocality, CTNNB1 mutation, gender and overall survival. Additionally, the present study investigated a recently described 16-gene signature characterizing HB known to correlate with prognosis. Compared with tumor-free liver tissue, tumorous tissue expressed TACR1 significantly higher for the truncated version (P=0.0301), and by trend also for the full-length version. Accordingly, the expression of fl-TACR1 correlated with the expression of the truncated version (P=0.0074). Furthermore, a low expression of fl-TACR1 correlated with characteristics of the 16-gene signature known to predict prognosis (P=0.0222). However, there was no correlation between tr-TACR1 and the tumor characteristics investigated, including outcome, although a clear trend was observed for some tumor characteristics. The current results reinforced the previously described findings that in HB, tr-TACR1 is overexpressed compared with tumor-free liver tissue. Furthermore, to the best of our knowledge, the present study demonstrated for the first time that tr-TACR1 is expressed ubiquitously among the different subsets of HB. Therefore, NK1R may serve as a potent anticancer target in a large variety of patients with HB, independent of tumor biology and clinical stage.
Keywords: neurokinin-1 receptor, aprepitant, substance P, antitumor target, apoptosis, hepatoblastoma
Introduction
Hepatoblastoma (HB) is the most common liver tumor of childhood (1). If complete surgical resection of the tumor is achieved, the prognosis of children with HB is favorable, with or without additional chemotherapy (standard-risk patients) (1). Despite recent advances in therapy for these children, prognosis remains poor for high-risk patients (1,2). Among high-risk children, chemotherapy is crucial in addition to surgical therapy. However, multi-drug resistance to chemotherapy significantly limits the ability to successfully treat these patients (1,3). Therefore, the employment of novel anticancer agents against HB is needed.
The use of neurokinin-1 receptor (NK1R; also known as TACR1) antagonists is a novel and promising approach for future anticancer strategies (4). The peptide substance P (SP; also known as TAC1) is a widely distributed neuronal transmitter that, after binding specifically to NK1R, triggers a broad variety of functions (5). It is known that SP can induce tumor cell proliferation, angiogenesis and migration via NK1R, and that the SP/NK1R complex is an integral part of the cancer cell itself, as well as its tumor microenvironment (6). Aprepitant, a non-peptide NK1R antagonist, is a clinical agent approved by the Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting. Its effects as an anticancer agent have been described extensively in vitro and in vivo (6–11). Notably, evidence indicates that it has limited toxic side effects even when administered in high doses (6,12).
In a previous study, we described that TACR1 is highly expressed in human HB, predominantly in its truncated form [truncated-TACR1 (tr-TACR1)] (8). Compared with full-length (fl-TACR1), tr-NK1R lacks 96 amino acids at the cytoplasmic C-terminus of the receptor that are responsible for intracellular signal transduction. Although this splice variant is considered to be able to couple G proteins, it exhibits decreased efficiency with respect to internalization and desensitization (8,13–15). The net result of this is a decreased ability for negative feedback inhibition, allowing constant activation despite saturation of the receptor complex (13–15). This, in turn, may contribute to the correlation of expression of this particular splice variant with cancer. In an experimental HB setting, NK1R antagonists acted as highly active anticancer agents in vitro and in vivo, and functioned synergistically with established chemotherapy agents in vitro (8).
In addition to these findings, a molecular 16-gene signature has been described for HB, in order to better classify molecular patterns and biological characteristics of these tumors (16,17). Using this signature, two tumor subclasses resembling distinct phases of liver development can be identified. Notably, this signature discriminates invasive and metastatic from localized HB and predicts prognosis with high accuracy (16). Additionally, it has recently been suggested that the expression of TACR1 may correlate with a clinically worse prognosis in some cancers (18–22). However, scientific evidence for such an association remains scarce. No study has previously focused on the expression of TACR1 and a possible association with the clinical prognosis in HB. Therefore, the present study analyzed the expression pattern of this target among human HB subsets and investigated whether it correlates with clinical characteristics, such as stage, biology and outcome, including a 16-gene molecular signature known to correlate with prognosis in these tumors. The current results showed that tr-TACR1 is overexpressed compared with tumor-free liver tissue in HB. Addtionally, tr-TACR1 was expressed ubiquitously among the different subsets of HB. Therefore, NK1R may serve as a potent anticancer target in a number of patients with HB, independent of tumor biology and clinical stage.
Patients and methods
Patients and tumor tissues
Analysis of tumor tissue samples from patients with HB (n=47) who were all part of the German Cooperative Pediatric Liver Tumor Registry Study HB99 and its subsequent Register for Pediatric Liver Tumors was performed. The two registries were multicentric and were initiated by the German Society for Pediatric Oncology and Hematology. They were open to registration for patients from Germany, Austria and Switzerland up to the age of 20 years with untreated HB. The registry protocols were assigned by the institutional Ethical Committee of the University Children's Hospital Basel (Basel, Switzerland) and the University of Bonn (Bonn, Germany), and written consent was obtained from the parents for treatment, data collection and analysis.
