Abstract
Endometriosis is a chronic gynecological disease with a wide spectrum of clinical manifestations that affects approximately 10% of women of reproductive age. Recent reviews have demonstrated the connection between endometriosis and breast cancer, which represents the most frequently diagnosed female cancer and the most common cause of cancer-related mortality among women worldwide. The aim of this study was to conduct a survey of available published epidemiological studies indicating the association between endometriosis and breast cancer, and simultaneously to categorize the results based on the strength of the association, with the intention of the critical evaluation of the existing data. We performed a rigorous search of the PubMed/Medline database, using the key words ‘endometriosis’ and ‘breast cancer’ for all studies published in the English language until September 2015. We found 4 retrospective cohort studies, 4 case-control studies and 3 case-cohort studies that demonstrated a notable risk for developing breast cancer among women with endometriosis. By contrast, we also found 5 case-control studies, 1 prospective cohort study, 1 case-cohort study and 1 cross-sectional study that demonstrated a negative association between endometriosis and breast cancer. In conclusion, as regards the clarification of a ‘robust’ or ‘weak’ association between endometriosis and breast cancer, no definite conclusions could be drawn, due to the limited number of studies and the limitations of each of these studies. New well-designed, prospective cohort or randomized control trials with long-term follow-up are warranted in order to provide evidence-based clinical recommendations for proper counseling, screening and treatment strategies for patients with endometriosis, and hence to improve public health.
Keywords: breast cancer, endometriosis, epidemiological studies, gynecology, risk of breast cancer
Introduction
Endometriosis is a chronic gynecological disease that has been widely investigated, due to its high prevalence and substantial complications. It is estimated to affect almost 10% of women of reproductive age and up to 25–40% of infertile women (1,2). Although dysmenorrhea, pelvic pain and infertility constitute the classic triad of symptoms used in the diagnosis of endometriosis, 20-25% of patients may be asymptomatic (3). Traditionally, endometriosis is defined as the presence of endometrial glands and stroma in ectopic sites, other than the uterine cavity, primarily on the pelvic peritoneum, the ovaries, the rectovaginal septum and the uterosacral ligaments. The pathogenesis of this benign disease remains obscure, as none of the described theories (retrograde menstruation, coelomic metaplasia, lymphatic or vascular spread and dysfunctional immune response) can offer a complete explanation (4,5). Continually, various risk factors, such as familial clustering, genetic mutations or polymorphisms and environmental toxins, have been implicated, although without clear evidence (6,7). In addition, special attention has been paid to the similar behavioral pattern between endometriosis and cancer, as they both exhibit uncontrolled, estrogen-dependent proliferation, invasion, neo-angiogenesis and metastases (5,8). As a matter of fact, recent studies have established a connection between endometriosis and certain types of malignancies, particularly ovarian cancer, breast cancer (BC), cutaneous melanoma and non-Hodgkin's lymphoma (9,10).
Nowadays, much interest is focused on the interconnection between endometriosis and BC, granted that the latter forms the most frequent type of female cancer worldwide (11,12). The latest GLOBOCAN statistics are really impressive, as 1.67 million new cases of BC and 522,000 deaths were estimated for the year 2012 (13). However, evidence linking endometriosis with BC is rather vague and relies on the hormonal dependence and common risk factors of both diseases (14,15). Scientific study is also pointed towards the interplay of BC medication in the progress of endometriosis and vice versa. As has been previously demonstrated, aromatase inhibitors, selective estrogen receptor modulators and antiprogestins are novel therapeutic agents used in the treatment of endometriosis (7,16), whereas oral contraceptives and progestins, which are used as standard therapies for endometriosis, may have an adverse effect on the breast (17,18).
The aim of this study was to conduct a survey of available published epidemiological studies indicating an association between endometriosis and BC, and simultaneously to categorize the results based on the strength of the association, with the intention of the critical evaluation of the existing data.
Data collection methods
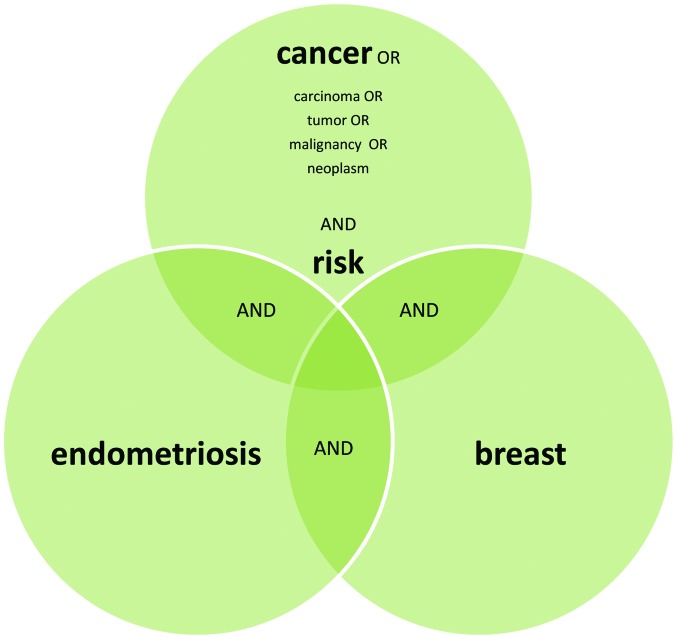
We performed a rigorous search of the PubMed/Medline database, using the key words ‘endometriosis’ and ‘BC’ for studies in the English language until September 2015. A supplementary literature search was carried out using the terms ‘endometriosis’ and ‘breast’ together with ‘cancer’ or ‘malignancy’ or ‘neoplasm’ or ‘tumor’ and ‘risk’. Basic science studies (molecular, genetic and functional), pathological studies, case reports and reviews were all excluded from our survey (Fig. 1).
