Abstract
This study was performed to explore the deterioration of physicochemical quality of beef hind limb during frozen storage at −20℃, affected by repeated freeze-thaw cycles. The effects of three successive freeze-thaw cycles on beef hind limb were investigated comparing with unfrozen beef muscle for 80 d by keeping at −20±1℃. The freeze-thaw cycles were subjected to three thawing methods and carried out to select the best one on the basis of deterioration of physicochemical properties of beef. As the number of repeated freeze-thaw cycles increased, drip loss decreased and water holding capacity (WHC) increased (p<0.05) till two cycles and then decreased. Cooking loss increased in cycle one and three but decreased in cycle two. Moreover, drip loss, WHC and cooking loss affected (p<0.05) by thawing methods within the cycles. However, pH value decreased (p<0.05), but peroxide value (p<0.05), free fatty acids value (p<0.05) and TBARS value increased (p<0.05) significantly as the number of repeated freeze-thaw cycles increased. Moreover, significant (p<0.05) interactive effects were found among the thawing methods and repeated cycles. As a result, freeze-thaw cycles affected the physicochemical quality of beef muscle, causing the degradation of its quality.
Keywords: physicochemical, lipid oxidation, freeze-thaw cycle, beef muscle
Introduction
Beef is widely available source of animal protein in Bangladesh; due to the livestock population in Bangladesh is well above the average from many other countries of the world (FAO, 2013). The nutritional elements of beef provides a major proportion of consumer requirements for protein (Breidenstein, 1987), some vitamins (Johnson, 1987) and minerals (Robinson, 2001). Thus, beef is becoming an important part of a balanced and varied diet (Whitney and Rolfes, 2008). Beef and beef products to be preserved under refrigerated or frozen conditions to stop physicochemical deterioration, enzymatic activity and further delay of lipid oxidation and extending the shelf life by inhibiting microbial spoilage. But during preservation of beef muscle, physicochemical deterioration can’t be stopped completely. However, beef muscle composed of much lipid than other meat, which made it more tasteful and acceptable to the consumer. These lipid undergo oxidation processes to a greater extent during repeated freeze-thaw cycles (Jun et al., 2012). Beef is biologically complex and ideal system (Lee et al., 2003) used in evaluating physicochemical variations and lipid oxidation during freezing. Beef muscle with greater lipid oxidation accelerated greater protein oxidation and metmyoglobin formation which lead variation in physicochemical quality of beef muscle (Insani et al., 2008). Chemical deterioration related to pH and lipid oxidations have greater effect in declining palatability and texture of beef and beef products. Fresh beef and beef products preserved under refrigeration or freezing state can still develop physicochemical deterioration slowly. The degree of lipid oxidations affected by various thawing methods during frozen state is still a potential problem and effective methods need to be explored (Park et al., 2007). Freezing commercially at −18℃ and domestically at −10℃ is now a standard of eating quality compared to fresh meat. Freezing temperature at −18 to −20℃ is an effective form both preservation of meat and further manufacturing of meat (Farouk et al., 2004; Soyer et al., 2010). From other reports it is found that the optimal temperature for freezing meat is to be −40℃, as only a minimum amount of water is unfrozen at this point (Estévez, 2011; Leygonie et al., 2012a). Thawing of meat at commercial and domestic level in refrigeration temperature (4℃) (Eastridge and Bowker, 2011), temperatures ranging from room temperature to 49℃ or microwave (Chemat et al., 2011) and running tap water (Rahman et al., 2014) is being practiced for long since. The most commonly used determinants to measure the degree of lipid oxidation of beef are free fatty acids (FFA), peroxide value (POV), and thiobarbituric acid reactive substance (TBARS) values. Repeated freeze-thaw cycles affected the physicochemical quality and increased lipid oxidation (Jun et al., 2012) and protein degradation of beef muscle, causing the degradation of beef quality (Li et al., 2012). The most studies were performed under refrigeration rather than frozen state in different thawing methods. However, frozen storage might not fully inhibit lipid oxidation and thawing methods can accelerate physicochemical deterioration and lipid oxidation of beef to a great extent. The current study has undertaken to know how frozen storage and thawing methods affect in physicochemical quality as well as degree of lipid oxidation and oxidative volatiles in fresh beef muscle. To our knowledge, this matter has not been extensively studied. From this point of view the current study has undertaken.