Clinical information, including demographic, therapeutic, tumor and clinical outcome variables, were retrieved from the two clinical studies. The treatment protocol consisted of preoperative chemotherapy followed by delayed surgery and postoperative chemotherapy according to two risk groups (standard- versus high-risk). The two risk groups were based on the International Childhood Liver Tumor Strategy Group risk criteria (23). Standard risk patients received two or three courses of neoadjuvant ifosfamide, cisplatin and doxorubicin (IPA) chemotherapy prior to surgery (1 g/m2 ifosfamide every 72 h, days 1–3; 20 mg/m2 cisplatin every 1 h, days 4–8; and 60 mg/m2 doxorubicin every 48 h, days 9–10). Radical surgery was conducted after the second or third course depending on the resectability. Postoperatively, another course of IPA was applied. In case of microscopically incomplete resection, two adjuvant courses of IPA were administered. Patients with small-extended tumors (PRETEXT stage I) (24) could be resected without neoadjuvant chemotherapy and were treated with two courses of IPA postoperatively. High-risk patients received up to seven courses of carboplatin-based chemotherapy preoperatively depending on tumor shrinkage and resectability. Patients were initially treated with two courses of carbo/VP16 (200 mg/m2 carboplatin every 24 h, days 1–4; and 100 mg/m2 etoposide every 24 h, days 1–4) followed by stem cell collection. In case of tumor response, the therapy was continued with high dose carboplatin and etoposide (500 mg/m2 per 24 h, days 8–5) following autologous stem cell transplantation. Patients without tumor response were treated with IPA. Resection was scheduled as soon as the tumor was determined to be completely resectable. In case of persisting non-resectability, liver transplantation was recommended. Lung metastases were resected if residual metastases were still observed on radiological images after chemotherapy.
Tumor specimens were reviewed by the local institution as well as the Institute of Pediatric Pathology, University of Kiel (Kiel, Germany), which served as a reference center. Matched adjacent liver tissue samples from the surgical specimens without macroscopic or microscopic tumors served as tumor-free controls (n=9). Clinical and molecular data, such as gender, age at diagnosis, PRETEXT staging (24), including vascular invasion and multifocality, metastatic disease, histology, CTNNB1 mutation, 16-gene signature and overall survival, were retrieved from the HB99 database and our recent exome sequencing study, respectively (25).
RNA extraction and reverse transcription
RNA extraction, complementary (c)DNA synthesis and quantitative polymerase chain reaction (qPCR) analysis were performed as previously described (26). Briefly, total RNA was isolated from all the samples using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and dissolved in RNase-free water. The purity and quality of the RNA was checked using a Nanodrop® 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA (2 µg) was reverse transcribed using SuperScript™ II reverse transcriptase (Invitrogen Life Technologies), according to the manufacturer's instructions. The amplification reactions were performed with 40 ng complementary DNA, 500 nM forward and reverse primers and iTaq SYBR®-Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) in a final reaction volume of 20 µl and were incubated at 95°C for 7 min, subjected to 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec, followed by a final extension cycle at 72°C for 7 min. All the reactions were conducted on ice to minimize the risk of RNA degradation. cDNA obtained was stored at −80°C.
Reverse transcription-qPCR (RT-qPCR)
According to the modified method of Bigioni et al, the prepared cDNA (2 µl) was used in a PCR with specific primers, based on the common sequence of the TACR1 (NK1R) human isoforms, which yield a 186-bp fragment (27). Specific primers were as follows: Forward, 5′-AACCCCATCATCTACTGCTGC-3′ and reverse, 5′-ATTTCCAGCCCCTCATAGTCG-3′ for fl-TACR1 (NM_001058.3); forward, 5′-GGGCCACAAGACCATCTACA-3′ and reverse, 5′-AAGTTAGCTGCAGTCCCCAC-3′ for tr-TACR1 (NM_015727.2); and forward, 5′-GCCCGAAACGCCGAATAT-3′ and reverse, 5′-CCGTGGTTCGTGGCTCTCT-3′ for the TBP housekeeping gene. The amplification reactions were performed with iTaq SYBR®-Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) in a final reaction volume of 20 µl and were incubated at 95°C for 7 min, subjected to 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec, followed by a final extension cycle at 72°C for 7 min. PCR was performed using a Mastercycler ep Gradient S (Eppendorf, Hamburg, Germany) and the transcript numbers were normalized according to the expression of the housekeeping gene. Relative quantification of gene expression was performed using the 2−∆∆Ct method, as described by Pfaffl (28).