Figure 1.
SmartArt graphics used to depict the search strategies that were applied in this study.
Results
Studies asserting a positive (direct) association between endometriosis and BC
The first study examining the association between hormonal-dependent medical disorders and BC was introduced in 1993 by Moseson et al (19). The participants in this small case-control study were 354 cases of BC and 747 controls, who were questioned via a telephone interview about a series of reproductive, menstrual and gynecological variables. Women with a reported history of endometriosis had a substantial increased risk of BC, particularly if they belonged to the premenopausal subgroup [odds ratio (OR), 4.3; 95% confidence interval (CI), 0.9–20.4]. However, a fundamental study indicating a significant correlation between endometriosis and BC was undertaken in 1997 by Schairer et al (20). In a case-cohort study, involving 15,844 Swedish women who underwent surgery for benign gynecological conditions, the risk of developing BC was evaluated with regard to the indication for surgery. Following data linkage to the National Swedish Cancer Registry, 295 cases of BC were recognized during a follow-up period of 12.2 years. Information about the type of surgery (oophorectomy and hysterectomy), age at surgery and the underlying medical conditions was also available. The authors concluded that endometriosis per se, as an exclusive indication for surgery, was associated with a >3-fold increase in the risk of developing BC when hysterectomy alone was performed [standardized morbidity ratio (SMR), 3.2; 95% CI, 1.2–8.0], whilst a slight increase was noticed when an oophorectomy was performed without a hysterectomy (SMR, 1.7; 95% CI, 0.7–4.1). Moreover, in 1997, Brinton et al accomplished a larger retrospective cohort study, including 20,686 Swedish women with a hospital discharge diagnosis of endometriosis (21). Record linkage to cancer registers allowed the identification of 297 patients with a subsequent diagnosis of BC at a mean follow-up of 11.4 years. The authors agreed that the total risk of developing BC was notably affected by the history of endometriosis [standardized incidence ratio (SIR), 1.3; 95% CI, 1.1–1.4]. The risk of developing BC was also related to the site of origin of endometriosis and was found to be higher among women with endometriosis arising in the pelvis (SIR, 1.79; 95% CI, 1.2–2.6).
In 1999, Weiss et al presented a population-based case-control study, concerning the influence of several medical conditions on the risk of developing BC (22). The authors collected questionnaires from 2,173 young American women, newly diagnosed with in situ or invasive BC, and 1,990 controls. Multivariate logistic regression analysis revealed a greater risk of developing BC among premenopausal women with endometriosis (OR, 1.68), particularly among those with recent surgery (OR 1–9 years, 2.38; 95% CI, 1.0–5.5). However, the risk was relatively lower among young women who had previously undergone surgery for endometriosis (OR, 1.14; 95% CI, 0.7–1.8). At the same time, Venn et al conducted a case-cohort study in 2,970 Australian in vitro fertilisation (IVF) candidates, in order to assess the incidence of invasive breast, ovarian and uterine cancer combined with the infertility cause and administration of superovulation drugs (23). As a result, infertile women with endometriosis were found to have a borderline increase in the risk of developing BC, particularly 12 months following exposure to fertility drugs and when 3–6 oocytes per ovulation cycle were collected (SIR, 1.04; 95% CI, 0.71–1.54). In 2004, Borgfeldt and Andolf evaluated the incidence of gynecological cancer in patients with benign ovarian cysts, functional ovarian cysts, or endometriosis (24). By the optimal use of the Swedish database, they designed a nested case-control study of 28,163 women with a hospital diagnosis of endometriosis each matched with 3 controls. Briefly, the authors detected a subtle increase in the risk of developing BC among women with endometriosis (OR, 1.10; 95% CI, 1.0–1.2). Utilizing Swedish Inpatient and Cancer records once again, in 2006, Melin et al conducted a larger retrospective cohort study for the purpose of examining cancer risk, particularly ovarian cancer risk, among women with endometriosis (25). Therefore, 64,492 women discharged from the hospital and coded for endometriosis as first time diagnosis, entered the study. Statistical analysis adjusted for age verified an important risk of developing BC when endometriosis was diagnosed in older women, particularly those aged between 50 and 60 years (SIR, 1.28; 95% CI, 1.13–1.45). Later on, Melin et al examined the effect of parity on the previously documented excess risk of developing certain malignancies among women with endometriosis (26). In a new cohort study, containing the two Swedish cohorts of Brinton et al in 1997 (21) and Melin et al in 2006 (25), overall, 63,630 women with a discharge diagnosis of endometriosis were recruited. Through the National Swedish Cancer Registry, 1,465 cases of BC were identified at a mean follow-up time of 13.4 years. Eventually, the study confirmed that the risk of developing BC was clearly increased in the women with endometriosis, but was not affected by parity or the lack of parity (SIR, 1.08; 95% CI, 1.02–1.13). In 2007, Bertelsen et al evaluated the association between endometriosis and BC on the grounds of medical history (27). In their large Danish case-cohort study, including 114,327 women, they encountered 236/1,978 cases of endometriosis, women who were diagnosed with BC during a mean follow-up period of 18 years. By Cox regression analysis, the authors observed that age at the time of diagnosis of endometriosis influenced the risk of developing BC, hence premenopausal (approximately ≥40) and postmenopausal women had a significantly elevated odds ratio (OR, 2.40; 95% CI, 1.43–4.01).
