Abstract
Atypical chronic myeloid leukemia (aCML) is a hematopoietic stem/progenitor cell disorder, predominantly involving neutrophils. At present, a limited number of studies regarding the treatment of aCML have been published, and the therapies that are currently available exhibit unsatisfactory outcomes. In the present study, the cases of two aCML patients treated with decitabine (DCA) therapy who achieved remission are presented. A 48-year-old male, who presented with fatigue and a cough that had lasted two months, and a 69-year-old male who presented with dizziness, fatigue and shortness of breath with exercise, were diagnosed with aCML following bone marrow examination, flow cytometry and chromosome banding analysis. The two patients were treated with four cycles of DCA chemotherapy (20 mg/m2, days 1–5) and remission was achieved in each patient. The present study evaluated the clinical manifestations, diagnostic criteria and relevant treatment regimens of aCML, which may provide insights for the treatment of affected patients. Routine blood and bone marrow examinations were performed weekly prior to each cycle. Symptoms were relieved in both patients after the first cycle and the two patients were followed up for 3 months after completion of the final cycle. The findings of the current case report indicate that DCA may present an efficacious treatment for aCML.
Keywords: myelodysplastic/myeloproliferative neoplasms, atypical chronic myeloid leukemia, decitabine, diagnosis, treatment
Introduction
Atypical chronic myeloid leukemia (aCML) is a hematopoietic stem/progenitor cell disorder that predominantly involves neutrophils (1) and is characterized by an increased number of mature neutrophils, myelocytes and metamyelocytes in the bone marrow, as well as evident granulocytic dysplasia. Patients with aCML also present with an elevated peripheral white blood cell (WBC) count, in addition to other symptoms of CML, including anemia and thrombocytopenia (1,2). However, aCML patients are negative for the Philadelphia (Ph) chromosome and BCR/ABL fusion gene (3). The incidence of the disease is unconfirmed, as the largest published study presented a series of 134 patients with aCML (4–7); however, it is reasonable to assume that the disease is rare. The median overall survival is 14–30 months and ~40% of patients progress to acute myeloid leukemia (4–7).
The prognosis of aCML sharply contrasts with CML, for which inhibitors specific to BCR/ABL have been developed (8). At present, a limited number of studies have investigated the treatment of aCML, and current treatments exhibit unsatisfactory outcomes (4–7). In the present study, the cases of two patients with aCML who were successfully treated with decitabine (DCA) are reported. Written informed consent was obtained from the two patients.
Case report
Case one
On March 1, 2013, a 48-year-old male was hospitalized at the Department of Hematology of The First Affiliated Hospital (Zhejiang University School of Medicine, Hangzhou, China), with fatigue and a cough that had persisted for two months. A physical examination revealed anemia, with no involvement of the superficial lymph nodes. No dry or wet rales were identified. The cardiac rhythm was regular, without murmurs. In addition, the patient's abdomen was soft and the liver was impalpable. No tenderness in the spleen or percussion pain in the renal region were observed. In addition, no edema was identified in the lower limbs.
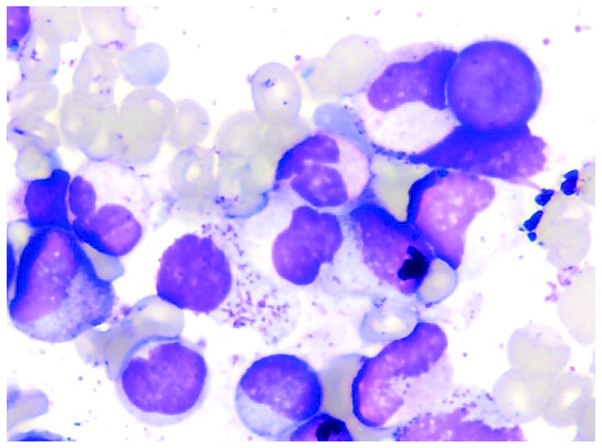
A routine blood test revealed a WBC count of 47.7×109/l (normal range, 4–10×109/l), with a differential of 10.5% lymphocytes (normal range, 20–40%), 76.4% neutrophils (normal range, 40–60%), 1.3% eosinophils (normal range, 0.5–5.0%) and 0.7% basophils (normal range, 0–1%), and a hemoglobin concentration and platelet count of 71 g/l (normal range, 120–160 g/l) and 239×109/l (normal range, 100–300 ×109/l), respectively. Additionally, an increased number of nucleated cells (10:1; normal range, 20:1) and significant granulocytic hyperplasia was observed upon morphological examination of the bone marrow, with 4.5% myeloblasts (normal range, 1–2%), 2% promyelocytes (normal range, 2–3%) and 68.5% granulocytes (normal range, 40–60%) detected in the total nucleated marrow cell count (Fig. 1).