Statistical analysis
Data are presented as mean ± SD. Mean and individual relative expression values of tumor and control samples are expressed in dot plots for each group. Statistical comparisons were performed with a standard t-test and Mann-Whitney U test using GraphPad Prism biostatistics software (version 5.0d; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 and P<0.01 indicated a statistically significant difference for all the comparisons. To differentiate between a high and low expression of TACR1 and its components, 3-fold of the mean of 9 tumor-free control samples was used as a cutoff for high expression. Kaplan-Meier estimates of specific survival time in the two groups were compared using the log-rank Mantel-Cox test.
Results
TACR1 is overexpressed in human HB patients
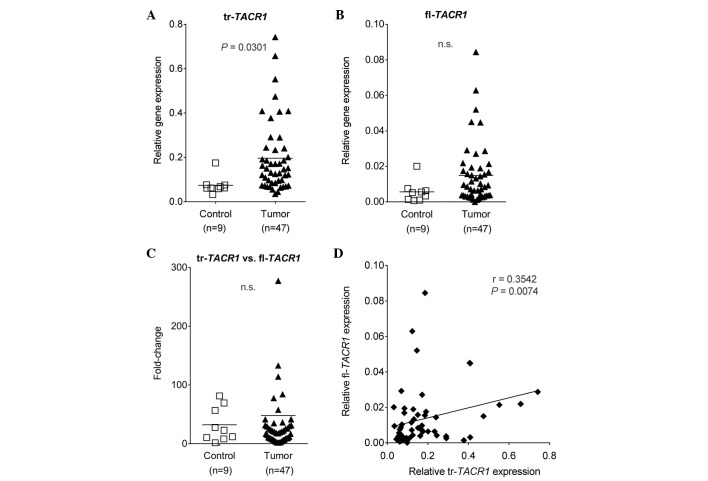
To address the aforementioned hypotheses, the gene expression pattern of fl-TACR1 and tr-TACR1 were analyzed in tumor tissue samples of HB and non-tumorous liver tissue. A significantly higher expression of tr-TACR1 was observed in HB compared with the control specimens (P=0.0301; Fig. 1A). Although not statistically significant, the expression of fl-TACR1 also tended to be higher in tumor specimens (P>0.05; Fig. 1B). These results correlated with our previous findings, in which in vitro and in vivo models of HB were used to demonstrate that tr-TACR1 is overexpressed in malignant HB cells. In turn, malignant HB cells were correlated with responsiveness to treatment with NK1R antagonists, such as aprepitant (8).
Figure 1.
Hepatoblastoma tumors overexpress TACR1 compared with normal liver tissue. (A) Statistically significant differences in the relative gene expression levels of tr-TACR1 in HB (n=47, black triangles) compared with normal liver tissue (n=9, white squares; P=0.0301). (B) No statistically significant differences in the gene expression levels of fl-TACR1 in the same samples as in (A). (C) Ratio of the gene expression values of (A) tr-TACR1 vs. (B) fl-TACR1, calculated for HB tumors and liver tissue samples. (D) Graphical representation of the correlation of (A) tr-TACR1 vs. (B) fl-TACR1 gene expression levels (black squares; P=0.0074, r=0.3542). tr/fl-TACR1, truncated/full-length-neurokinin-1 receptor; n.s., not significant.
Expression of tr-TACR1 correlates with fl-TACR1
Due to the wide range of gene expression, the ratio of tr-TACR1 versus fl-TACR1 expression was presented. It was found that the ratios were comparable between the tumor and control samples (Fig. 1C), suggesting a positive correlation of the two splice variants. When analyzed in-depth, a statistically significant weak correlation was identified between tr-TACR1 and fl-TACR1 expression (r=0.3542), potentially indicating a mutual dependency (Fig. 1D).