Contrariwise, in 2011, Nichols et al, by a population-based case-control study that was held in Wisconsin, Massachusetts and New Hampshire, emphasized that women diagnosed with endometriosis under the age of 35 years had a borderline significant increase in the risk of developing BC (OR, 1.83; 95% CI, 0.95–3.51), particularly those with an intact uterus and ovaries (28). Ultimately, in 2015, a new article, discussing the risk-spectrum of ovarian, endometrial, breast and colorectal cancer in women with recently diagnosed endometriosis, was published (29). Kok et al performed a population-based cohort study by using data from the Taiwan National Health Insurance Research Database during the years 2003–2005. The participants had a follow-up for cancer occurrence until December 2008. Of the 2,266 women in the endometriosis cohort and 9,064 women in the comparison cohort, they extracted 18 and 51 cases of BC, respectively. Cox regression analysis adjusted to miscellaneous variants apart from parity, led to the aggregate outcome of a ‘marginal’ risk of developing BC among women with surgically confirmed endometriosis [hazard ratio (HR), 1.15; 95% CI, 0.61–2.15].
Studies asserting a negative (null or inverse) association between endometriosis and BC
Although the majority of the aforementioned studies demonstrate a rather significant association between endometriosis and the risk of developing BC, several other studies are supportive of a negative correlation. First of all, Moseson et al (19) in the small case-control study of 354 BC cases and 747 controls described above, noted a non-significant protective association in postmenopausal women with a self-reported diagnosis of endometriosis (OR, 0.5; 95% CI, 0.1–2.6). In 2001, Baron et al, through a large American case-control study including 5,659 cases of BC and 5,928 controls, investigated the association between BC and metabolic disorders (30). Following detailed telephone interviews composed of a series of questions on reproductive and medical history, overall, 303 cases of endometriosis were identified. Eventually, their study demonstrated that women who reported endometriosis had a modest reduction in the risk of developing BC (OR, 0.8; 95% CI, 0.7–1.00). A year later, Olson et al announced the results of the Iowa Women's Health Study which was a large prospective cohort study of 37,434 participants, aiming to pinpoint risk factors for cancer in postmenopausal women (31). In total, 1,392 women with a self-reported history of endometriosis were followed-up for 13 years for cancer incidence. Among the 1,795 new cases of BC, solely 67 (3.7%) cases were detected in the endometriosis group. By using Cox proportional hazards and multivariate adjustment, the authors found that endometriosis was not associated with a significant risk of breast carcinoma [relative risk (RR), 0.96; 95% CI, 0.75–1.23]. In the above-quoted case-cohort study of Bertelsen et al (27) examining the association between different medical conditions and BC, overall, 236 cases of endometriosis were counted among 16,983 women who laterally developed BC between 1978 and 1998. In a Cox regression analysis adjusted for confounding variables, a neutral association between endometriosis and BC was declared (RR, 0.97; 95% CI, 0.85–1.11). Moreover, women who were diagnosed with endometriosis at a young age (>40 years) had a lower relative risk of developing BC than the older subgroups (RR, 0.77; 95% CI, 0.61–0.96).
In a more recent US cross-sectional study published in 2010, Gemmill et al examined the hypothesis that women with surgically confirmed endometriosis had a higher prevalence of other concurrent disorders, such as cancer, endocrine diseases and infections (32). On this scope, questionnaires from 4,331 members of the Endometriosis Association provided efficient data for comparison with national cancer statistics. The authors isolated only 16 women diagnosed with BC at a mean age of 40.9 years (across all 75 cancer cases), and thus realized that BC is less frequent in patients with endometriosis than in the general population [prevalence odds ratio (POR), 0.54; 95% CI, 0.32–0.90; P=0.016]. Likewise, the previously reported case-control study by Nichols et al indicated an inverse association between endometriosis and BC (28). The main purpose of that study was to determine whether benign indications for bilateral oophorectomy, such as uterine fibroids and endometriosis, modify the risk of developing BC post-menopause. The respondents to a telephone interview were 4,935 women with a first diagnosis of invasive BC, between the period from 1992 to 1995, and 5,111 controls. In total, 198 cases of endometriosis were found from the survey. Following multivariate logistic regression analysis and adjustment for potential confounders, there was no assurance of a significant risk of developing BC among American women with a history of endometriosis (OR, 0.99; 95% CI, 0.80–1.21). The authors also affirmed that the risk of developing BC was not affected by the history of bilateral oophorectomy with hysterectomy nor by the history of an intact uterus and ovaries (OR, 0.82; 95% CI, 0.60–1.10). Of note, a strong BC risk reduction (58% lower BC risk odds) related to bilateral oophorectomy with hysterectomy at age ≤40 years versus no surgery, was observed in the endometriosis pool (OR, 0.42; 95% CI, 0.21–0.87; P=0.03).