Figure 1.
Case one: Morphological examination of the bone marrow showing atypical chronic myeloid leukemia. Readily identifiable marrow blasts, a relatively high proportion of neutral promyelocytes displaying a pathological phenotype, and reduced cytoplasmic granules in the granulocytes are observed. Wright's stain; magnification, ×100.
Morphological examination of the bone marrow also revealed unbalanced cell development and marked erythroid hyperplasia (23.5% erythrocytes and 6.5% lymphocytes), similar to the second case. The patient's peripheral blood smear showed 14% neutrophilic myelocytes (normal range, 4–8%), 10% neutrophilic metamyelocytes (normal range, 6–9%), 26% neutrophilic bands (normal range, 20–27%), 18% segmented neutrophils (normal range, 7–12%), 2% eosinophils (normal range, 2–3%), 3% monocytes (normal range, 0–1%), 4% polychromatic erythrocytes (normal range, 5–10%), 12% orthochromatic erythrocytes (normal range, 8–13%) and 11% mature lymphocytes (normal range, 15–30%). Bone marrow biopsy demonstrated dyshematopoiesis, with an increased number of nucleated cells and marked granulocytic hyperplasia. In addition, megakaryocytes were visible on bone marrow biopsy, with few lymphocytes and no evidence of fibrous proliferation.
Flow cytometry was used to reveal the phenotypes of these cells. Fluorescence intensity lower than the isotype controls was determined as negative expression (−), while fluorescence intensity higher than the isotype control was qualitatively determined as partially +, +, ++ or +++ (8). The aberrant granulocytes accounted for 79.94% of non-erythrocytes, with the following phenotype: Cluster of differentiation (CD)19(+), CD33(+), CD64(+), CD56(+), CD11b(+), CD13(+), CD1α(+), CD35(+), CD15(++), CD7(−), CD36(−), CD117(−), CD34(−), CD14(−), CD61(−), CD2(−), CD71(−) and HLA-DR(−). Flow cytometry analysis also revealed a small number of original myeloid cells with the following phenotype: CD56(+), CD117(++), CD34(++), CD13(+), HLA-DR(+++), CD7(−), CD19(−), CD64(−), CD11b(−), CD36(−), CD14(−), CD61(−), CD10(−), CD71(−), CD35(−), CD15(−) and CD2(−) (8). Cytogenetic and molecular biological analyses demonstrated a normal chromosome karyotype, 46,XY[20]. Additionally, the patient was negative for the Ph chromosome, BCR/ABL fusion gene, Janus kinase 2 (JAK2) mutations and IP1L1-PDGFRα. Based on these findings, aCML was diagnosed.
