Abstract
Angiotensin-converting enzyme (ACE) inhibitory activity was evaluated for the low-molecular-weight fraction (<3 kDa) obtained from milk fermentation by Bifidobacterium longum KACC91563. The ACE inhibitory activity in this fraction was 62.3%. The peptides generated from the <3 kDa fraction were identified by liquid chromatography-electrospray ionization quantitative time-of-flight mass spectrometry analysis. Of the 28 peptides identified, 11 and 16 were identified as β-casein (CN) and αs1-CN, respectively. One peptide was identified as κ-CN. Three peptides, YQEPVLGPVRGPFPIIV, QEPVLGPVRGPFPIIV, and GPVRGPFPIIV, from β-CN corresponded to known antihypertensive peptides. We also found 15 peptides that were identified as potential antihypertensive peptides because they included a known antihypertensive peptide fragment. These peptides were as follows: RELEELNVPGEIVE (f1-14), YQEPVLGPVRGPFP (f193-206), EPVLGPVRGPFPIIV (f195-206), PVLGPVRGPFPIIV (f196-206), VLGPVRGPFPIIV (f197-206), and LGPVRGPFPIIV (f198-206) for β-CN; and APSFSDIPNPIGSENSEKTTMPLW (f176-199), SFSDIPNPIGSENSEKT- TMPLW (f178-199), FSDIPNPIGSENSEKTTMPLW (f179-199), SDIPNPIGSENSEKTTMPLW (f180-199), DIPNPIGSENSEKTTMPLW (f181-199), IPNPIGSENSEKTTMPLW (f182-199), PIGSENSEKTTMPLW (f185-199), IGSENSEKTTMPLW (f186-199), and SENSEKTTMPLW (f188-199) for αs1-CN. From these results, B. longum could be used as a starter culture in combination with other lactic acid bacteria in the dairy industry, and/or these peptides could be used in functional food manufacturing as additives for the development of a product with beneficial effects for human health.
Keywords: B. longum, antihypertensive peptide, angiotensin converting enzyme
Introduction
Probiotic bifidobacteria such as Bifidobacterium longum (B. longum), Bifidobacterium breve, Bifidobacterium animalis, and Bifidobacterium bifidum are commonly used as starter cultures in the dairy industry e.g., fermented milk, cheese and infant formulas (Chang et al., 2013; Davidson et al., 2000; Martín-Diana et al., 2003; McBrearty et al., 2001; Saavedra et al., 2004) because of their beneficial effects for human health. The various beneficial effects include the pathogenic species inhibition, diminution of colon cancer risk, immune response for protection effect on their function, regulation of gut microflora (Arunachalam, 1999; Chang et al., 2013; Collins and Gibson, 1999; Leahy et al., 2005).
As lactic acid bacteria (LAB) including Lactococcus lactis (L. lactis), Lactobacillus rhamnosus (Lb. rhamnosus), Streptococcus thermophilus (St. thermophilus), Lactobacillus lactis (Lb. lactis), Lactobacillus helveticus (Lb. helveticus), and Lactobacillus subsp. bulgaricus (Lb. bulgaricus), bifidobacteria strains for their growth also use milk protein as a nitrogen source, and their proteolytic system can produce peptides in milk (Chang et al., 2013; Janer et al., 2005). The proteolytic system of LAB as St. thermophilus, Lb. rhamnosus, L. lactis, Lb. bulgaricus, Lb. helveticus is composed of 3 steps which contain the cell envelope protease (CEP), a transporter of oligopeptides and small peptides, and the various intracellular peptidases (Genay et al., 2009; Gilbert et al., 1996; Miclo et al., 2012; Pastar et al., 2003; Sadat-Mekmene et al., 2011b; Siezen, 1999).
The proteolytic system of bifidobacteria is not well known. Some studies have shown that peptidases of the genus Bifidobacterium can hydrolyze milk protein directly, including those of Bifidobacterium animalis subsp. lactis (Janer et al., 2005), Bifidobacterium longum Bl 536 (Donkor et al., 2007), and B. longum KACC 91563 (Chang et al., 2013) generating the biological peptides. Hydrolysis of milk protein by microorganisms during fermentation can generate various peptides, including biologically active peptides, e.g., angiotensin I-converting enzyme (ACE) inhibitory peptides, antioxidant peptides, opiates, antimutagens, immunomodulatory peptides, antimicrobial peptides, or peptides with mineral binding activity. The bioactive peptides produced by the activity of proteases or peptidases of microorganisms are well documented in the literature (Korhonen, 2009). The most studied commercial fermented milk products are “Calpis” and “Evolus”, including two ACE-inhibitory tripeptides, VPP and IPP generated from β-casein by fermentation with Lb. helveticus including Saccharomyces cerevisiae for Calpis and Lb. helveticus for Evolus have been commercialized (Korhonen, 2009; Seppo et al., 2002; Takano, 2002).
In the case of bifidobacteria, the antioxidative peptides produced by B. longum were reported by Chang et al. (2013). Another study showed that the bioactive peptides of B. longum Bl 536 presented in vitro ACE-inhibitory activity, indicating that these are potential hypotensive peptides (Donkor et al., 2007); however, the peptides were not identified. Inhibition of ACE activity is regarded as an important component for the treatment of patients with hypertension because ACE leads to an increase in blood pressure by conversion of angiotensin I to angiogestin II or by bradykinin hydrolysis (Gobbetti et al., 2000; Hayes et al., 2007a; Miguel et al., 2009; Petrillo and Ondetti, 1982). Recently, some novel ACE-inhibitory peptides, including LVYPFP, were identified in Bifidobacterium bifidum (Gonzalez-Gonzalez et al., 2013). However, no hypotensive peptides have been identified in B. longum to date. Thus, we sought to investigate the antihypertensive activity and identify the peptides released from casein (CN) during fermentation of milk by B. longum KACC 91563.
Material and Methods
Materials
Chemical reagents, including hippuryl-histydil-leucine(HHL), captopril, ACE (2 mU; EC 3.4.15.1, 5.1 U mg−1), and lung acetone powder from rabbit were purchased from Sigma Aldrich (USA), and all other chemicals used were of analytical grade.