In 2013, Matta et al, through a case-control study of 991 Puerto Rican women, attempted to explore the interrelation between endometriosis and BC, from the prospective of DNA repair capacity (33). In their study, among the 385 cases of BC and 606 controls recruited over a 5-year period, primary BC was diagnosed in only 20 participants with surgically confirmed endometriosis (n=80 cases of endometriosis). Following multiple logistic regression adjusted for confounders, it was manifested that BC cases had 50% lower odds of having a history of endometriosis (OR, 0.5; 95% CI, 0.3–0.9; P=0.038) compared to the controls. Using a similar methodological approach, Morales et al presented another case-control study of 1,126 adult female Puerto Rican residents, evaluating the major risk factors for BC (34). In this larger incidence-case study, 465 cases of recently diagnosed BC and 661 controls were included. Statistical analysis revealed the beneficial effect of endometriosis, since the risk of developing BC was decreased by 39% (OR, 0.61; 95% CI, 0.3–1.0; P=0.039) in women with a history of endometriosis.
Discussion
Indeed there is an extensive literature on the issue ‘endometriosis and BC’, indicative of the still indefinable relevance between them. Apart from the aforementioned studies, numerous other publications have highlighted various noteworthy aspects of this topic. For instance, Melin et al underlined that endometriosis plays a pivotal role in cancer survival, since there was a statistically significant improved survival for women with endometriosis and BC (HR 0.86) (35). Other studies, such as the one by Chalas et al, proved that both premenopausal (RR, 1.9; 95% CI, 1.35–2.70) and postmenopausal (RR, 1.9; 95% CI, 1.29–5.58) women administered tamoxifen for the treatment/prevention for BC were almost 2-fold more likely to develop endometriosis compared to women on the placebo (36). Moreover, Matalliotakis et al, in a retrospective study on a Yale series emphasized an elevated risk associated with a family history of BC among women with endometriosis (37).
Taking the above-mentioned data into account, in this study, we attempted to separate our results into two different categories, based on the criterion of the strength of the association between endometriosis and BC, in terms of RR, OR, HR, POR, SIR or SMR. Collectively, from our survey, we found 4 retrospective cohort studies (21,25,26,29), 4 case-control studies (19,22,24,28) and 3 case-cohort studies (20,23,27) that demonstrate a notable risk of developing BC among women with endometriosis (Table I). By contrast, we gathered 5 case-control studies (19,28,30,33,34), 1 prospective cohort study (31), 1 case-cohort study (27) and 1 cross-sectional study (32) that showed a negative association between endometriosis and BC (Table II).
Table I.
Overview of studies asserting a positive (direct) association between endometriosis and breast cancer.
| Authors/(Refs.) year | Study design (study period) | Study size | No. of person year | Mean follow-up (years) | No. of endometriosis cases | No. of BC cases | No. of countrols | Median age at entry (years) | Excludes 1st year of follow-up | Association |
|---|---|---|---|---|---|---|---|---|---|---|
| Moseson et al (19) | Case-control | N/A | N/A | 2.3 | 6 | 354 | 747 | 55–64 | No | OR, 1.7 (95% CI, 0.6–5.1) P=0.33 |
| 1993 | (1977–1981) | 4a | OR, 4.3 (95% CI, 0.9–20.4) P=0.07a | |||||||
| Schairer et al (20) | Case-cohort | 15,844 | 193,083 | 12.2 | N/A | 295 | 1,235 | 45.7 | No | SMR, 3.2 (95% CI, 1.2–8.0)b |
| 1997 | (1965–1983) | SMR, 1.7 (95% CI, 0.7–4.1)c | ||||||||
| Brinton et al (21) | Cohort | 21,398 | 216,851 | 11.4 | 20,686 | 297 | N/A | 38.8 | Yes | SIR, 1.3 (95% CI, 1.1–1.4) |
| 1997 | (1969–1983) | SIR, 1.79 (95% CI, 1.2–2.6)d | ||||||||
| Weiss et al (22) | Case-control | N/A | N/A | N/A | 53 | 2173 | 1,990 | <55 | No | RR, 1.14 (95% CI, 0.7–1.8)e |
| 1999 | (1990–1992) | RR, 1.68 (95% CI, 0.9–3.0)f | ||||||||
| RR, 1.37 (95% CI, 0.7–2.5)g | ||||||||||
| Venn et al (23) | Case-cohort | 29,700 | 148,672 | 7 | 3613 | 25 | 9,044 | 31 | No | SIR, 1.04 (95% CI, 0.71–1.54)h |