Materials and Methods
Experimental samples
The samples were obtained from hindlimb of a bull which is around 2.5 year old with weighing live weight of 300±5 kg. After removal of fat, tendons, ligaments and bone from beef muscles, they were randomly divided into twenty seven samples for Completely Randomized Design (CRD) model. The physicochemical properties like drip loss, cooking loss, WHC, pH and lipid oxidation as peroxide value, free fatty acids value, TBARS value were analyzed. The fresh beef muscle which has not been frozen and thawed was used as control.
Freezing and thawing
The samples were frozen at −20℃ with a wind speed of 2.6 m/s in a blast freezer. The freezing temperature was checked regularly. During the total storage period of 80 d, the samples were stored for 42±2 d for first cycle, first cycle 21±2 d for second cycle, and second cycle 14± 2 d for third cycle at −20±1℃ before thawing. The three methods of thawing were achieved using two thawing mediums and various temperatures. Thawing methods are 4±1℃ in a refrigerator, under tap water (26±1℃) and warm water (40±1℃) and thaw for minimum time.
Physicochemical properties of beef
Preparation of sample
The raw beef samples (100±5 g) from three thawing methods were prepared for determining drip loss, cooking loss, WHC, pH, free fatty acids (FFA) value, peroxide value and thiobarbituric acid reacting substances (TBARS) value. These were accomplished at 42 d and repeated in 21 and 14 d intervals up to the end of frozen storage at −20±1℃.
Drip loss
Beef muscle samples are cut from the carcass and immediately weighed (approximately 80-100 g). Drip loss was determined by suspending individually weighed steaks in inflated polyethylene bags (taking care that samples did not touch the sides of the bags) for 24 h at 4℃. After 24 h, samples were removed, gently blotted dry and weighed; drip loss was calculated as the percentage of weight lost.
Drip loss (%): [(sample weight (g) − 24 hrs after sample weight (g)) / sample weight (g)] × 100
Cooking loss
To determine cooking loss, weighed 5±1 g samples and wrapped in a heat-stable foil paper and kept in water bath at 80℃ for 30 min. The internal temperature was not measured, but from a previous study (Sultana et al., 2008) it was estimated that the optimum internal meat temperature (75-80℃) would be gained by 30 min. Samples are dried and weighed out. Cook loss was calculated after draining the drip coming from the cooked meat as follows:
Cook loss (%) = [(w2 − w3) / w2] × 100
where, w2 = meat weight before cooking (g) and w3 = meat weight after cooking (g).
Water holding capacity (WHC)
Measurement of WHC of muscle was determined by the filter paper press method (Grau and Hamm 1953). Each piece of meat (1 × 1 × 1.5 cm3) was covered with eight sheets of filter paper and pressed with a 12 kg load for two minutes. The water holding capacity was calculated as follows:
WHC (%) = [1 − {(meat weight before pressing − meat weight after pressing) / (meat weight before pressing × moisture content in gram)}] × 100
pH value
The pH value of beef samples were measured with a pH meter (Metter Toledo Delta 320, Switzerland). For this, 5 g samples were dissolved in 45 mL aquadest in a glass beaker with a homogenizer. Then pH value was determined from the reading of pH meter.
Peroxide value
The peroxide value (POV) was determined according to the method of Sallam et al. (2004). The samples (3 g) were weighed in a 250-mL glass stopper Erlenmeyer flask. Then it was heated for 3 min at 60℃ in a water bath to melt the fat. After that the flask thoroughly agitated for 3 min with 30 mL acetic acid-chloroform solution (3:2 v/v) to dissolve the fat. Whatman filter paper number 1 was used in filtration process to remove beef particles from the filtrate. After adding saturated potassium iodide solution (0.5 mL) to filtrate and continued with addition of starch solution as indicator. The titration was continued against standard solution of sodium thiosulfate. POV was calculated by following equation and expressed as milli equivalent peroxide per kilogram of sample:
POV (meq / kg) = {(S × N) / W} × 100
Where “S” is the volume of titration (ml), “N” is the normality of sodium thiosulfate solution (N=0.01) and “W” is the sample weight (g).