TACR1 expression does not correlate with biological characteristics
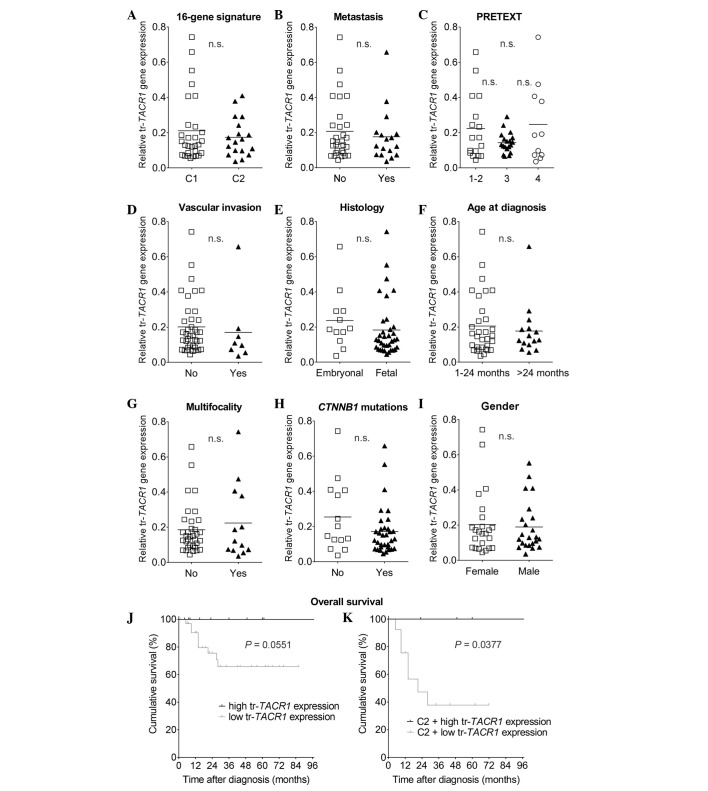
To improve understanding of whether splice variants or their ratio correlate with the biological features of the tumor, TACR1 expression was analyzed accordingly (Table I; Figs. 2–4). First, the truncated variant was investigated, due to its significance in HB as a potential therapeutic target (8). In order to accomplish this, the relative expression of tr-TACR1 was correlated with a recently described 16-gene molecular signature known to be associated with prognosis (16). Similar to the original description of this signature, the current cohort was separated into 29 patients with HB that exhibited the C1 signature (poor prognosis; 61.7%) and 18 exhibited the C2 signature (improved prognosis; 38.3%) (Table I). Relative gene expression analysis of tr-TACR1 revealed no significant difference between patients with C1 and C2 signatures (Fig. 2A). The same features were then analyzed in correlation to the gene expression of fl-TACR1 (Fig. 3A). A significant correlation was identified between low fl-TACR1 expression and the C2 population of the 16-gene signature (P=0.0222; Fig. 4A). It is of note that 55.6% of the specimens grouped into the C2 population exhibited extremely low levels of fl-TACR1.
Table I.
Clinical, biological and histological outcome characteristics of 47 patients with hepatoblastoma.
| Characteristic | Patients, n (%) |
|---|---|
| 16-gene signature | |
| C1 | 29 (61.7) |
| C2 | 18 (38.3) |
| Metastasis | |
| Yes | 17 (36.2) |
| No | 30 (63.8) |
| PRETEXT | |
| I–II | 17 (36.2) |
| III | 19 (40.4) |
| IV | 11 (23.4) |
| Vascular Invasion | |
| Yes | 8 (17.1) |
| No | 39 (82.9) |
| Histology | |
| Fetal | 35 (74.5) |
| Embryonal | 12 (25.5) |
| Age at diagnosis, months | |
| 1–24 | 32 (68.1) |
| >24 | 15 (31.9) |
| Multifocality | |
| Yes | 13 (27.7) |
| No | 34 (72.3) |
| CTNNB1 status | |
| Wild-type | 14 (29.8) |
| Mutated | 33 (70.2) |
| Gender | |
| Female | 24 (51.1) |
| Male | 23 (48.9) |
Figure 2.
tr-TACR1 expression is not significantly associated with biological, clinical and histological parameters. Relative gene expression of tr-TACR1 was correlated to the (A) 16-gene signature, (B) metastasis, (C) the preoperative staging system PRETEXT, (D) vascular invasion, (E) histology, (F) age at diagnosis, (G) multifocality, (H) CTNNB1 mutation status (no represents wild-type, yes represents mutated β-catenin) and (I) gender; however, the differences were not significant (P>0.05). (J) Overall survival for high and low tr-TACR1 expression revealed a non-significant difference in survival (P=0.0551). (K) Low expression of tr-TACR1 was associated with significantly lower overall survival in hepatoblastoma tumors harboring the C2 signature (P=0.0377). tr-TACR1, truncated-neurokinin-1 receptor; n.s., not significant.
Figure 4.
Ratio of tr-TACR1 and fl-TACR1 does not predict clinical prognosis. As in Figs. 2 and 3, ten different clinical features were analyzed with regard to the tr-TACR1:fl-TACR1 ratio gene expression. No significant difference were detected in the (A) 16-gene signature, (B) metastasis, (D) vascular invasion, (E) histology, (F) age at diagnosis, (G) multifocality, (H) CTNNB1 mutation status and (I) gender or (J) overall survival (P>0.05). (C) However, PRETEXT 1–2 significantly correlated with a higher expression ratio compared with PRETEXT 3 (P<0.05). (K) Low expression of the tr-TACR1:fl-TACR1 ratio was associated with lower overall survival in hepatoblastoma tumors harboring the C2 signature, however, the results were not significant (P<0.05). tr/fl-TACR1, truncated/full-length-neurokinin-1 receptor; n.s., not significant.