Preparation and growth of Bifidobacterium longum KACC91563
B. longum KACC91563 was isolated from infant feces in Korea. Identification of this strain was performed according to previously described methods (Chang et al., 2013; Ham et al., 2011). After isolation, B. longum was grown according to the conditions described by Ruiz et al. (2009). B. longum KACC91563 was grown in de Man, Rogosa, and Sharpe (MRS) broth (BD Biosciences, USA) containing cysteine (0.05% final concentration), and the cells (bacteria) were harvested by centrifugation (Beckman Coulter, USA) at 3,200 g for 30 min at 4℃. The cells were incubated and stored in reconstituted skim milk (10% w/v) at 80℃.
Milk fermentation
To obtain the fermentate fraction, fermentation was performed in skimmed milk for 24 h by inoculation of 1% B. longum after preculture at 37℃. The fermentate was retrieved by centrifugation at 3,200 g for 15 min at 4℃ when the fermentation was complete. The obtained fractions were separated using an ultrafiltration membrane system (Millipore, USA) with a molecular weight cut-off of 3 kDa.
Measurement of ACE inhibitory activity
ACE inhibitory activity was measured using a spectrophotometric assay as previously described (Cushman and Cheung, 1971) with slight modifications. First, crude ACE was prepared and extracted from 1 g of rabbit lung acetone powder (L0756, Sigma Aldrich) by gentle mixing of 40 mL of 0.1 M sodium borate buffer (pH 8.3) containing 0.3 M NaCl over 24 h. The supernatant containing ACE was obtained by centrifugation a 3,200 g for 40 min at 4℃, and was used for ACE inhibitory activity. A 50 μL sample was added to 100 μL of 0.1 M sodium borate buffer (pH 8.3) containing 0.3 M NaCl, and 50 μL of crude ACE was obtained. The reaction mixture was pre-incubated at 37℃ for 5 min. Fifty microliters of 12.5 mM HHL (H-1635, Sigma Aldrich) solubilized in 0.1 M sodium borate buffer (pH 8.3) containing 0.3 M NaCl was added to the reaction mixture and left to stand for 30 min at 37℃ after vortexing. To stop the enzyme reaction, 250 μL of 1 N HCl was added. Next, 1.5 mL of ethyl acetate was added to this reaction mixture and vortexed for 15 s. One milliliter of supernatant was retrieved by centrifugation at 3,000 g for 5 min and evaporated using Concentrator Plus (Eppendorf, Germany) at 60℃ for 30 min. The product was resupended in 1 mL of distilled water and its absorbance was measured at 228 nm using a spectrophotometer (Molecular Devices, USA). All assays were carried out in triplicate and the values represent the average and standard errors. Captopril (C-4042, Sigma Aldrich) was used as a positive control. The ACE inhibitory activity was calculated as follows: ACE inhibition (%) = [(C − B) − (S − B)] × 100/(C − B), where S is the absorbance of the ACE, ACE-inhibitory sample, and HHL; B is the absorbance of ACE and sodium borate buffer (pH 8.3) without HHL; and C is the absorbance of ACE, sodium borate buffer (pH 8.3), and HHL.
Identification of peptides by mass spectrometry
Liquid chromatography-electrospray ionization-quantitative time-of-flight tandem mass spectrometry experiments (LC-ESI-TOF-MS/MS) were performed at the National Instrumentation Center for Environmental Management (NICEM) of Seoul National University in Korea, according to the method described by Chang et al. (2013).
MS analysis experiments were carried out using an integrated system consisting of an auto-switching nano pump, autosampler (TempoTM nano LC system; MDS SCIEX, Canada), and a hybrid quadrupole-time-of-flight (TOF) mass spectrometer (QStar Elite; Applied Biosystems, USA) fitted with a fused silica emitter tip (New Objective, USA). For ionization, the nano-electrospray ionization (ESI) was applied. Two μL fractions were injected into the LC-nano ESI-MS/MS system.
Samples were first trapped on a ZORBAX 300SB-C18 trap column (300-μm i.d × 5 mm, 5-μm particle size, 100 pore size, Agilent Technologies, part number 5065-9913) and washed for 6 min with gradient with 98% solvent A and 2% solvent B at a flow rate of 5 μL/min. The solvent A and B consisted in [water/acetonitrile (98:2, v/v), 0.1% formic acid] and [Water/acetonitrile (2:98, v/v), 0.1% formic acid]. Separation was carried out on a ZORBAX 300SB-C18 capillary column (75-μm i.d × 150 mm, 3.5-μm particle size, 100 pore size, part number 5065-9911) at a flow rate of 300 nL/min with gradient at 2% to 35% solvent B over 30 min, then from 35% to 90% over 10 min, followed by 90% solvent B for 5 min, and finally 5% solvent B for 15 min. Electrospray through a coated silica tip (FS360-20-10-N20-C12, PicoTip emitter, New Objective) was performed at an ion spray voltage of 2,000 eV. Peptides were analyzed automatically using Analyst QS 2.0 software (Applied Biosystems, USA). The range of m/z values was 200-2000.
Results and Discussion
Determination of in vitro ACE inhibitory activity in low molecular weight fermentate
Two fractions were prepared to evaluate the ACE inhibitory activity. One was the fraction obtained after B. longum KACC9156 fermentation in skimmed milk for 24 h. Fermentates were fractionated at a molecular weight cutoff of 3 kDa using a centrifugal ultrafiltration membrane system. This cutoff value was chosen because Gonzalez-Gonzalez et al. (2013) demonstrated that the <3 kDa fraction of B. bifidum MF 20/5-fermented milk showed higher ACE inhibitory activity than that of the >3-kDa fraction. Similarly, Miguel et al. (2009) showed that the 50% inhibitory concentration of ACE was higher (5.5 μg/mL) in the <3 kDa fraction than in the > 3 kDa fractions of the bovine CN hydrolysate and whole hydrolysate without molecular weight fractionation. Confirming these results, in the present study, the ACE inhibitory activity determined from the fermentate obtained from milk fermentation with B. longum was 62.3% (Table 1), which was higher than that of the CN hydrolysate (28.3% ACE inhibitory activity) obtained from a 0.1% CN solution in 0.05 M sodium phosphate buffer (pH 7.0) at the same incubation time (data not shown). This ACE inhibitory activity was similar to that reported previously (63.7%) in milk fermented with B. longum B1 536 (Donkor et al. 2007). During fermentation, the casein could be degraded by the protolytic system of B. longum which was detected on peptidase activity of their cell surface (Chang et al., 2013). However, the cell envelop protease of B. longum which is the first step of casein hydrolysis was not found. From this reason, the ACE inhibitory activity in this work could be resulted from the potential cell wall peptidase.