Free fatty acid value
Free fatty acid (FFA) value was determined according to the method of Rukunudin et al. (1998). The sample (5 g) was dissolved with 30 mL chloroform using a homogenizer at 10,000 rpm for 1 min. Whatman filter paper number 1 was used in filtration process to remove beef particles from the filtrate. After addition of five drops 1% ethanolic phenolphthalein as indicator to filtrate, the titration was continued with 0.01 N ethanolic potassium hydroxide solutions and FFA value was calculated as follows:
FFA (%) = (mL titration × Normality of KOH × 28.2) / g of sample
Thiobarbituric acid reactive substance (TBARS)
The 2-thiobarbituric acid (TBA) values were determined by the method described by Schmedes and Holmer (1989). The samples (5 g) were blended with 25 mL of 20% trichloroacetic acid solution (200 g/L of trichloroacetic acid in 135 mL/L phosphoric acid solution) in a homogenizer for 30 s. The homogenized samples were filtered through Whatman filter paper number 4 to remove beef particles from the filtrate. Then 2 mL of 0.02 M aqueous TBA solution (3 g/L) was added to 2 mL of filtrate in a test tube. After that, test tubes were incubated at 100℃ for 30 min and cooled with running tap water. The absorbance of supernatant solutions was measured at 532 nm using a UV-VIS spectrophotometer (UV-1200, Shimadzu, Japan). The TBA values were calculated from a standard curve and expressed as mg malonaldehyde per kilogram (MA/kg) of beef sample.
Statistical analysis
The three treatments (T1= 4℃, T2= 40℃ and T3= tap water) were resulted from three repeated freeze-thaw cycles. Different tests were repeated thrice for each cycle. Data were statistically analyzed using Completely Randomized Design (CRD) model procedure by JMP, SAS Statistical Discovery software, NC, USA. The 3×3 factorial design was used for cycle-treatment interaction analysis. Tukey HSD test was used to determine the significance of differences among treatments means.
Results
Drip loss
The initial drip loss of fresh sample was 12.5%. Drip loss was decreased as the number of freeze-thaw cycles increased. Thawing at 4℃ had the highest and thawing in tap water had the lowest drip loss observed in every cycle. Table 1, 2, 3 and 4 shows that drip loss was affected (p<0.01) by thawing methods within cycles and among the cycles. Table 5 also shows that there were significant interactive effects (p<0.01) on drip loss in different cycles and thawing methods. Thawing at 4℃ in cycle one had the highest and thawing in tap water in cycle three had the lowest drip loss among interactions (Table 5).
Table 1. Changes of physicochemical properties (mean±SE) in thawed beef samples compared to control in freeze-thaw cycle-1.
| Thawing methods | Drip loss (%) | Cooking loss (%) | WHC (%) |
|---|---|---|---|
| Control | 12.5b±0.11 | 47.30b±0.38 | 69.78d±0.06 |
| 4℃ | 13.55a±0.14 | 55.54a±1.01 | 75.74b±0.47 |
| 40℃ | 6.54c±0.14 | 40.75d±1.01 | 77.67a±0.47 |
| Tap water | 5.55d±0.14 | 45.49c±1.01 | 72.91c±0.47 |
| Level of significance | ** | ** | ** |
Columns having different superscripts differed significantly (**p<0.01).
Table 2. Changes of physicochemical properties (mean±SE) in thawed beef samples compared to control in freeze-thaw cycle-2.
| Thawing methods | Drip loss (%) | Cooking loss (%) | WHC (%) |
|---|---|---|---|
| Control | 12.5a±0.11 | 47.30b±0.38 | 69.78d±0.06 |
| 4℃ | 10.45b±0.23 | 48.60a±0.42 | 73.71b±0.27 |
| 40℃ | 5.77c±0.23 | 42.29d±0.42 | 76.85a±0.27 |
| Tap water | 4.95d±0.23 | 46.33c±0.42 | 72.65c±0.27 |
| Level of significance | ** | ** | ** |
Columns having different superscripts differed significantly (**p<0.01).