| 1999 | (1986–1994) | |||||||||
| Borgfeldt and Andolf (24) | Nested case-control | 88,378 | N/A | N/A | 28,163 | 427 | 71165 | N/A | Yes | OR, 1.10 (95% CI, 1.0–1.2) |
| 2004 | (1969–1996) | |||||||||
| Melin et al (25) | Cohort | 64,492 | 766,556 | 12.7 | N/A | 1,288 | N/A | 39.4 | Yes | SIR, 1.28 (95% CI 1.13–1.45)i |
| 2006 | (1969–2000) | SIR, 1.23 (95% CI, 0.82–1.78)j | ||||||||
| Melin et al (26) | Cohort | 63,630 | 792,013 | 13.4 | N/A | 1,465 | N/A | 39.5 | Yes | SIR, 1.08 (95% CI, 1.02–1.13) |
| 2007 | (1969–2002) | |||||||||
| Bertelsen et al (27) | Case-cohort | 114,327 | 2,031,811 | 17.8 | 1978 | 236 | N/A | 40.6 | Yes | OR, 1.10 (95% CI, 0.90–1.34)k |
| 2007 | (1978–1998) | OR, 2.40 (95% CI, 1.43–4.01)l | ||||||||
| Nichols et al (28) | Case-control | N/A | N/A | N/A | 26m | 4,935 | 5,111 | 66.2 | No | OR, 1.83(95% CI, 0.95–3.51)m |
| 2011 | (1992–1995) | |||||||||
| Kok et al (29) | Cohort | N/A | 9,842 | 3 | 2266 | 18 | 9,064 | 31–50 | No | HR, 1.15 (95% CI, 0.61–2.15) |
| 2015 | (2003–2005) |
The table includes study design and size, number of person-years, follow-up, number of endometriosis cases, number of breast cancer cases, number of controls, median age, exclusion of first year of follow-up and the association, in terms of OR or RR or HR or SIR or SMR.
Premenopausal women;
hysterectomy without ovarian ablation;
oophorectomy without hysterectomy;
origin of endometriosis in the pelvis;
young women ever diagnosed;
premenopausal women ever diagnosed;
exposed and unexposed to IVF treatment with ovarian stimulation combined;
women between 50–60 years who were hospitalized and coded for the first time for endometriosis;
women between 60–70 years who were hospitalized and coded for the first time for endometriosis;
age at endometriosis 40–49 years (1 year latency);
age at endometriosis ≥50 years (1 year latency);
number of endometriosis cases with intact uterus and ovaries diagnosed at age <35 years; N/A, not available; OR, odds ratio; RR, relative risk; HR, hazard ratio; SMR, standardized morbidity ratio.
Table II.
Overview of studies asserting negative (null or inverse) association between endometriosis and breast cancer.
| Authors/(Refs.) year | Study design (study period) | Study size | No. of person-Years | Mean follow-up (years) | No. of endometriosis cases | No. of BC cases | No. of controls | Median age at entry (years) | Excludes 1st year of follow-up | Association |
|---|---|---|---|---|---|---|---|---|---|---|
| Moseson et al (19) | Case-control | N/A | N/A | 2.3 | 2a | 354 | 747 | 55–64 | No | OR, 0.5 (95% CI, 0.1–2.6) P=0.40a |
| 1993 | (1977–1981) | |||||||||
| Baron et al (30) | Case-control | N/A | N/A | N/A | 303 | 5,659 | 5,928 | 50–79 | No | OR, 0.8 (95% CI, 0.7–1.0) |
| 2001 | (1990–1994) | |||||||||
| Olson et al (31) | Cohort | 37434 | N/A | 13 | 1392 | 67 | N/A | >55 | No | RR, 0.96 (95% CI, 0.75–1.23) |
| 2002 | (1986–1998) | |||||||||
| Bertelsen et al (27) | Case-cohort | 114,327 | 2,031,811 | 17.8 | 236 | 16,983 | N/A | 40.6 | No | RR, 0.97 (95% CI, 0.85–1.11)b |
| 2007 | (1978–1998) | RR, 0.77 (95% CI, 0.61–0.96)c | ||||||||
| Gemmill et al (32) | Cross-sectional | 4,745 | N/A | N/A | 4,331 | 16 | N/A | 40.9 | No | POR, 0.54 (95% CI, 0.32–0.90) P=0.016 |
| 2010 | (1998–2010) | |||||||||
| Nichols et al (28) | Case-control | N/A | N/A | N/A | 198d | 4,935 | 5,111 | 66.2 | No | OR, 0.99 (95% CI, 0.80–1.21)d |
| 2011 | (1992–1995) | 18e | OR, 0.42 (95% CI, 0.21–0.87) P=0.03e | |||||||
| Matta et al (33) | Case-control | 991 | N/A | 5 | 20 | 385 | 606 | 41–60 | No | OR, 0.5 (95% CI, 0.3–0.9) P=0.038 |
| 2013 | (2006–2012) | |||||||||
| Morales et al (34) | Case-control | 1,126 | N/A | 5 | 26 | 465 | 661 | 56.4 | No | OR, 0.61 (95% CI, 0.3–1.0) P=0.039 |
| 2013 | (2006–2012) |
The table presents the studies asserting negative (null or inverse) association between endometriosis and breast cancer, including study design and size, number of person-years, follow-up, number of endometriosis cases, number of breast cancer cases, number of controls, median age, exclusion of first year of follow up and the association, in terms of OR or POR or RR.