Table 1. ACE-inhibitory activities in the low-molecular-weight fraction (<3 kDa) of the fermentate obtained after incubation of milk by Bifidobacterium longum KACC 91563.
| Control | Fermentate | |
|---|---|---|
| A228 | 0.715±0.027 | 0.375±0.011 |
| Activity (%) | 0 | 62.3 |
All assays were carried out in triplicate.
Using LC-ESI-MS/MS, the further study was proceeded to identify the peptides and to search the antihypertensive peptides generated by fermentation with B. longum KACC 9156 in milk.
Peptides generated from milk casein during fermentation by Bifidobacterium longum KACC9156
The peptides generated from fermentates were identified by LC-ESI-TOF-MS/MS. As shown in Table 2, the peptides in the fermentate were identified to have been generated from CN. The results also indicated that CN was the preferential substrate of B. longum in spite of the presence of whey protein in skim milk, similar to a strain of St. thermophilus (Chang et al., 2014). A total of 28 peptides were generated, corresponding to 11 β-CN, 16 αs1-CN, and 1 κ-CN peptide. This observation was slightly different from that reported by Chang et al. (2013), who identified 33 peptides (19 β-CN, 12 αs1-CN, and 2 κ-CN) in bovine CN hydrolysates obtained from a 0.1% CN solution with the same strain of B. longum. This difference could be explained by a difference in the accessibility of peptides for hydrolysis by B. longum or in a structural difference of milk protein in the different matrices used, i.e., milk versus buffer.
Table 2. Casein-derived peptides identified by liquid chromatography electrospray ionization time-of-flight tandem mass spectrometry in the <3 kDa fraction of the 24 h fermentates obtained from fermentation of milk by Bifidobacterium longum KACC91563.
| Names | Peptide sequence | Prec MW | Prec m/z | Theor MW | Theor m/z | z |
|---|---|---|---|---|---|---|
| β-CN | f1-25, RELEELNVPGEIVE | 1623.8091 | 812.9118 | 1623.8468 | 812.9307 | 2 |
| f109-125, MPFPKYPVEPFTESQSL | 1995.9216 | 998.9681 | 1995.9652 | 998.9899 | 2 | |
| f193-206, YQEPVLGPVRGPFP | 1554.7750 | 778.3948 | 1554.8195 | 778.4170 | 2 | |
| f193-209, YQEPVLGPVRGPFPIIV | 1880.0123 | 941.0134 | 1880.0560 | 941.0353 | 2 | |
| f194-209, QEPVLGPVRGPFPIIV | 1699.9307 | 850.9726 | 1699.9662 | 850.9904 | 2 | |
| f195-206, EPVLGPVRGPFP | 1263.6628 | 632.8387 | 1263.6975 | 632.8561 | 2 | |
| f195-209, EPVLGPVRGPFPIIV | 1588.9012 | 795.4579 | 1588.9341 | 795.4743 | 2 | |
| f196-209, PVLGPVRGPFPIIV | 1459.8593 | 730.9369 | 1459.8915 | 730.9530 | 2 | |
| f197-209, VLGPVRGPFPIIV | 1362.8054 | 682.4100 | 1362.8387 | 682.4266 | 2 | |
| f198-209, LGPVRGPFPIIV | 1263.7390 | 632.8768 | 1263.7704 | 632.8925 | 2 | |
| f199-209, GPVRGPFPIIV | 1150.6611 | 576.3378 | 1150.6863 | 576.3504 | 2 | |
| αS1-CN | f11-21, LPQEVLNENLL | 1302.6389 | 652.3267 | 1302.6796 | 652.3470 | 2 |
| f11-23, LPQEVLNENLLRF | 1583.8263 | 792.9204 | 1583.8672 | 792.9409 | 2 | |
| f26-34, APFPEVFGK | 990.4937 | 496.2541 | 990.5175 | 496.2660 | 2 | |
| f176-190, APSFSDIPNPIGSEN | 1565.6588 | 783.8367 | 1565.6974 | 783.8560 | 2 | |
| f176-197, APSFSDIPNPIGSENSEKTTMP | 2318.0234 | 1160.0190 | 2318.0737 | 1160.0441 | 2 | |
| f176-199, APSFSDIPNPIGSENSEKTTMPLW | 2633.1953 | 878.7391 | 2633.2319 | 878.7513 | 3 | |
| f177-197, PSFSDIPNPIGSENSGKTTMP | 2202.9614 | 1102.4880 | 2203.0103 | 1102.5125 | 2 | |
| f178-199, SFSDIPNPIGSENSEKTTMPLW | 2449.0955 | 1225.5550 | 2449.1472 | 1225.5808 | 2 | |
| f179-199, FSDIPNPIGSENSEKTTMPLW | 2362.0474 | 1182.0310 | 2362.1152 | 1182.0648 | 2 | |
| f180-199, SDIPNPIGSENSEKTTMPLW | 2214.9893 | 1108.5020 | 2215.0466 | 1108.5306 | 2 | |
| f181-199, DIPNPIGSENSEKTTMPLW | 2127.9514 | 1064.9830 | 2128.0146 | 1065.0146 | 2 | |
| f182-198, IPNPIGSENSEKTTMPL | 1808.8452 | 905.4299 | 1808.8978 | 905.4562 | 2 | |
| f182-199, IPNPIGSENSEKTTMPLW | 2028.9335 | 1015.4740 | 2028.9827 | 1015.4986 | 2 | |
| f185-199, PIGSENSEKTTMPLW | 1704.7677 | 853.3911 | 1704.8029 | 853.4087 | 2 | |
| f186-199, IGSENSEKTTMPLW | 1613.6985 | 807.8565 | 1613.7372 | 807.8759 | 2 | |
| f188-199, SENSEKTTMPLW | 1421.6108 | 711.8127 | 1421.6497 | 711.8321 | 2 | |
| κ-CN | f151-169, EVIESPPEINTVQVTSTAV | 2033.9755 | 1017.9950 | 2034.0133 | 1018.0139 | 2 |
CN: casein, m/z = mass to charge ratio, where z = number of positively charged ions.