Table 3. Changes of physicochemical properties (mean±SE) in thawed beef samples compared to control in freeze-thaw cycle-3.
| Thawing methods | Drip loss (%) | Cooking loss (%) | WHC (%) |
|---|---|---|---|
| Control | 12.5a±0.11 | 47.30b±0.38 | 69.78a±0.06 |
| 4℃ | 9.55b±0.13 | 46.23c±0.42 | 53.55d±0.25 |
| 40℃ | 5.18c±0.13 | 45.24d±0.42 | 55.45b±0.25 |
| Tap water | 4.74d±0.13 | 50.14a±0.42 | 54.12c±0.25 |
| Level of significance | ** | ** | * |
Columns having different superscripts differed significantly (**p<0.01 and *p<0.05).
Table 4. Changes of physicochemical properties (mean±SE) in thawed beef samples compared to control in repeated cycles.
| Thawing methods | Drip loss (%) | Cooking loss (%) | WHC (%) |
|---|---|---|---|
| Control | 12.5a±0.106 | 47.30b±0.38 | 69.78c±0.06 |
| Cycle 1 | 8.15b±0.101 | 46.38c±0.39 | 76.46a±0.20 |
| Cycle 2 | 6.42c±0.101 | 45.65d±0.39 | 74.39b±0.20 |
| Cycle 3 | 5.39d±0.101 | 48.12a±0.39 | 54.86d±0.20 |
| Level of significance | ** | ** | ** |
Columns having different superscripts differed significantly (**p<0.01).
Table 5. Freezing-thawing interactive effects on physicochemical properties (mean±SE) of thawed beef muscle in different cycles and thawing methods.
| Interactions | Drip loss (%) | Cooking loss (%) | WHC (%) | pH |
|---|---|---|---|---|
| C1 × T1 | 13.95a±0.17 | 57.00a±0.68 | 77.19b±0.35 | 6.037a±0.044 |
| C1 × T2 | 7.13d±0.17 | 41.40f±0.68 | 78.45a±0.35 | 5.920b±0.044 |
| C1 × T3 | 6.16e±0.17 | 45.37e±0.68 | 72.75c±0.35 | 5.870bc±0.044 |
| C2 × T1 | 11.33b±0.17 | 49.61c±0.68 | 73.13c±0.35 | 5.723c±0.044 |
| C2 × T2 | 5.56f±0.17 | 41.27f±0.68 | 78.28ab±0.35 | 5.477cd±0.044 |
| C2 × T3 | 4.91fg±0.17 | 47.95cd±0.68 | 73.09c±0.35 | 5.387cd±0.044 |
| C3 × T1 | 9.95c±0.17 | 47.46cd±0.68 | 53.84e±0.35 | 5.480d±0.044 |
| C3 × T2 | 4.41g±0.17 | 45.24e±0.68 | 55.14d±0.35 | 5.397cd±0.044 |
| C3 × T3 | 4.40g±0.17 | 50.53b±0.68 | 54.79de±0.35 | 5.240e±0.044 |
| Level of Significance | ** | ** | ** | * |
Columns having different superscripts differed significantly (**p<0.01, *p>0.05).
Note: C=Cycle (C1=Cycle 1; C2=Cycle 2; C3=Cycle 3) and T=Treatment/Thawing methods (T1=4℃; T2=40℃; T3=Tap water).
Cooking loss
The initial cooking loss of fresh sample was 47.30%. As the number of freeze-thaw cycles increased cooking loss was also increased slightly. Thawing at 4℃ had the highest and thawing at 40℃ had the lowest cooking loss in every cycle except cycle three. Cooking loss was affected (p<0.01) by thawing methods within cycles and among the cycles (Table 1, 2, 3, and 4). Table 5 shows that there were significant interactive effects (p<0.01) on cooking loss in different cycles and thawing methods. Thawing at 4℃ in cycle one had the highest and thawing at 40℃ in cycle two had the lowest cooking loss among the interactions (Table 5).