Figure 3.
fl-TACR1 expression displays no significant difference in the majority of biological, clinical and histological features. (A-I) Analogous to Fig. 2, relative gene expression of fl-TACR1 was compared with the same ten parameters. (A) The C2 signature significantly correlated with low expression of the fl-TACR1 gene (P=0.0222). P-values for (B) metastasis, (C) PRETEXT staging, (D) vascular invasion, (E) histology, (F) age at diagnosis, (G) multifocality, (H) CTNNB1 mutation status and (I) gender did not reveal any statistically significant differences (P>0.05). (J) Analysis of overall survival for high and low fl-TACR1 expression revealed no significant difference in survival (P>0.05). (K) High expression of fl-TACR1 was associated with a worse outcome in hepatoblastoma tumors harboring the C2 signature, however, the trend was not significant (P>0.05). fl-TACR1, full-length neurokinin-1 receptor; n.s., not significant.
The ratio of fl-TACR1 to tr-TACR1 was then calculated to determine whether it could be correlated with the 16-gene signature (16). Notably, extremely low ratios were identified in the favorable C1 population and extremely high ratios were evident for the C2 population; however, this effect was not statistically significant (data not shown). Populations with a C2 signature were previously demonstrated to be associated with a poor prognosis of HB (16).
Expression of TACR1 does not correlate with clinical characteristics
The expression patterns of fl-TACR1 and tr-TACR1 were correlated with clinical, biological and histological characteristics, including metastasis (Figs. 2B and 3B), the preoperative classification PRETEXT (Figs. 2C and 3C), vascular invasion (Figs. 2D and 3D), histology (Figs. 2E and 3E), onset period (Figs. 2F and 3F), multifocality (Figs. 2G and 3G), CTNNB1 mutations (Figs. 2H and 3H) and gender (Figs. 2I and 3I). Of the entire cohort, it was found that 63.8% exhibited no metastasis at the time of diagnosis, 82.9% had no vascular invasion and only 27.7% were multifocal. Gender was equally distributed (51.1% female vs. 48.9% male), the majority tumors had a fetal histology (74.5 vs. 25.5% embryonal) and, as expected, 70.2% of tumors possessed a β-catenin (CTNNB1) mutation. The age of diagnosis was predominantly within the first 24 months of life (68.1%) and the specimens were classified as PRETEXT 1–2 (36.2%), PRETEXT 3 (40.4%) and PRETEXT 4 (23.4%).
When analyzing tr-TACR1 expression in detail, no statistically relevant differences were identified with respect to the aforementioned clinical features. Notably, a high expression of tr-TACR1 correlated with a better overall survival (P=0.0551; Fig. 2J), although this was only a trend as it did not reach statistical significance.
Similarly, when analyzing the pattern of fl-TACR1 expression with metastasis, PRETEXT, vascular invasion, histology, age at diagnosis, multifocality, CTNNB1 mutations or gender, no significant correlation was observed (P>0.05; Fig. 3B–I). When clustered into groups of high versus low expression of fl-TACR1, overall survival curves did not deviate from each other (P>0.05; Fig. 3J), contrary to the finding for tr-TACR1 (Fig. 2J).
Use of the ratio of the two variants (tr-TACR1:fl-TACR1), revealed no statically significant differences with regard to the majority of the characteristics (Fig. 4A–I). The only exception to this was that a higher tr-TACR1:fl-TACR1 ratio, which occurred predominantly in PRETEXT 1–2 compared with PRETEXT 3 (P=0.0459; Fig. 4C). Similar to the analysis with tr-TACR1 alone, overall survival was worse with a low ratio of tr-TACR1:fl-TACR1 (P>0.05; Fig. 4J).
As the original description of the 16-gene signature by Cairo et al (16) suggested a worse prognosis for the C2 signature, the present study aimed to investigate whether either factor (tr-TACR1, fl-TACR1 or the ratio thereof) could refine the predictive value in our set of tumors. Therefore, overall survival within the C2 HB tumors was re-analyzed, and their outcome with respect to high versus low expression of TACR1 or its ratio was investigated. It was identified that low tr-TACR1 predicted a poor prognosis for C2 tumors with a higher significance than tr-TACR1 alone (P=0.0377; Fig. 2K). Although not significant, high fl-TACR1 suggested a worse outcome (P>0.05; Fig. 3K), and the ratio of the two variants had the same trend as truncated alone and as analyzed in the whole cohort, but with a clear tendency towards a worse prognosis for low tr/fl-TACR1 in the C2 group (P>0.05; Fig. 4K).
Taken together, no strong correlation of tr-TACR1, fl-TACR1 or tr-TACR1:fl-TACR1 gene expression with clinical and histological data was identified. However, low tr-TACR1 or a low ratio was associated with a worse prognosis, particularly when associated with the C2 signature. By contrast, no significance was identified in fl-TACR, with a marginal trend towards a worse outcome in C2 and high fl-TACR1 expression.