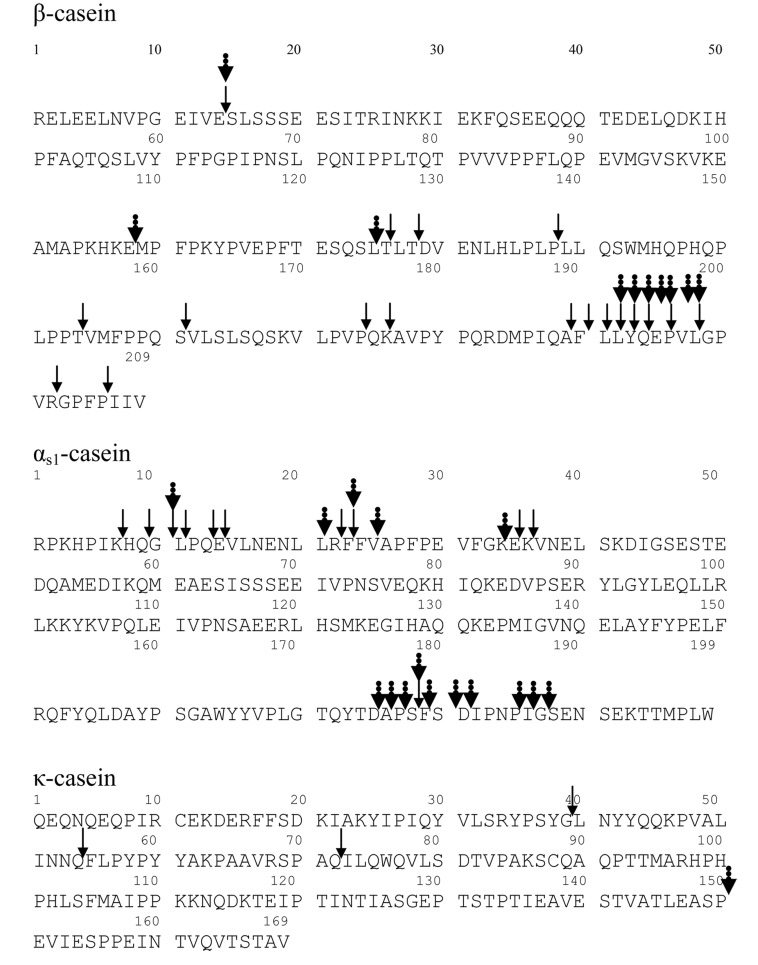
Nonetheless, the pattern of CN hydrolysis determined in the present study was similar to that reported by Chang et al. (2013). In the case of β-CN peptides, the C terminus was more hydrolyzed than the N-terminal (Fig. 1). A large number of peptides were also found generated in this region from Lactobacillus helveticus (Sadat-Mekmene et al., 2011a), Lactobacillus delbrueckii subsp. lactis CRL 581 (Hebert et al., 2008), Lactobacillus bulgaricus (Tsakalidou et al., 1999), Lactobacillus lactis subsp. cremoris (Reid et al., 1991), and S. thermophilus (Miclo et al., 2012). On the other hand, the N terminus of β-CN was resistant to hydrolysis by B. longum, as previously reported by Chang et al. (2013). The C terminus of β-CN, which was determined to be a hydrophobic region in this study, is more accessible for hydrolysis by B. longum (Chang et al., 2013) and by the proteases of S. thermophilus (Chang et al., 2014; Miclo et al., 2012) and L. helveticus (Sadat-Mekmene et al., 2011a).
Fig. 1. Cleavage sites of peptide bonds on bovine β-, αs1-, and κ-casein hydrolyzed after fermentation with Bifidobacterium longum KACC9156. The dot arrows cleaved peptide bonds in this study. The line arrow cleaved peptide bonds reported by Chang et al. (2013).
For αs1-CN, the hydrolysis pattern was also consistent with the results of Chang et al. (2013), who reported that B. longum hydrolyzed the N-terminal region to a greater extent than the C-terminal region. In spite of the similar hydrolysis patterns, the observed cleavage pattern (Fig. 1) was slightly different to the result obtained by Chang et al. (2013) for B. longum, in which we observed relatively more cleavage sites on αs1-CN. The reason for the different cleavage patterns might be that the structure of αs1-CN was changed during fermentation in milk to make it more accessible for hydrolysis by B. longum. A similar result was reported by Chang et al. (2014) and Sadat-Mekmene et al. (2011b), who also found that the proteases PrtS and PrtH of S. thermophilus and L. helveticus, respectively, were more accessible at the N terminus than the C terminus.
In this study, regions found to be resistant to hydrolysis were also identified in the β-CN and αs1-CN sequences. This may be due to the presence of phosphoserine residues in these regions, which leads to high resistance to hydrolysis (Chang et al., 2014; Kaspari et al., 1996). In present work, five and eight phosphoserine residues were identified in the regions that were not hydrolyzed on β-CN and αs1-CN, respectively.
In the case of κ-CN, the cleavage site (Fig. 1) only showed the generation of one peptide, whereas Chang et al. (2013) found two peptides in this region. Furthermore, Zahraa (2010) reported that the glycomacropeptide (f106-169) region, composed of glycan chains, was difficult to hydrolyze because of the presence of hydrophilic amino acids and a negative charge, leading to increased electrostatic repulsion. However, in the present study, only one peptide, f150-151, of this casein was obtained. Fermentation with B. longum could induce a structural change due to weak bond of glycan chains on this glycomacropeptide region that would allow B. longum to access κ-CN for hydrolysis.