Water holding capacity
A significant difference was noted for WHC among beef samples subjected to repeated freeze-thaw cycles. The initial WHC of fresh sample was 69.78%. Table 1, 2, 3 and 4 showing that WHC was affected (p<0.01) by thawing methods within cycles and among the cycles. Moreover, WHC increased in cycle one and two but decreased greatly (p<0.05) in cycle three. Table 5 also showing that there were significant interactive effects found (p<0.01) on WHC in different cycles and thawing methods. Thawing at 40℃ in cycle one had the highest value of WHC and thawing at 4℃ in cycle three had the lowest value of WHC among interactions (Table 5).
pH
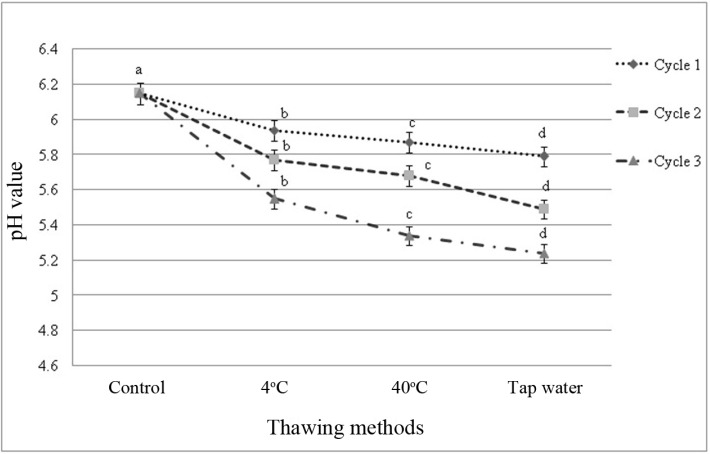
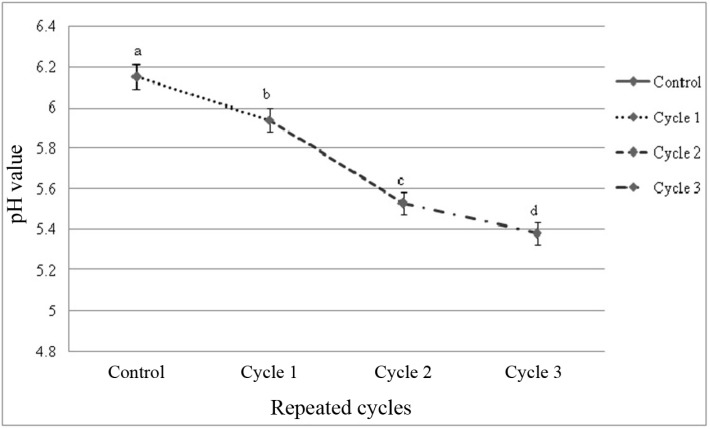
The initial pH of fresh beef sample was 6.15 indicating the normal pH of beef within 2 h of slaughter. The pH was almost similar to fresh beef (control) which was not frozen and thawed but slightly varied by thawing methods within and among the cycles (Fig. 1 and 2). Different thawing methods affected (p<0.05) pH value in each cycle. The final pH observed in this study was 5.24; thawing in tap water in cycle 1 (Fig. 1). Among the cycles significant (p<0.01) change on pH value was observed (Fig. 2) and interactive effects (p<0.05) were found on pH value in different cycles-thawing methods. In this study, thawing at 4℃ in cycle 1 had the highest value and thawing in tap water in cycle 3 had the lowest value of pH among the interactions (Table 5).
Fig. 1. Changes of pH value (mean±SE) in thawed beef samples compared to control in freeze-thaw cycle-1, 2 and 3.
Fig. 2. Changes of pH value (mean±SE) in thawed beef samples compared to control in repeated cycles.
Lipid oxidation
Fresh beef undergoes major undesirable changes during preservation both at refrigeration and freezing temperatures. In this study acid value, peroxide and TBA value were chosen as representative of lipid hydrolysis, primary and secondary lipid oxidation respectively.