Postmenopausal women;
all ages at BC diagnosis (without 1-year-latency);
age at endometriosis 30–39 years;
endometriosis women who either reported bilateral oophorectomy and hysterectomy or no surgery;
bilateral oophorectomy and hysterectomy performed at age ≤40 years; N/A, not available; OR, odds ratio; RR, relative risk.
On balance, our results seem to be contradictory and require interpretation in a careful and prudent manner. To begin with, a broad comparison between Tables I and II shows a numerical superiority of the studies confirming a positive association between endometriosis and BC. Nonetheless, this is a spurious argument, as three of these studies are included in both tables due to the ambiguous results of each one (19,27,28). Moreover, safe conclusions cannot be drawn due to the wide range of the association (between 4.3–1.04 in terms of OR, RR, HR, SIR and SMR), the different study designs and the inherent weaknesses of such studies.
At a glance, strong evidence that women with endometriosis are more vulnerable to later develop BC is shown in 3 case-control studies (19,22,28), 2 case-cohort studies (20,27) and in only 1 retrospective cohort study (21). Certainly, the first case-control study of Moseson et al (19) is not of statistical importance, by reason of an elevated odds ratio (OR, 4.3 with wide-range CI, 0.9–20.4) pertaining to a small number of premenopausal women (n=4), in opposition to the larger case-cohort study of Schairer et al (20), which clarifies that women with endometriosis who underwent hysterectomy with ovarian retention had a higher morbidity ratio (SMR, 3.2; CI, 1.2–8.0) for BC. Next, in the case-cohort study by Bertelsen et al (27), higher odds ratios (OR, 2.40) for BC were recorded in women diagnosed with endometriosis at an older age (over 40 years), whereas in the case-control study of Nichols et al (28), comparable odds (OR, 1.83) were found in those diagnosed before the age of 35. The cohort study of Brinton et al (21) further underlines the effect of the localization of endometriosis, suggesting a significant incidence ratio (SIR, 1.79) for BC in the case of the pelvic origin of the disease. Subsequently, the wide case-control study by Weiss et al (22) indicated a significant relative risk of developing BC in premenopausal women ever diagnosed with endometriosis (RR, 1.68), particularly in those who underwent recent surgery (RR, 1.37). Lastly, the remaining 2 retrospective cohort studies of Melin et al (25,26) illustrated a minor increase in the risk of developing BC (SIR, 1.08–1.28) related to an advanced age (>50 years) at the time of the diagnosis of endometriosis, but not to parity.
Beyond the potential selection and detection bias of these studies, several limitations basically concerning the population under study and the cofounding variables, can greatly hinder the data evaluation process. As an example, selection bias in some studies may arise from the particular selection of women who underwent surgery for endometriosis (20,22,29). Detection bias in other studies may occur by the use of hospital discharge diagnosis of endometriosis, since only the serious cases of the disease could have been included (21,22,24–26,29). Selection and detection bias in conjunction with the exclusion of a large number of cancer patients as ineligible candidates may have resulted in an underestimation of the true risk of developing BC. The self-reported history of endometriosis by questionnaires or telephone interviews may also have led to recall bias (19,28), and consequently to an overestimation of the overall risk. A point often overlooked is that women participants in certain studies were in their vast majority, postmenopausal (19,28) or premenopausal (20,27), whereas other studies included entirely young women (23). The intervals of follow-up, as well, differ amidst studies with a wide range from 2.3 to 17.8 years (19,27). Particularly problematic in the majority of studies is the analysis with adjustment for numerous confounders, such as age, race, parity, hysterectomy status, family history of BC, mammograms and body mass index (BMI) (22,27). Another drawback is the lack of data on endometriosis staging by the revised American Society for Reproductive Medicine (rASRM), on the histological type of BC, as well as on treatment regimens. After all, the increased risk of developing BC in postmenopausal women may be attributed to common risk factors between endometriosis and BC or to hormone replacement treatment or to altered endogenous estrogens (20,27). Comparatively, young women on danazol/GnRH agonists treatment for endometriosis may have a long term protection against BC (27). On the other hand, we found only 1 prospective cohort study (31), 1 case-cohort study (27) and 1 case-control study (28) supporting a null association between endometriosis and BC. As can be seen, the major limitation in the study of Olson et al (31) is that the population under study was strictly menopausal (>55 years) at entry, and thus a possible degradation of the true BC must be taken into account, given the short latency period between endometriosis and BC occurrence. Another weak point of this cohort is the self-reports and not the surgical confirmation of endometriosis, which may have also affected the risk estimates. Furthermore, we collected 5 case-control studies (19,28,30,33,34) and 1 cross-sectional study (32), providing evidence that women with endometriosis are less likely to develop BC. A closer look at these reveals participant groups of premenopausal and postmenopausal women, short follow-up intervals and variation in effect sizes (in OR) between 0.42 (28) and 0.8 (30). Two of these studies also refer exclusively to Puerto Rican women (33,34). In fact, several limitations related to implicit sources of bias in such studies preclude the acceptance of an inverse association between endometriosis and BC.