On March 13, 2013, the patient was administered with a first cycle of DCA [20 mg/m2 intravenously (i.v.), days 1–5; Xian-Janssen Pharmaceutical Ltd., Beijing, China] as an induction treatment. On April 17, 2013, a second morphological examination of the bone marrow was performed, which revealed that the bone marrow contained 0.5% promyelocytes with no evidence of myeloblasts. The residual leukemic cells (0.475%) were identified by flow cytometry using fluorescent labels for CD71, CD117, CD45 and HLA-DR. On April 20, 2013, the patient received a second cycle of DCA (20 mg/m2) for 5 consecutive days as consolidation therapy for aCML. On May 17, 2013, an additional bone marrow examination was performed, showing 1% myeloblasts and 1.5% promyelocytes, with 0.782% residual leukemic cells. On May 24, 2013, a second course of consolidation chemotherapy with DCA (third cycle; 20 mg/m2 i.v.) was administered for 5 consecutive days. On June 15, 2013, examination of the bone marrow revealed 2% myeloblasts and 1.5% promyelocytes, with 0.475% residual leukemic cells. On June 24, 2013, a fourth course of the DCA chemotherapy regimen (20 mg/m2 i.v.) was administered for 5 consecutive days. On July 31, 2013, a bone marrow examination revealed 2.5% myeloblasts and 1.5% promyelocytes, with 0.528% residual leukemic cells, as analyzed by flow cytometry. Following completion of the four cycles of chemotherapy, the patient was in good general health with a normal peripheral hemogram. At present, the patient is undergoing further clinical observations and follow-up.
Case two
On August 28, 2012, a 69-year-old male was admitted to the Department of Hematology of Zhejiang (Tongde Hospital, Hangzhou, China) with dizziness, fatigue and shortness of breath with exercise. Upon physical examination, the patient was severely anemic without the presence of enlarged superficial lymph nodes. Tenderness of the breastbone was not reported and hemorrhagic spots were scattered throughout the body. The patient presented with normal cardiac and pulmonary functions and the liver was non-palpable. No tenderness in the spleen was identified, however, a tender mass was present.
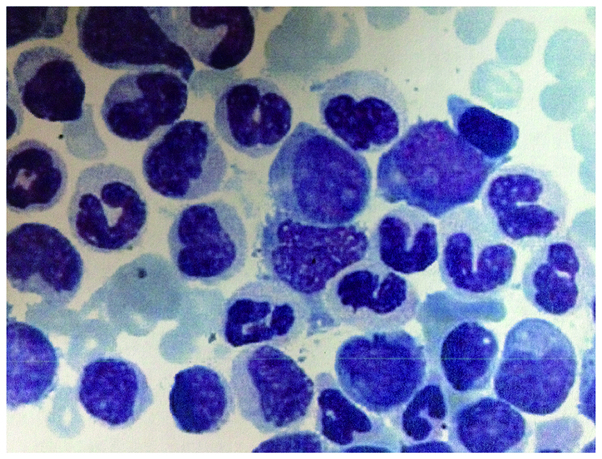
A routine blood test revealed a WBC count of 31.9×109/l with a differential of 11.8% lymphocytes, 80.2% neutrophils, 1.1% eosinophils and 1.8% basophils, and a hemoglobin concentration and platelet count of 54 g/l and 16×109/l, respectively. Overactive bone marrow with marked granulocytic hyperplasia were identified on bone marrow morphologic examination, and the presence of 15.5% myeloblasts, 27% promyelocytes and 91.5% granulocytes in the total nucleated marrow cells was observed, as well as a granulocyte to erythrocyte ratio of 13.8 (Fig. 2).
Figure 2.
Case two: Morphological examination of the bone marrow revealing abnormalities, which indicated atypical chronic myeloid leukemia. Active bone marrow hyperplasia, a relatively high proportion granulocytes with unbalanced development, and obvious marrow blasts are observed. Wright's stain; magnification, ×100.
Additionally, the bone marrow examination revealed that the nucleoplasm was in a state of unbalanced growth, with abundant cytoplasmic granules in the granulocytes. The patient exhibited 4% myeloblasts, 12% promyelocytes and 78% segmented neutrophils on the peripheral blood smear. A subsequent bone marrow biopsy demonstrated an increased number of nucleated cells and a reduced fat level. Additionally, marked granulocytic hyperplasia was observed on bone marrow biopsy, and an increased scatter distribution of myeloblasts and a marked increase in the number of other granulocytes with a normal distribution were found. Immature erythrocytes and lymphocytes were present in small numbers, and megakaryocytes were occasionally observed in the bone marrow, with no evidence of fibrous proliferation.