Peroxide value
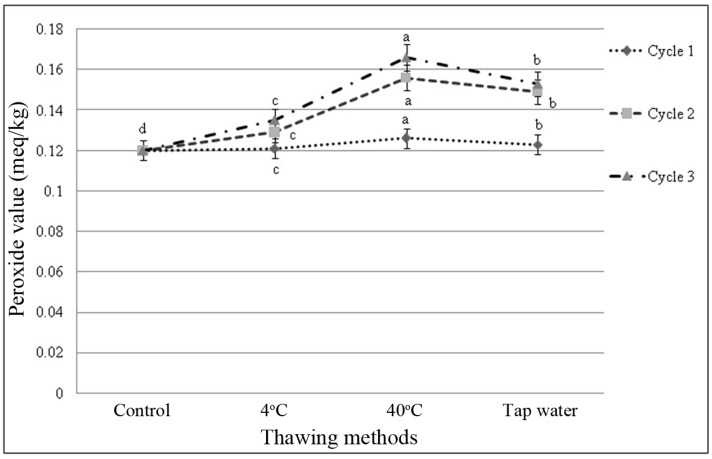
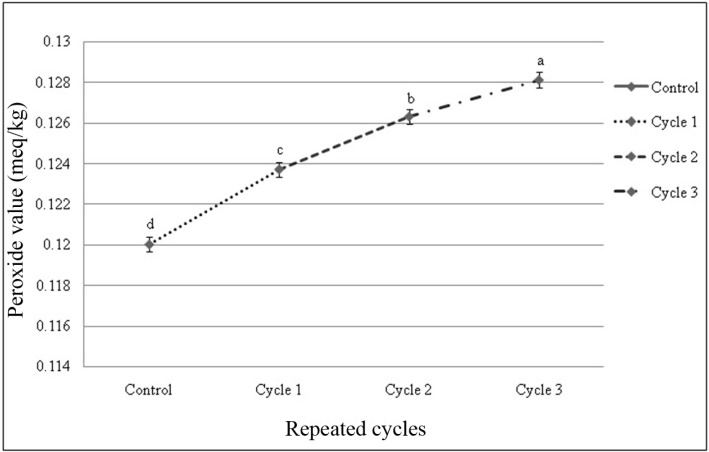
Peroxide value of fresh beef sample was 0.12 meq/kg at initially and ranging within 0.12 to 0.166 meq/kg. There was significant (p<0.05) difference of peroxide values of the beef samples, subjected to different thawing methods within cycles and among the cycles observed (Fig. 3 and 4). Peroxide value was increased slightly as the number of freeze-thaw cycles increased. No interactive effects (p>0.05) on peroxide value were observed in different cycles and thawing methods. Thawing at 40℃ in cycle 3 had the highest value and thawing at 4℃ in cycle 1 had the lowest peroxide value among the interactions (Table 6).
Fig. 3. Changes of peroxide value (mean±SE) in thawed beef samples compared to control in freeze-thaw cycle-1, 2 and 3.
Fig. 4. Changes of peroxide value (mean±SE) in thawed beef samples compared to control in repeated cycles.
Table 6. Freezing-thawing interactive effects on Lipid oxidation (mean ± SE) of frozen beef muscle in different cycle and thawing methods.
| Interactions | Peroxide value | FFA (%) | TBARS value (mg MA/kg) |
|---|---|---|---|
| C1 × T1 | 0.121±0.001 | 0.115e±0.008 | 0.291f±0.008 |
| C1 × T2 | 0.126 ±0.001 | 0.134c±0.008 | 0.351d±0.008 |
| C1 × T3 | 0.123±0.001 | 0.147ab±0.008 | 0.299e±0.008 |
| C2 × T1 | 0.125±0.001 | 0.125d±0.008 | 0.315de±0.008 |
| C2 × T2 | 0.129±0.001 | 0.145ab±0.008 | 0.503b±0.008 |
| C2 × T3 | 0.126±0.001 | 0.129cd±0.008 | 0.461c±0.008 |
| C3 × T1 | 0.126±0.001 | 0.132c±0.008 | 0.354d±0.008 |
| C3 × T2 | 0.130±0.001 | 0.149a±0.008 | 0.572a±0.008 |
| C3 × T3 | 0.128±0.001 | 0.140b±0.008 | 0.506b±0.008 |
| Level of Significance | NS | * | ** |
Same column having similar superscript did not differ significantly (**p<0.01, *p>0.05) and different superscript differ significantly (p<0.05). NS means not significance.