As the previously study by Chang et al. (2013) who reported that no peptide detected from αs2-CN from 0.1% CN solution in 0.05 M sodium phosphate buffer (pH 7.0), none of the peptides identified were found in <3 kDa fraction from fermentate. This observation could be explained by the results of Tauzin et al. (2002), who found a protected region due to the formation of a tetrameric complex from CN that was more resistant to hydrolysis. Miclo et al. (2012) also suggested that accessibility of this region depends on its protein structure change.
Milk proteins play role precursors to release many peptides relating biological activity (Korhonen, 2009), i.e., ACE inhibitory peptide as this work.
Thirty two peptides relating ACE inhibitory obtained from casein have been reported in literature (Table 3). Out of 28 peptides generated from bovine casein during fermentation in present study, only 3 peptides from β-CN identified through MS/MS analysis (ESI-Q-TOF), YQEP-VLGPVRGPFPIIV, QEPVLGPVRGPFPIIV, GPVRGPFPIIV corresponded to ACE inhibitory bioactive peptides (Table 3A). These peptides were consistent with the previously studies reviewed by Yamamoto et al. (1994), Gobbetti et al. (2002) and Gomez-Ruiz et al. (2002), respectively. However no ACE inhibitory peptide released from other casein reported in previously study was present. These 3 peptides identified in this study were known the antihypertensive peptide in the literature. Thus, ACE inhibitory activity shown in Table 1 might result from these peptides.
Table 3. Antihypertensive and potential antihypertensive peptides generated by fermentation by Bifidobacterium longum KACC 91563 after 24 h at 37℃.
| Sequence | Fragment | Source | Proteolytic agent | References |
|---|---|---|---|---|
| A. Bioactive peptides clearly identified in the literature | ||||
| LNVPGEIVE | β-CN(f6-14) | milk | Lb. bulgaricus | Gobbetti et al., 2000 |
| DKIHPF | β-CN(f47-52) | milk | L. lactis subsp. cremoris | Gobbetti et al., 2000 |
| LVYPFP | β-CN(f58-63) | milk | B. bifidum | Gonzalez-Gonzalez et al., 2013 |
| NIPPLTQTPV | β-CN(f73-82) | milk | Lb. bulgaricus | Gobbetti et al., 2000 |
| EMPFPK | β-CN(f108-113) | casein | milk starter+pepsin and trypsin | Pihlanto-Leppälä et al., 1998 |
| HLPLPLL | β-CN(f134-140) | casein | pepsin | Del Mar Contreras et al., 2009 |
| SQSKVLPVPQ | β-CN(f166-175) | sodium caseinate | Lb. animalis | Hayes et al., 2007a |
| SKVLPVPQ | β-CN(f168-175) | β-casein | protease of Lb.helveticus | Yamamoto et al., 1994 |
| KVLPVPQ | β-CN(f169-175) | β-casein | protease of Lb.helveticus | Maeno et al., 1996 |
| RDMPIQAF | β-CN(f183-190) | β-casein | protease of Lb.helveticus | Yamamoto et al., 1994 |
| LLYQEPVLGPVRGPFPIIV | β-CN(f191-209) | β-casein | protease of Lb.helveticus | Yamamoto et al., 1994 |
| YQEPVL | β-CN(f193-198) | casein | milk starter +pepsin and trypsin | Pihlanto-Leppälä et al., 1998 |
| YQEPVLGPVR | β-CN(f193-202) | milk | Lb casei ssp. rhamnosus | Rokka et al., 1997 |
| YQEPVLGPVRGPFPI | β-CN(f193-208) | casein | trypsin | Maruyama and Suzuki, 1982 |
| YQEPVLGPVRGPFPIIVa, | β-CN(f193-209) | β-casein | protease of Lb.helveticus | Yamamoto et al., 1994 |
| QEPVLGPVRGPFPIIVa, | β-CN(f194-209) | milk | L. lactis + chymosin/trypsin/chymotrypsin | Gobbetti et al., 2002 |
| GPVRGPFPIIVa, | β-CN(f199-209) | Manchego cheese | protease in Manchego | Gomez-Ruiz et al., 2002 |
| AVPYPQR | β-CN(f176-182) | milk | lactic acid bacterias | Hernandez-Ledesma et al., 2004 |
| YQEP | β-CN(f191-198) | Gouda cheese | Proteases from Cynara cardunculus | Silva et al., 2006 |
| GPFPIIV | β-CN(f203-209) | milk | protease of Lb.helveticus | Yamamoto et al., 1994; |
| Hayes et al., 2007b | ||||
| RPKHPIKHQ | αs1-CN(f1-9) | Gouda cheese | Proteases from Cynara cardunculus | Silva et al., 2006 |
| FF | αs1-CN(f23-24) | casein | trypsin | Maruyama and Suzuki, 1982 |
| FFVAP | αs1-CN(f23-27) | casein | trypsin | Maruyama and Suzuki, 1982 |
| FFVAPFPEVFGK | αs1-CN(f23-34) | sodium caseinate | Lb. animalis | Hayes et al., 2007a |
| YKVPQL | αs1-CN(f104-109) | αs1-casein | protease of Lb.helveticus | Maeno et al., 1996 |
| LAYFYP | αs1-CN(f142-147) | casein | milk starter +pepsin and trypsin | Pihlanto-Leppälä et al., 1998 |
| DAYPSGAW | αs1-CN(f157-164) | casein | milk starter +pepsin and trypsin | Pihlanto-Leppälä et al., 1998 |
| TTMPLW | αs1-CN(f194-199) | casein | trypsin | Maruyama and Suzuki, 1982; |
| Pihlanto-Leppälä et al., 1998 | ||||
| FALPQYLK | αs2-CN(f174-181) | αs2-casein | trypsin | Tauzin et al., 2002 |
| AMKPWIQPK | αs2-CN(f189-197) | αs2-casein | protease of Lb.helveticus | Maeno et al., 1996 |
| MKPWIQPK | αs2-CN(f190-197) | αs2-casein | protease of Lb.helveticus | Maeno et al., 1996 |
| TKVIP | αs2-CN(f198-202) | αs2-casein | protease of Lb.helveticus | Maeno et al., 1996 |
| B. Potential ACE inhibitory peptides | ||||
| Sequence | Fragment | Present studya | References | |
| LNVPGEIVE | β-CN(f6-14) | f1-14, RELEELNVPGEIVE | Gobbetti et al., 2000 | |
| YQEPVLGPVR | β-CN(f193-202) | f193-206, YQEPVLGPVRGPFP | Rokka et al., 1997 | |
| GPVRGPFPIIV | β-CN(f199-209) | f195-209, EPVLGPVRGPFPIIV | Gomez-Ruiz et al., 2002 | |
| β-CN(f199-209) | f196-209, PVLGPVRGPFPIIV | |||
| β-CN(f199-209) | f197-209, VLGPVRGPFPIIV | |||
| β-CN(f199-209) | f198-209, LGPVRGPFPIIV | |||
| TTMPLW | αs1-CN(f194-199) | f176-199, APSFSDIPNPIGSENSEKTTMPLW | Maruyama and Suzuki, 1982; | |
| Pihlanto-Leppälä et al., 1998 | ||||
| αs1-CN(f194-199) | f178-199, SFSDIPNPIGSENSEKTTMPLW | |||
| αs1-CN(f194-199) | f179-199, FSDIPNPIGSENSEKTTMPLW | |||
| αs1-CN(f194-199) | f180-199, SDIPNPIGSENSEKTTMPLW | |||
| αs1-CN(f194-199) | f181-199, DIPNPIGSENSEKTTMPLW | |||
| αs1-CN(f194-199) | f182-199, IPNPIGSENSEKTTMPLW | |||
| αs1-CN(f194-199) | f185-199, PIGSENSEKTTMPLW | |||
| αs1-CN(f194-199) | f186-199, IGSENSEKTTMPLW | |||
| αs1-CN(f194-199) | f188-199, SENSEKTTMPLW | |||
aPeptides obtained from the fermentates in milk with B. longum in the present study.