Discussion
Little is known regarding the expression profile of TACR1 and its associations with clinical outcome. NK1R is a crucial component of cancer development and progression. Thus, NK1R is a promising anticancer target in a multitude of cancer types, including HB (7,8). In the present study, in-depth analysis of the expression pattern of TACR1 in HB was performed, and the findings were correlated with the patients' clinical tumor stage, biology and outcome. It was determined that, compared with tumor-free liver tissue, tumorous tissue expressed significantly more tr-TACR1. Although the difference was not significant, HB tissues also tended to express marginally more of fl-TACR1. This is in accordance with our recent description of this receptor in HB (8). Within the tumorous tissue, expression of fl-TACR1 correlated with the expression of tr-TACR1. Furthermore, the expression of fl-TACR1 was lower in the C2 (poor prognosis) compared with the C1 (improved prognosis) population of the 16-gene signature. When analyzing the expression of low versus high fl-TACR1 within the C2 population only, no difference was found. There was also no correlation between tr-TACR1 expression alone and any of the tumor characteristics investigated. However, a low expression of tr-TACR1 demonstrated a clear trend towards worse prognosis but did not reach statistical significance. Thus, the current data provide evidence that HB ubiquitously expresses TACR1, supporting recent studies that NK1R antagonists may be promising anticancer agents against a wide variety HB subsets (8,29).
It has previously been proposed that a correlation exists between the expression rate of the NK1R/SP complex and prognosis in various types of cancer (18–22). Garcia-Recio et al identified that SP contributes to persistent transmodulation of the ErbB receptors, epidermal growth factor receptor (EGFR) and human epidermal growth factor 2 (HER2), in breast cancer, acting to enhance malignancy and therapeutic resistance. Both TACR1 and TAC1 (SP) were highly expressed in HER2+ primary breast tumors and correlated with poor prognosis factors (18). These findings are in contradiction to the current results in HB, which indicated that worse prognosis was associated with a low expression of tr-TACR1. However, it should be noted that two separate tumor entities were investigated. In addition, Garcia-Recio et al (18) made no distinction between the truncated and the full-length variant of the receptor. Notably, following treatment of xenografted mice bearing HER2+ or HER2− human breast carcinoma, Garcia-Recio et al (18) only observed a therapeutic effect for HER2+ tumors, suggesting that the antitumor effects of NK1R inhibition in carcinoma of the breast depend on the modulatory properties of NK1R signaling on the activity of HER2 and EGFR (18).
In a different study, Gillespie et al identified that it was the expression of tr-TACR1 and not fl-TACR1 that predicted the progression from quiescent colitis to high-grade dysplasia and cancer in colitis-associated cancer (13). This is in accordance with the current results for HB, in which tr-TACR1 was observed to be upregulated in cancer cells but not in non-tumorous tissue.
Cairo et al recently described two tumor subclasses within HB, resembling distinct phases of liver development and containing a discriminating 16-gene signature. Furthermore, it was found that β-catenin, a key protein of the Wnt signaling pathway, activated different transcriptional programs in the two distinct tumor subpopulations, C1 and C2. Notably, when separated into the two subpopulations by this 16-gene signature, clinical prognosis could be predicted for these children with an extremely high accuracy (16,17). Considering these findings, the HB tumor bank was screened in the present study and each tumor was classified according to this specific 16-gene signature. Subsequently, the findings were correlated with the expression of fl-TACR1 and tr-TACR1, and it was determined that fl-TACR1 expression was lower in the C2 signature compared with the C1 group. A low expression of tr-TACR1 was associated with a worse prognosis, although this was only a trend and not significant (P=0.0551). Of note, when analysis of the C2 signature population was performed separately, a low expression of tr-TACR1 was significantly associated with a worse prognosis (P=0.0377). Therefore, it can be concluded that TACR1 alone does not serve as a clinical marker for aggressiveness or potential to metastasize in HB. However, tr-TACR1 may facilitate the identification of tumors that have a very poor prognosis, potentially alone but in particular within the C2 signature patient population. More in-depth analysis of such a C2 tr-TACR1low tumor cohort is necessary to demonstrate the value of this distinction. Additionally, when making such distinction, it should be understood that ‘low’ expression in the present study is in reference to ‘high’ expression, as defined in the Patients and methods section. Thus, a tumor with tr-TACR1low expression may, on average, express significantly more tr-TACR1 compared with non-tumorous tissue.
Previous studies identified that overexpression of tr-TACR1 is associated with malignancy, including in HB (6–8,30). The present finding of low tr-TACR1 correlating with worse prognosis initially appears to contradict this finding. Numerous reasons exist that possibly account for this discrepancy. One possible explanation is that tumors that express low levels of tr-TACR1 represent an advanced stage in tumorigenesis characterized by profound immaturity, highlighted by the fact that a low expression of α-fetoprotein (AFP), the only accepted tumor marker for HB, correlates with poor prognosis (1). It is proposed that this correlation occurs because tumor cells that are unable to produce AFP are more immature than AFP-producing tumor cells, leading to the recognized poor prognosis. However, this hypothesis cannot be adequately addressed by the data of the current study at this time.