Flow cytometry revealed that 97.5% of the total nucleated cells were myeloblasts and myeloid cells, with 2.5% myeloblasts showing a relatively concentrated distribution and prominent expression on myeloid lineages, 2.5% monocytes with a mature phenotype, 91.5% granulocytes with an unbalanced nucleoplasm, and a significantly reduced number of lymphocytes. In addition, the results of cytogenetic and molecular biology analyses demonstrated a normal chromosome karyotype, 46,XY[20]. The patient was negative for the Ph chromosome, BCR/ABL fusion gene and JAK2 mutations, and positive for the CEBPA mutations, which have been shown to be associated with the prognosis of acute myeloid leukemia (AML) (4–7,9). The patient was subsequently diagnosed with aCML.
On September 14, 2012, the patient received the first DCA cycle (20 mg/m2 i.v., days 1–5) as induction therapy. On October 18, 2012, a second bone marrow specimen revealed 51% myeloblasts. Flow cytometry was used to reveal the phenotypes of these cells. Fluorescence intensity was classified, as described for case one. The phenotype was as follows: CD33(+), CD64(+), CD56(+) (partially), CD11b(+), CD117(+) (partially), CD13(+), CD65s(++), CD35(+), CD15(+), CD34(−), CD7(−), CD19(−) and CD14(−) (8); among the non-erythrocytes (myelocytes, metamyelocytes, bands and segmented neutrophils, negative reaction for CD10 and CD14), 92.7% were aberrant granulocytes. On October 23, 2012, the patient received a second cycle of consolidation therapy with DCA (20 mg/m2) for 5 consecutive days. On November 28, 2012, an additional bone marrow examination revealed 2% myeloblasts. Following fluorescent labeling for CD65, CD15, CD11b and CD45, flow cytometry revealed that the non-erythrocytes were composed of 66.442% aberrant granulocytes with normal CD65, CD15 and CD11b expression. On February 2, 2013, a third cycle of chemotherapy was administered. On March 26, 2013, a bone marrow examination revealed 2% myeloblasts. In addition, flow cytometry analysis identified that 39.07% of the non-erythrocytes were aberrant granulocytes with normal expression of CD65, CD15 and CD11b. On March 29, 2013, an additional course of chemotherapy (fourth cycle) was administered and on May 6, 2013, a bone marrow examination revealed that 2.5 and 45.27% of the non-erythrocytes were myeloblasts and aberrant granulocytes with normal expression of CD65, CD15, and CD11b, respectively, as analyzed by flow cytometry. The treatment was stopped when the patient presented with a normal peripheral hemogram and a good general condition. At present, the patient is undergoing follow-up examinations.
Discussion
aCML is classified as a CML according to the morphological diagnostic criteria proposed by the French-American-British Cooperatives Leukemia Group in 1994 (2). Notably, a previous study showed that aCML exhibits dyshematopoiesis and myeloproliferative features (10). aCML is categorized as a myelodysplastic syndrome (MDS)/myeloproliferative disease, according to the World Health Organization classification of tumors of hematopoietic and lymphatic tissues (2001) (1) and as a MDS/myeloproliferative neoplasm based on the revised WHO classification (2008) (11).
Chromosomal abnormalities, including +8, del(20q), del(11q), del(5q), t(6,8), and t(8,9) are found in ~80% of patients with aCML, however, none of these abnormalities are specific for aCML (12,13). Hernández et al (12) analyzed the clinical, hematological and cytogenetic characteristics of 11 aCML patients and found that 2 patients were positive for CEBPA mutations [chromosome 19q13.1; encoding CCAAT/enhancer-binding protein α (C/EBPα)]. C/EBPα is an important transcription factor for the maintenance of granulocytic differentiation in the hematopoietic system and the regulation of balance between cell proliferation and differentiation (12). The first type of mutation (including BCR-ABL, TEL-PDGFRb, RAS mutants, point mutations in FLT3-ITD and its activation loop and C-KIT mutation) predominantly manifest as sustained activation of tyrosine kinase that can affect downstream growth factors and thereby induce hyper-proliferation of the hematopoietic system. By contrast, the second type of mutation induces loss of function via gene fusion or point mutation in important transcription factors that are involved in the maintenance of granulocytic differentiation in the hematopoietic system. These mutations influence the differentiation of hematopoietic cells and the subsequent apoptotic events (1). CEBPA mutations belong to the second type of mutation. Notably, CEBPA mutations may be an optimal prognostic factor for aCML. However, at present, no studies regarding CEBPA mutations in aCML have been published, and further studies are required to investigate the association between aCML and CEBPA mutations.