In summary, our conclusions are in accordance with those of previously published surveys on the same issue thus far, confirming the lack of data and inconsistent results among studies (38–40). As regards the clarification of a ‘robust’ or ‘weak’ association between endometriosis and BC, no definite conclusions can be drawn from our survey, due to the limited number of studies and the limitations of each of these studies. Importantly, new studies are urgently required to investigate whether women with endometriosis have a predisposition to develop BC, taking into consideration the high mortality rate associated with BC worldwide. Large well-designed, adequately powered, prospective cohort or randomized control trials with long term follow-up periods are thus warranted in order to provide evidence-based clinical recommendations for proper counseling, screening and treatment strategies for such patients, and hence to improve public health.
Glossary
Abbreviations
- BC
breast cancer
- RR
relative risk
- OR
odds ratio
- POR
prevalence odds ratio
- HR
hazard ratio
- SIR
standardized incidence ratio
- SMR
standardized morbidity ratio
- CI
confidence interval
- IVF
in vitro fertilization
- rASRM
revised American Society for Reproductive Medicine
- BMI
body mass index
References
- 1.Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: An update for clinicians. Hum Reprod Update. 2006;12:179–189. doi: 10.1093/humupd/dmi049. [DOI] [PubMed] [Google Scholar]
- 2.Ozkan S, Murk W, Arici A. Endometriosis and infertility: Epidemiology and evidence-based treatments. Ann NY Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007. [DOI] [PubMed] [Google Scholar]
- 3.Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27:441–447. doi: 10.1007/s10815-010-9436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515. doi: 10.1155/2014/179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 7.Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7:349–357. [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas EJ, Campbell IG. Evidence that endometriosis behaves in a malignant manner. Gynecol Obstet Invest. 2000;50(Suppl 1):2–10. doi: 10.1159/000052872. [DOI] [PubMed] [Google Scholar]
- 9.Kokcu A. Relationship between endometriosis and cancer from current perspective. Arch Gynecol Obstet. 2011;284:1473–1479. doi: 10.1007/s00404-011-2047-y. [DOI] [PubMed] [Google Scholar]
- 10.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA. Endometriosis: A high-risk population for major chronic diseases? Hum Reprod Update. 2015;21:500–516. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy D, Morgan M, Yoo C, Deoraj A, Roy S, Yadav VK, Garoub M, Assaggaf H, Doke M. Integrated bioinformatics, environmental epidemiologic and genomic approaches to identify environmental and molecular links between endometriosis and breast cancer. Int J Mol Sci. 2015;16:25285–25322. doi: 10.3390/ijms161025285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung JN, Holdsworth-Carson SJ, Sapkota Y, Zhao ZZ, Jones L, Girling JE, Paiva P, Healey M, Nyholt DR, Rogers PA, Montgomery GW. Functional evaluation of genetic variants associated with endometriosis near GREB1. Hum Reprod. 2015;30:1263–1275. doi: 10.1093/humrep/dev051. [DOI] [PubMed] [Google Scholar]
- 13.Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys. 2014 Dec 28; doi: 10.1007/s12013-014-0459-6. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Shah R, Rosso K, Nathanson SD. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J Clin Oncol. 2014;5:283–298. doi: 10.5306/wjco.v5.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SourcePDQ Cancer Information Summaries [Internet] Bethesda (MD): National Cancer Institute (US); Jul 14, 2002. Breast Cancer Screening (PDQ®): Health Professional Version. Authors PDQ Screening and Prevention Editorial Board. 2015. [Google Scholar]
- 16.Goyeneche AA, Telleria CM. Antiprogestins in gynecological diseases. Reproduction. 2015;149:R15–R33. doi: 10.1530/REP-14-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gierisch JM, Coeytaux RR, Urrutia RP, Havrilesky LJ, Moorman PG, Lowery WJ, Dinan M, McBroom AJ, Hasselblad V, Sanders GD, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931–1943. doi: 10.1158/1055-9965.EPI-13-0298. [DOI] [PubMed] [Google Scholar]
- 18.Prentice RL. Postmenopausal hormone therapy and the risks of coronary heart disease, breast cancer, and stroke. Semin Reprod Med. 2014;32:419–425. doi: 10.1055/s-0034-1384624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moseson M, Koenig KL, Shore RE, Pasternack BS. The influence of medical conditions associated with hormones on the risk of breast cancer. Int J Epidemiol. 1993;22:1000–1009. doi: 10.1093/ije/22.6.1000. [DOI] [PubMed] [Google Scholar]