Furthermore, tumors, and particularly tumors of the liver, have been shown to be significantly heterogeneous (31). Only one sample was analyzed per tumor in the current analysis, which may not be representative for other areas of the cancer. In addition, gene expression does not always correlate with protein expression. Therefore, it may be useful to determine whether an immunohistochemical (IHC) staining classification of HB may indicate more favorable clinical features and improved prognosis. However, IHC staining of the different splice variants of the NK1R/SP-complex remains a challenge and presents a major obstacle to this endeavor. Additionally, according to current understanding of the NK1R/SP-complex, SP is a critical ligand required for its function. The present study did not investigate the gene expression level of SP within the tumor or circulating protein of it; therefore, this should be performed in the future. Finally, all but four patients enrolled into this retrospective registry had received chemotherapy prior to surgery [8.8 vs. 27.7% in the original description of the 16-gene signature by Cairo et al(16)]. This is important to consider, as the exposure to chemotherapy could potentially alter the expression pattern of TACR1 and its splice variants. Our previous study demonstrated that the expression of TACR1 did not change with prior chemotherapy treatment in children with HB (8). However, these data did not distinguish between the full-length and the truncated version of the receptor (8). Therefore, the influence of systemic chemotherapy on the expression of the NK1R complex remains, to date, an unsolved question.
In conclusion, the results of the present study do not indicate that the TACR1 expression pattern depends on or predicts the clinical stage and behavior of HB. However, two splice variants of TACR1 were demonstrated to be ubiquitously overexpressed in HB. Furthermore, the current analysis suggests that the prediction of overall survival in the C2 signature-expressing HB subgroup may be refined by tr-TACR1 and fl-TACR1, identifying a C2-TACR1low population with particularly poor prognosis. Overall, the present data further support the potential of the NK1R/SP complex as an ideal target in a wide variety of HB subsets.
Acknowledgements
We are grateful to Mrs. Fatemeh Promoli for technical assistance. Dr Michael Berger and Dr Matthias Ilmer were supported by postdoctoral stipends from the German Academic Exchange Program. Dr Michael Berger was additionally funded by the Friedrich-Baur Foundation Munich, Münchener Medizinische Wochenschrift, as well as the Funding for Research and Teaching of Ludwig Maximilian University of Munich. Professor Roland Kappler obtained funding from the Bettina Bräu Foundation (Munich, Germany) and the Gänseblümchen-Voerde Foundation (Voerde, Germany).
References
- 1.von Schweinitz D. Hepatoblastoma: Recent developments in research and treatment. Semin Pediatr Surg. 2012;21:21–30. doi: 10.1053/j.sempedsurg.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Haeberle B, Schweinitz D. Treatment of hepatoblastoma in the German cooperative pediatric liver tumor studies. Front Biosci (Elite Ed) 2012;4:493–498. doi: 10.2741/E395. [DOI] [PubMed] [Google Scholar]
- 3.Warmann S, Hunger M, Teichmann B, et al. The role of the MDR1 gene in the development of multidrug resistance in human hepatoblastoma: Clinical course and in vivo model. Cancer. 2002;95:1795–1801. doi: 10.1002/cncr.10858. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz M, Rosso M, Coveñas R. The NK-1 receptor: A new target in cancer therapy. Curr Drug Targets. 2011;12:909–921. doi: 10.2174/138945011795528796. [DOI] [PubMed] [Google Scholar]
- 5.Palma C, Bigioni M, Irrissuto C, et al. Anti-tumour activity of tachykinin NK1 receptor antagonists on human glioma U373 MG xenograft. Br J Cancer. 2000;82:480–487. doi: 10.1054/bjoc.1999.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosso M, Muñoz M, Berger M. The role of neurokinin-1 receptor in the microenvironment of inflammation and cancer. ScientificWorldJournal. 2012;2012:381434. doi: 10.1100/2012/381434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz M, Rosso M, Coveñas R. A new frontier in the treatment of cancer: NK-1 receptor antagonists. Curr Med Chem. 2010;17:504–516. doi: 10.2174/092986710790416308. [DOI] [PubMed] [Google Scholar]
- 8.Berger M, Neth O, Ilmer M, et al. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J Hepatol. 2014;60:985–994. doi: 10.1016/j.jhep.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz M, Pérez A, Rosso M, Zamarriego C, Rosso R. Antitumoral action of the neurokinin-1 receptor antagonist L-733 060 on human melanoma cell lines. Melanoma Res. 2004;14:183–188. doi: 10.1097/01.cmr.0000129376.22141.a3. [DOI] [PubMed] [Google Scholar]