Actually, peptides which have the high ACE inhibitory activity contain several amino acids (Trp, Phe, Tyr, or Pro) at the extremity of C-terminal and Ala, Val, IIe and Ser called aliphatic amino acids at the N-terminal (Jao et al., 2012). The three peptides, YQEPVLGPVRGPFPIIV, QEPVLGPVRGPFPIIV, GPVRGPFPIIV identified in present study contain these amino acids except for 4 amino acids, Gln, Glu, Leu and Gly. Thus, from these results, the peptides identified were reasonable to show the antihypertensive activity.
Other peptides as potential antihypertensive peptides which were included the fragment having ACE inhibitory activity were also identified in this study. These peptides were listed in Table 3B displaying 6 for β-CN and 9 for αs1-CN. Peptide, RELEELNVPGEIVE (f1-14) released from β-CN identified in present study contains LNVPGEIVE reviewed by Gobbetti et al. (2000) as ACE inhibitory peptide. Another peptide, YQEPVLGPVRGPFP (f193-206) from β-CN, contains also the ACE inhibitory peptide, YQEPVLGPVR (Rokka et al., 1997). The others from β-CN, the fragment GPVRGPFPIIV reviewed by Gomez-Ruiz et al. (2002) as antihypertensive peptide was included in 4 peptides, EPVLGPVRGPFPIIV, PVLGPVRGPFPIIV, VLGPVRGPFPIIV, LGPVRGPFPIIV.
For peptides obtained from αs1-CN, the 9 peptide, APSFSDIPNPIGSENSEKTTMPLW (f176-199), SFSDIPNPIGSENSEKTTMPLW (f178-199), FSDIPNPIGSENSEKTTMPLW (f179-199), SDIPNPIGSENSEKTTMPLW (f180-199), DIPNPIGSENSEKTTMPLW (f181-199), IPNPIGSENSEKTTMPLW (f182-199), PIGSENS EKTTMPLW (f185-199), IGSENSEKTTMPLW (f186-199), SENSEKTTMPLW (f188-199) was released (Table 3B). These peptides contain the peptide TTMPLW (f154-199) which have reviewed that this peptide has a biological activity to inhibit ACE (Maruyama and Suzuki 1982; Pihlanto-Leppälä et al., 1998). The antihypertensive peptide, SDIPNPIGSENSEKTTMPLW (f180-199) occurring naturally in milk, was also reviewed by Islam et al. (2014).
From our results, these 15 peptides obtained from β-CN and αs1-CN during fermentation with B. longum may be presented the antihypertensive activity (Table 3B) during digestion in vivo. Chang et al. (2014) have reported that some peptides containing bioactive peptide fragment could be released during digestion. To verify whether these peptides show the ACE inhibitory activity as novel antihypertensive peptide after synthesis of these peptides, whether these peptides during digestion with gastro-intestinal enzyme i.e., pepsin, trypsin, chymotrypsin, pancreatin etc. will be short and generated as antihypertensive peptide reviewed in literature and also whether peptides generated during digestion will be novel antihypertensive peptide, the further study will need.
Conclusions
Antihypertensive activity was demonstrated in the low-molecular-weight fraction (<3 kDa) obtained from the fermentate after milk fermentation with B. longum. This fraction was used to identify the CN-derived ACE inhibitory peptides. Using mass spectrometry analysis, three peptides showing antihypertensive activity and 15 peptides with potential antihypertensive activity were identified from CN. Thus, our results suggest that, given its capacity to generate antihypertensive peptides, B. longum KACC 91563 could be used as a starter culture with other lactic acid bacteria in the dairy industry and/or these peptides could be used in functional food manufacturing as antihypertensive agents, owing to their beneficial effects for human health.
Acknowledgments
This study was sponsored by Postdoctoral Fellowship Program and Research Grant (PJ008585) from National Institute of Animal Science (NIAS), Rural Development Administration of Korea (RDA) of Republic of Korea.