Note: C=Cycle (C1=Cycle 1; C2=Cycle 2; C3=Cycle 3) and T=Treatment/Thawing methods (T1=4℃; T2=40℃; T3=Tap water).
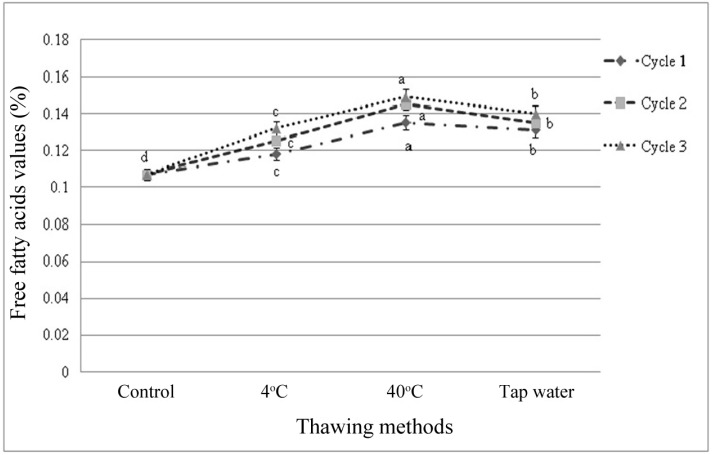
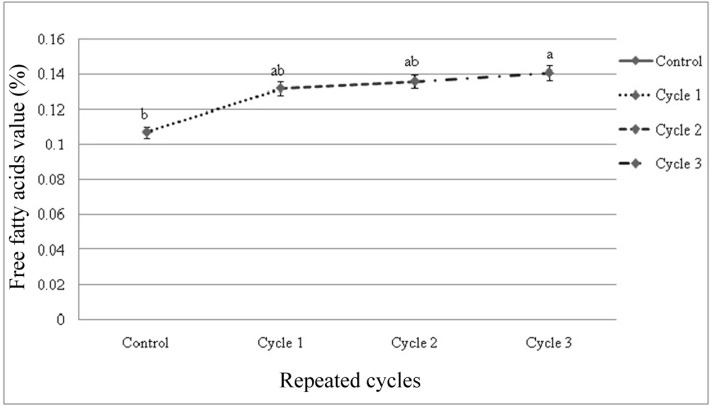
Free fatty acids (FFA) value
There were significant differences among the samples subjected to repeated freeze-thaw cycles in free fatty acids (FFA) value ranging between 0.107-0.149. FFA was affected (p<0.01) by thawing methods within cycles and among the cycles (Figs. 5, 6). FFA value was also increased slightly as the number of freeze-thaw cycles increased. Significant interactive effects (p<0.05) on FFA value were observed in different cycles and thawing methods. Thawing at 40℃ in cycle 3 had the highest value and thawing at 4℃ in cycle 1 had the lowest FFA value among
Fig. 5. Changes of free fatty acids value (mean±SE) in thawed beef samples compared to control in freeze-thaw cycle-1, 2 and 3.
Fig. 6. Changes of free fatty acids value (mean±SE) in thawed beef samples compared to control in repeated cycles.