- 20.Schairer C, Persson I, Falkeborn M, Naessen T, Troisi R, Brinton LA. Breast cancer risk associated with gynecologic surgery and indications for such surgery. Int J Cancer. 1997;70:150–154. doi: 10.1002/(SICI)1097-0215(19970117)70:2<150::AID-IJC2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 21.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–579. doi: 10.1016/S0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 22.Weiss HA, Brinton LA, Potischman NA, Brogan D, Coates RJ, Gammon MD, Malone KE, Schoenberg JB. Breast cancer risk in young women and history of selected medical conditions. Int J Epidemiol. 1999;28:816–823. doi: 10.1093/ije/28.5.816. [DOI] [PubMed] [Google Scholar]
- 23.Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet. 1999;354:1586–1590. doi: 10.1016/S0140-6736(99)05203-4. [DOI] [PubMed] [Google Scholar]
- 24.Borgfeldt C, Andolf E. Cancer risk after hospital discharge diagnosis of benign ovarian cysts and endometriosis. Acta Obstet Gynecol Scand. 2004;83:395–400. doi: 10.1111/j.0001-6349.2004.00305.x. [DOI] [PubMed] [Google Scholar]
- 25.Melin A, Sparén P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006;21:1237–1242. doi: 10.1093/humrep/dei462. [DOI] [PubMed] [Google Scholar]
- 26.Melin A, Sparén P, Bergqvist A. The risk of cancer and the role of parity among women with endometriosis. Hum Reprod. 2007;22:3021–3026. doi: 10.1093/humrep/dem209. [DOI] [PubMed] [Google Scholar]
- 27.Bertelsen L, Mellemkjaer L, Frederiksen K, Kjaer SK, Brinton LA, Sakoda LC, van Valkengoed I, Olsen JH. Risk for breast cancer among women with endometriosis. Int J Cancer. 2007;120:1372–1375. doi: 10.1002/ijc.22490. [DOI] [PubMed] [Google Scholar]
- 28.Nichols HB, Visvanathan K, Newcomb PA, Hampton JM, Egan KM, Titus-Ernstoff L, Trentham-Dietz A. Bilateral oophorectomy in relation to risk of postmenopausal breast cancer: Confounding by nonmalignant indications for surgery? Am J Epidemiol. 2011;173:1111–1120. doi: 10.1093/aje/kwq510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kok VC, Tsai HJ, Su CF, Lee CK. The risks for ovarian, endometrial, breast, colorectal, and other cancers in women with newly diagnosed endometriosis or adenomyosis: A population-based study. Int J Gynecol Cancer. 2015;25:968–976. doi: 10.1097/IGC.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 30.Baron JA, Weiderpass E, Newcomb PA, Stampfer M, Titus-Ernstoff L, Egan KM, Greenberg ER. Metabolic disorders and breast cancer risk (United States) Cancer Causes Control. 2001;12:875–880. doi: 10.1023/A:1013796112348. [DOI] [PubMed] [Google Scholar]
- 31.Olson JE, Cerhan JR, Janney CA, Anderson KE, Vachon CM, Sellers TA. Postmenopausal cancer risk after self-reported endometriosis diagnosis in the Iowa Women's Health Study. Cancer. 2002;94:1612–1618. doi: 10.1002/cncr.10370. [DOI] [PubMed] [Google Scholar]
- 32.Gemmill JA, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Cancers, infections, and endocrine diseases in women with endometriosis. Fertil Steril. 2010;94:1627–1631. doi: 10.1016/j.fertnstert.2009.07.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matta JL, Flores I, Morales LM, Monteiro J, Alvarez-Garriga C, Bayona M. Women with endometriosis have a higher DNA repair capacity and diminished breast cancer risk. Mol Cancer Biol. 2013;1:1. doi: 10.9777/mcb.2013.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales L, Alvarez-Garriga C, Matta J, Ortiz C, Vergne Y, Vargas W, Acosta H, Ramírez J, Perez-Mayoral J, Bayona M. Factors associated with breast cancer in Puerto Rican women. J Epidemiol Glob Health. 2013;3:205–215. doi: 10.1016/j.jegh.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melin A, Lundholm C, Malki N, Swahn ML, Sparen P, Bergqvist A. Endometriosis as a prognostic factor for cancer survival. Int J Cancer. 2011;129:948–955. doi: 10.1002/ijc.25718. [DOI] [PubMed] [Google Scholar]
- 36.Chalas E, Costantino JP, Wickerham DL, Wolmark N, Lewis GC, Bergman C, Runowicz CD. Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am J Obstet Gynecol. 2005;192:1230–1239. doi: 10.1016/j.ajog.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 37.Matalliotakis IM, Cakmak H, Mahutte N, Goumenou AG, Koumantakis G, Arici A. The familial risk of breast cancer in women with endometriosis from Yale series. Surg Oncol. 2008;17:289–293. doi: 10.1016/j.suronc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Munksgaard PS, Blaakaer J. The association between endometriosis and gynecological cancers and breast cancer: A review of epidemiological data. Gynecol Oncol. 2011;123:157–163. doi: 10.1016/j.ygyno.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Vlahos NF, Economopoulos KP, Fotiou S. Endometriosis, in vitro fertilisation and the risk of gynaecological malignancies, including ovarian and breast cancer. Best Pract Res Clin Obstet Gynaecol. 2010;24:39–50. doi: 10.1016/j.bpobgyn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Anifantaki FI, Boutas I, Kalampokas T, Kalampokas E, Sofoudis C, Salakos N. Association of endometriosis and breast cancer: Mini review of the literature. Arch Gynecol Obstet. 2015 Jul 03; doi: 10.1007/s00404-015-3809-8. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]