References
- 1.Arunachalam K. D. Role of bifidobacteria in nutrition, medicine and technology. Nutr. Res. 1999;19:1559–1597. [Google Scholar]
- 2.Chang O. K., Roux E., Awussi A. A., Miclo L., Jardin J., Jameh N., Dary A., Humbert G., Perrin C. Use of a free form of the Streptococcus thermophilus cell envelope protease PrtS as a tool to produce bioactive peptides. Int. Dairy J. 2014;38:104–115. [Google Scholar]
- 3.Chang O. K., Seol K. H., Jeong S. G., Oh M. H., Park B. Y., Perrin C., Ham J. S. Casein hydrolysis by Bifidobacterium longum KACC91563 and antioxidant activities of peptides derived therefrom. J. Dairy Sci. 2013;96:5544–5555. doi: 10.3168/jds.2013-6687. [DOI] [PubMed] [Google Scholar]
- 4.Collins M. D., Gibson G. R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 5.Cushman D. W., Cheung H. S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 6.Davidson R. H., Duncan S. E., Hackney C. R., Eigel W. N., Boling J. W. Probiotic culture survival and implications in fermented frozen yogurt characteristics. J. Dairy Sci. 2000;83:666–673. doi: 10.3168/jds.S0022-0302(00)74927-7. [DOI] [PubMed] [Google Scholar]
- 7.Del Mar Contreras M., Carron R., Montero M., Recio I. Novel casein-derived peptide with anti-hypertensive activity. Int. Dairy J. 2009;19:566–573. [Google Scholar]
- 8.Donkor O. N., Henriksson A., Singh T. K., Vasiljevic T., Shah N. P. ACE-inhibitory activity of probiotic yoghurt. Int. Dairy J. 2007;17:1321–1331. [Google Scholar]
- 9.Genay M., Sadat L., Gagnaire V., Lortal S. prtH2, not prtH, is the ubiquitous cell wall proteinase gene in Lactobacillus helveticus. Appl. Environ. Microbiol. 2009;75:3238–3249. doi: 10.1128/AEM.02395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert C., Atlan D., Blanc B., Portalier R., Germond G. J., Lapierre L., Mollet B. A new cell surface proteinase: Sequencing and analysis of the prtB gene from Lactobacillus delbrueckii subsp. bulgaricus. J. Bacteriol. 1996;178:3059–3065. doi: 10.1128/jb.178.11.3059-3065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobbetti M., Ferranti P., Smacchi E., Goffredi F., Addeo F. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp.bulgaricus SS1 and Lactococcuslactis subsp. cremoris FT4. Appl. Environ. Microbiol. 2000;66:3898–3904. doi: 10.1128/aem.66.9.3898-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobbetti M., Stepaniak L., De Angelis M., Corsetti A., Di Cagno R. Latent bioactive peptides in milk proteins: proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002;42:223–239. doi: 10.1080/10408690290825538. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Ruiz J. A., Ramos M., Recio I. Angiotensin-converting enzyme-inhibitory peptides in Manchego cheeses manufactured with different starter cultures. Int. Dairy J. 2002;12:697–706. [Google Scholar]
- 14.Gonzalez-Gonzalez C., Gibson R., Jauregi P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. Int. J. Food Microbiol. 2013;167:131–137. doi: 10.1016/j.ijfoodmicro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Ham J. S., Lee T., Byun M. J., Lee K. T., Kim M. K., Han G. S., Jeong S. G., Oh M. H., Kim D. H., Kim H. Complete genome sequence of Bifidobacterium longum subsp. longum KACC 91563. J. Bacteriol. 2011;193:5044. doi: 10.1128/JB.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes M., Stanton C., Slattery H., O'Sullivan O., Hill C., Fitzgerald G. F., Ross R. P. Casein fermentate of Lactobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors. Appl. Environ. Microbiol. 2007a;73:4658–4667. doi: 10.1128/AEM.00096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes M., Ross R. P., Fitzgerald G. F., Stanton C. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part II: Bioactive peptide functions. Biotechnol. J. 2007b;2:435–449. doi: 10.1002/biot.200700045. [DOI] [PubMed] [Google Scholar]
- 18.Hebert E. M., Mamone G., Picariello G., Raya R. R., De Giori G. S., Ferranti P., Addeo F. Characterization of the pattern of as1- and b-casein breakdown and release of a bioactive peptide by a cell envelope proteinase from Lactobacillus delbrueckii subsp. lactis CRL 581. Appl. Environ. Microbiol. 2008;74:3682–3689. doi: 10.1128/AEM.00247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez-Ledesma B., Amigo L., Ramos M., Recio I. Application of high-performance liquid chromatography-tandem mass spectrometry to the identification of biologically active peptides produced by milk fermentation and simulated gastrointestinal digestion. J. Chromatogr. A. 2004;1049:107–114. [PubMed] [Google Scholar]
- 20.Islam M. A., Alam M. K., Islam M. N., Khan M. A. S., Ekeberg D., Rukke E. O., Vegarud G. E. Principal milk components in buffalo, holstein cross, indigenous cattle and red chittagong cattle from bangladesh. Asian-Aust. J. Anim. Sci. 2014;27:886–897. doi: 10.5713/ajas.2013.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janer C., Arigoni F., Lee B. H., Pelaez C., Requena T. Enzymatic ability of Bifidobacterium animalis ssp. lactis to hydrolyze milk proteins: Identification and characterization of endopeptidase O. Appl. Environ. Microbiol. 2005;71:8460–8465. doi: 10.1128/AEM.71.12.8460-8465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jao C. L., Huang S. L., Hsu K. C. Angiotensin I-converting enzyme inhibitory peptides: Inhibition mode, bioavailability, and antihypertensive effects. Biomedicine. 2012;2:130–136. [Google Scholar]
- 23.Kaspari A., Diefenthal T., Grosche G., Schierhorn A., Demuth H. U. Substrates containing phosphorylated residues adjacent to proline decrease the cleavage by proline-specific peptidases. Biochim. Biophys. Acta. 1996;1293:147–153. doi: 10.1016/0167-4838(95)00238-3. [DOI] [PubMed] [Google Scholar]