- 10.Mayordomo C, García-Recio S, Ametller E, et al. Targeting of substance P induces cancer cell death and decreases the steady state of EGFR and Her2. J Cell Physiol. 2012;227:1358–1366. doi: 10.1002/jcp.22848. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz M, Berger M, Rosso M, et al. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int J Oncol. 2014;44:137–146. doi: 10.3892/ijo.2013.2164. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie E, Leeman SE, Watts LA, et al. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc Natl Acad Sci USA. 2011;108:17420–17425. doi: 10.1073/pnas.1114275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramkissoon SH, Patel PS, Taborga M, Rameshwar P. Nuclear factor-kappaB is central to the expression of truncated neurokinin-1 receptor in breast cancer: Implication for breast cancer cell quiescence within bone marrow stroma. Cancer Res. 2007;67:1653–1659. doi: 10.1158/0008-5472.CAN-06-3813. [DOI] [PubMed] [Google Scholar]
- 15.Patel HJ, Ramkissoon SH, Patel PS, Rameshwar P. Transformation of breast cells by truncated neurokinin-1 receptor is secondary to activation by preprotachykinin-A peptides. Proc Natl Acad Sci USA. 2005;102:17436–17441. doi: 10.1073/pnas.0506351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairo S, Armengol C, De Reyniès A, et al. Hepatic stem-like phenotype and interplay of Wnt/β-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14:471–484. doi: 10.1016/j.ccr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Cairo S, Armengol C, Buendia MA. Activation of Wnt and Myc signaling in hepatoblastoma. Front Biosci (Elite Ed) 2012;4:480–486. doi: 10.2741/E393. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Recio S, Fuster G, Fernandez-Nogueira P, et al. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res. 2013;73:6424–6434. doi: 10.1158/0008-5472.CAN-12-4573. [DOI] [PubMed] [Google Scholar]
- 19.Esteban F, Muñoz M, González-Moles MA, Rosso M. A role for substance P in cancer promotion and progression: A mechanism to counteract intracellular death signals following oncogene activation or DNA damage. Cancer Metastasis Rev. 2006;25:137–145. doi: 10.1007/s10555-006-8161-9. [DOI] [PubMed] [Google Scholar]
- 20.Esteban F, Gonzalez-Moles MA, Castro D, et al. Expression of substance P and neurokinin-1-receptor in laryngeal cancer: Linking chronic inflammation to cancer promotion and progression. Histopathology. 2009;54:258–260. doi: 10.1111/j.1365-2559.2008.03193.x. [DOI] [PubMed] [Google Scholar]
- 21.Castro TA, Cohen MC, Rameshwar P. The expression of neurokinin-1 and preprotachykinin-1 in breast cancer cells depends on the relative degree of invasive and metastatic potential. Clin Exp Metastasis. 2005;22:621–628. doi: 10.1007/s10585-006-9001-6. [DOI] [PubMed] [Google Scholar]
- 22.Misawa K, Kanazawa T, Misawa Y, et al. Frequent promoter hypermethylation of tachykinin-1 and tachykinin receptor type 1 is a potential biomarker for head and neck cancer. J Cancer Res Clin Oncol. 2013;139:879–889. doi: 10.1007/s00432-013-1393-5. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard J, Brown J, Shafford E, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: A successful approach - results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–3828. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 24.Roebuck DJ, Aronson D, Clapuyt P, et al. International Childrhood Liver Tumor Strategy Group: PRETEXT: A revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–132. doi: 10.1007/s00247-006-0361-5. quiz 249–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichenmüller M, Trippel F, Kreuder M, et al. The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J Hepatol. 2014;61:1312–1320. doi: 10.1016/j.jhep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Eichenmüller M, Gruner I, Hagl B, Häberle B, Müller-Höcker J, von Schweinitz D, Kappler R. Blocking the hedgehog pathway inhibits hepatoblastoma growth. Hepatology. 2009;49:482–490. doi: 10.1002/hep.22649. [DOI] [PubMed] [Google Scholar]
- 27.Bigioni M, Benzo A, Irrissuto C, Maggi CA, Goso C. Role of NK-1 and NK-2 tachykinin receptor antagonism on the growth of human breast carcinoma cell line MDA-MB-231. Anticancer Drugs. 2005;16:1083–1089. doi: 10.1097/00001813-200511000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muñoz M, Coveñas R. Neurokinin-1 receptor: A new promising target in the treatment of cancer. Discov Med. 2010;10:305–313. [PubMed] [Google Scholar]
- 30.Muñoz M, Rosso M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Invest New Drugs. 2010;28:187–193. doi: 10.1007/s10637-009-9218-8. [DOI] [PubMed] [Google Scholar]
- 31.Nault JC, Villanueva A. Intratumor molecular and phenotypic diversity in hepatocellular carcinoma. Clin Cancer Res. 2015;10:1786–1788. doi: 10.1158/1078-0432.CCR-14-2602. [DOI] [PubMed] [Google Scholar]