aCML is associated with a poor prognosis and a mean survival time of <20 months with conventional therapy. In addition, 25–40% of aCML patients develop acute leukemia (13). However, at present no standard treatment exists for aCML, and previous studies have reported poor outcomes with conventional chemotherapies, including hydroxyurea, busulfan and interferon (4,14,15). Additionally, the tyrosine kinase inhibitor, imatinib, is unlikely to be useful in the treatment of aCML (10). A previous study, which included 10 aCML patients, found that following chemotherapy with cytarabine alone or in combination with demethoxydaunorubicin or mitoxantrone, no patients achieved complete remission (10). Koldehoff et al (16) evaluated the outcomes of allogeneic bone marrow transplantation (BMT) in 9 patients with aCML (<60 years of age) and found that the median follow-up was 55 months post-transplantation; thus, BMT may improve the prognosis of aCML. The treatment of aCML therefore remains a challenge. To the best of our knowledge, DCA has not previously been used to treat aCML. In the present two cases, after four cycles of chemotherapy with DCA, each patient achieved remission, as shown by bone marrow examination and evident satisfactory effects on aCML, such as relieved fatigue, no anemia, normal blood routine examination results and <5% marrow blasts.
DCA is a novel drug that is approved by the Food and Drug Administration for the treatment of MDS. Due to its S-phase specificity, DCA can cause DNA hypomethylation and cellular differentiation or apoptosis by inhibiting the activity of DNA methyltransferase, resulting in terminal differentiation and loss of clonality in human leukemic cells (17). DCA has been found to exhibit good clinical effects in malignant hematonosis and has been proven to be effective in patients with an intermediate/high risk for MDS, refractory/recurrent AML and accelerated/blast phase CML (18–20). In the present study, DCA therapy was an effective treatment for aCML. However, further clinical observations are required to determine its long-term efficacy. Additionally, additional studies with large sample sizes are also required to verify the effects of DCA, which may present a novel treatment modality for aCML.
In conclusion, following the achievement of remission in two patients treated with of DCA chemotherapy, the current case report revealed that DCA is effective in the treatment of aCML.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. Atypical chronic myeloid leukaemia, BCR-ABL1 negative. In: Swerdlow SH, Harris NL, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th. Lyon, France: IARC Press; 2008. pp. 80–81. [Google Scholar]
- 2.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick H, Sultan C, Cox C. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid and chronic myelomonocytic leukaemia. Proposals by the French-American-British Cooperative Leukaemia Group. Br J Haematol. 1994;87:746–754. doi: 10.1111/j.1365-2141.1994.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 3.Galton DA. Haematological differences between chronic granulocytic leukaemia, atypical chronic myeloid leukaemia, and chronic myelomonocytic leukaemia. Leuk Lymphoma. 1992;7:343–350. doi: 10.3109/10428199209049789. [DOI] [PubMed] [Google Scholar]
- 4.Breccia M, Biondo F, Latagliata R, Carmosino I, Mandelli F, Alimena G. Identification of risk factors in atypical chronic myeloid leukemia. Haematologica. 2006;91:1566–1568. [PubMed] [Google Scholar]