- 24.Korhonen H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods. 2009;1:177–187. [Google Scholar]
- 25.Leahy S. C., Higgins D. G., Fitzgerald G. F., van Sinderen D. Getting better with bifidobacteria. J. Appl. Microbiol. 2005;98:1303–1315. doi: 10.1111/j.1365-2672.2005.02600.x. [DOI] [PubMed] [Google Scholar]
- 26.Maeno M., Yamamoto N., Takano T. Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP 790. J. Dairy Sci. 1996;79:1316–1321. doi: 10.3168/jds.S0022-0302(96)76487-1. [DOI] [PubMed] [Google Scholar]
- 27.Martín-Diana A. B., Janer C., Peláez C., Requena T. Development of a fermented goat’s milk containing probiotic bacteria. Int. Dairy J. 2003;13:827–833. [Google Scholar]
- 28.Maruyama S., Suzuki H. A peptide inhibitor of angiotensin I-converting enzyme in the tryptic hydrolysate of casein. Agric. Biol. Chem. 1982;46:1393–1394. [Google Scholar]
- 29.McBrearty S., Ross R. P., Fitzgerald G. F., Collins J. K., Wallace J. M., Stanton C. Influence of two commercially available bifidobacteria cultures on cheddar cheese quality. Int. Dairy J. 2001;11:599–610. [Google Scholar]
- 30.Miclo L., Roux E., Genay M., Brusseaux E., Poirson C., Jameh N., Perrin C., Dary A. Variability of hydrolysis of β-, αs1-, and αs2-caseins by 10 strains of Streptococcus thermophilus and resulting bioactive peptides. J. Agric. Food Chem. 2012;60:554–565. doi: 10.1021/jf202176d. [DOI] [PubMed] [Google Scholar]
- 31.Miguel M., Contreras M. M., Recio I., Aleixandre A. ACE-inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009;112:211–214. [Google Scholar]
- 32.Pastar I., Tonic I., Golic N., Kojic M., van Kranenburg R., Kleerebezem M., Topisirovic L., Jovanovic G. Identification and genetic characterization of a novel proteinase, PrtR, from the human isolate Lactobacillus rhamnosus BGT10. Appl. Environ. Microbiol. 2003;69:5802–5811. doi: 10.1128/AEM.69.10.5802-5811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrillo E. W., Ondetti M. A. Angiotensin-converting enzyme inhibitors: Medicinal chemistry and biological actions. Med. Res. Rev. 1982;2:1–41. doi: 10.1002/med.2610020103. [DOI] [PubMed] [Google Scholar]
- 34.Pihlanto-Leppälä A., Rokka T., Korhonen H. Angiotensin I converting enzyme inhibitory peptides derived from bovine milk proteins. Int. Dairy J. 1998;8:325–331. [Google Scholar]
- 35.Reid J. R., Ng K. H., Moore C. H., Coolbearm T., Pritchard G. G. Comparison of bovine β-casein hydrolysis by PI and PIII type proteinases from Lactobacillus lactis subsp. cremoris. Appl. Microbiol. Biotechnol. 1991;36:344–351. doi: 10.1007/BF00208154. [DOI] [PubMed] [Google Scholar]
- 36.Rokka T., Syväoja E. L., Tuominen J., Korhonen H. Release of bioactive peptides by enzymatic proteolysis of Lactobacillus GG fermented UHT milk. Milchwissenschaft. 1997;52:675–678. [Google Scholar]
- 37.Ruiz L., Sánchez B., de los Reyes-Gavilán C. G., Gueimonde M., Margolles A. Coculture of Bifidobacterium longum and Bifidobacterium breve alters their protein expression profiles and enzymatic activities. Int. J. Food Microbiol. 2009;133:148–153. doi: 10.1016/j.ijfoodmicro.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Saavedra J. M., Abi-Hanna A., Moore N., Yolken R. H. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am. J. Clin. Nutr. 2004;79:261–267. doi: 10.1093/ajcn/79.2.261. [DOI] [PubMed] [Google Scholar]
- 39.Sadat-Mekmene L., Genay M., Atlan D., Lortal S., Gagnaire V. Original features of cell-envelope proteinases of Lactobacillus helveticus: A review. Int. J. Food Microbiol. 2011a;146:1–13. doi: 10.1016/j.ijfoodmicro.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 40.Sadat-Mekmene L., Jardin J., Corre C., Mollé D., Richoux R., Delage M. M., Lortal S., Gagnaire V. Simultaneous presence of PrtH and PrtH2 proteinases in strains improves breakdown of the pure αs1-casein. Appl. Environ. Microbiol. 2011b;77:179–186. doi: 10.1128/AEM.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seppo L., Kerojoki O., Suomalainen T., Korpela R. The effect of a Lactobacillus helveticus LBK-16 H fermented milk on hypertension - a pilot study on humans. Milchwissenschaft. 2002;57:124–127. [Google Scholar]
- 42.Silva S. V., Pihlanto A., Malcata F. X. Bioactive peptides in ovine and caprine cheeselike systems prepared with proteases from Cynara cardunculus. J. Dairy Sci. 2006;89:3336–3344. doi: 10.3168/jds.S0022-0302(06)72370-0. [DOI] [PubMed] [Google Scholar]
- 43.Siezen R. J. Multi-domain, cell-envelope proteinases of lactic acid bacteria. Antonie van Leeuwoenhoek. 1999;76:139–155. [PubMed] [Google Scholar]
- 44.Takano T. Anti-hypertensive activity of fermented dairy products containing biogenic peptides. Antonie van Leeuwenhoek. 2002;82:333–340. [PubMed] [Google Scholar]
- 45.Tauzin J., Miclo L., Gaillard J. L. Angiotensin-I-converting enzyme inhibitory peptides from tryptic hydrolysate of bovine αs2-casein. FEBS Lett. 2002;531:369–374. doi: 10.1016/s0014-5793(02)03576-7. [DOI] [PubMed] [Google Scholar]
- 46.Tsakalidou E., Anastasiou R., Vandenberghe I., Vanbeeumen J., Kalantzopoulos G. Cell-wall-bound proteinase of Lactobacillus delbrueckii subsp. lactis ACA-DC 178: characterization and specificity for β-casein. Appl. Environ. Microbiol. 1999;65:2035–2040. doi: 10.1128/aem.65.5.2035-2040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto N., Akino A., Takano T. Antihypertensive effect of peptides derived from casein by an extracellular proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1994;77:917–922. doi: 10.3168/jds.S0022-0302(94)77026-0. [DOI] [PubMed] [Google Scholar]
- 48.Zahraa N. Le peptide κ-CN(f106-109) du lait: propriétés nutritionnelles, biologiques et techno-fonctionnelles. Mémoire de M2, UHP Nancy 1; 2010. [Google Scholar]