- 5.Cannella L, Breccia M, Latagliata R, Frustaci A, Alimena G. Clinical and prognostic features of patients with myelodysplastic/myeloproliferative syndrome categorized as unclassified (MDS/MPD-U) by WHO classification. Leuk Res. 2008;32:514–516. doi: 10.1016/j.leukres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Broseus J, Alpermann T, Wulfert M, et al. MPN and MPNr-EuroNet (COST Action BM0902): Age, JAK2(V617F) and SF3B1 mutations are the main predicting factors for survival in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2013;27:1826–1831. doi: 10.1038/leu.2013.120. [DOI] [PubMed] [Google Scholar]
- 7.Wang SA, Hasserjian RP, Fox PS, Rogers HJ, Geyer JT, Chabot-Richards D, Weinzierl E, Hatem J, Jaso J, Kanagal-Shamanna R, et al. Atypical chronic myeloid leukemia is clinically distinct from unclassifiable myelodysplastic/myeloproliferative neoplasms. Blood. 2014;123:2645–2651. doi: 10.1182/blood-2014-02-553800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland DR, Nayyar R, Acton E, Giftakis A, Dean S, Mosiman VL. Comparison of two single-platform ISHAGE-based CD34 enumeration protocols on BD FACSCalibur and FACSCanto flow cytometers. Cytotherapy. 2009;11:595–605. doi: 10.1080/14653240902923161. [DOI] [PubMed] [Google Scholar]
- 9.Piazza R, Valletta S, Winkelmann N, et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nature Genetics. 2013;45:18–24. doi: 10.1038/ng.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oscier DG. Atypical chronic myeloid leukaemia, a distinct clinical entity related to the myelodysplastic syndrome? Br J Haematol. 1996;92:582–586. doi: 10.1046/j.1365-2141.1996.396933.x. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe ES, Harrris NL, Stein H, et al. World Health Organization Classfication of Tumours. Lyon, France: IARC Press; 2001. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 12.Hernández JM, del Cañizo MC, Cuneo A, et al. Clinical, hematological and cytogenetic characteristics of atypical chronic myeloid leukemia. Ann Oncol. 2000;11:441–444. doi: 10.1023/A:1008393002748. [DOI] [PubMed] [Google Scholar]
- 13.Malcovati L, Cazzola M. Myelodysplastic/myeloproliferative disorders. Haematologica. 2008;93:4–6. doi: 10.3324/haematol.11374. [DOI] [PubMed] [Google Scholar]
- 14.Pabst T, Mueller BU, Zhang P, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet. 2001;27:263–270. doi: 10.1038/85820. [DOI] [PubMed] [Google Scholar]
- 15.Montefusco E, Alimena G, Lo Coco F, et al. Ph-negative and bcr-negative atypical chronic myelogenous leukemia: biological features and clinical outcome. Ann Hematol. 1992;65:17–21. doi: 10.1007/BF01715120. [DOI] [PubMed] [Google Scholar]
- 16.Koldehoff M, Beelen DW, Trenschel R, et al. Outcome of hematopoietic stem cell transplantation in patients with atypical chronic myeloid leukemia. Bone Marrow Transplant. 2004;34:1047–1050. doi: 10.1038/sj.bmt.1704686. [DOI] [PubMed] [Google Scholar]
- 17.Issa JP, Kantarjian HM. Introduction: emerging role of epigenetic therapy: focus on decitabine. Semin Hematol. 2005;42(3 Suppl 2):S1–S2. doi: 10.1053/j.seminhematol.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, O'Brien S, Giles F, et al. Decitabine low-dose schedule (100 mg/m2/course) in myelodysplastic syndrome (MDS). Comparison of three different dose schedules. Blood. 2005;106:708. [Google Scholar]
- 19.Cashen A, Shah A, Helget A, Todt L, Fisher N, DiPersio J. A phase I pharmacokinetic trial of decitabine administered as a 3-hour infusion to patients with acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) Blood. 2005;106:527–528. [Google Scholar]
- 20.Liu YL, Emanuel PD, Castleberry RP. Decitabine, a potential targeted therapeutic for juvenile myelomonocytic leukemia. Blood. 2005;106:706. [Google Scholar]