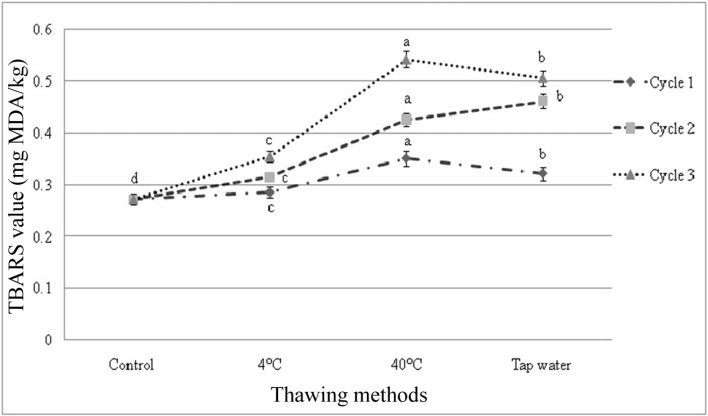
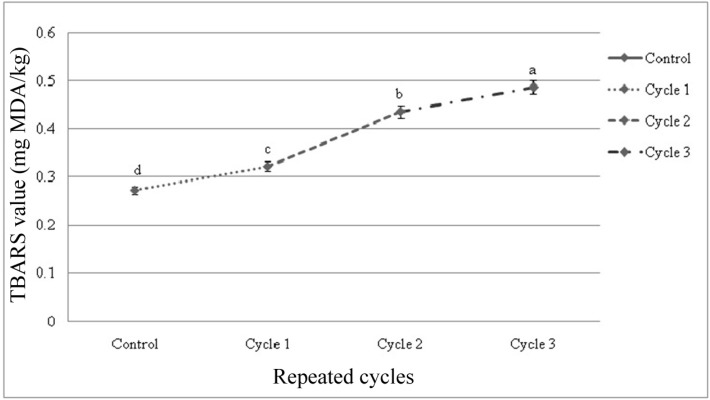
TBARS value
There were significant differences among the samples subjected to repeated freeze-thaw cycles in TBARS value. The initial TBARS value of fresh beef sample was 0.272 mg MDA/kg beef, ranging within 0.272 to 0.542 mg MDA/kg beef. TBARS value was affected (p<0.01) within cycles and among the cycles by different thawing methods (Fig. 7 and 8). Significant interactive effects (p<0.01) were found on TBARS value in different cycles and thawing methods. Thawing at 40℃ in cycle 3 had the highest value and thawing at 4℃ in cycle 1 had the lowest TBARS value among the interactions (Table 6).
Fig. 7. Changes of TBARS value (mean±SE) in thawed beef samples compared to control in freeze-thaw cycle-1, 2 and 3.
Fig. 8. Changes of TBARS value (mean±SE) in thawed beef samples compared to control in repeated cycles.
Discussions
Drip loss
Drip loss in all treatments showed a significant (p<0.01) change over the course of trial. The result of the current study agreed to Ambrosiadis et al. (1994) and Rahman et al. (2014) reported that rapid thawing of meat by submergence in water decreased the drip loss and in case of refrigerated thawing (28 h), which resulted in the highest drip loss. In another study up to 18.27% drip loss is observed by freeze-thawing pork (Xia et al., 2009) which is similar to the result of this study. Traore et al. (2012) reported that exudates (drip or thaw loss) are closely related to muscle protein oxidation and denaturation which are responsible for muscle pH decline, discoloration, and toughness which is related to present study. Moreover, changes of drip loss are also interlinked with the rate of pH decline post mortem, the rate of post mortem glycolysis, the degree of actomyosin cross-linking during rigor mortis, residual ATP levels, final pH and the activity of a multitude of enzymes (Lawrie, 1998). On the other hand, the loss of exudates (drip or thaw loss) from beef is unavoidable, because some loss of moisture occurs due to the presence of water in a free form in muscle tissue (Joo and Kim, 2011) during freeze-thaw. Amount of exudates can be reduced to a minimum by controlling WHC (Jeong et al., 2011).
Cooking loss
Both drip loss and cooking loss were affected by freeze-thawing (p<0.01) cycles reported by Rahman et al. (2014) agreed to the result of the present study. Loss of moisture due to cooking has been reported not to differ between fresh and frozen meat samples, as well as for samples frozen and thawed at different rates reported by Leygonie et al. (2012b) and Rahman et al. (2014) which are similar to this study. The cooking loss was not affected by the freeze-thaw cycles, as the water expelled during cooking originates mostly from chemically bound water (10% of the total fluid) and from the fat that melts, which is not affected by freezing and thawing (Vieira et al., 2009). Moreover, cooking loss in pre-rigor muscle decreased due to exposure of hydrophilic groups of myofibrillar protein, resulting in greater hydrogen bonding of water (Macfarlane, 1973).
Water holding capacity
Another important functional property of beef is water-holding capacity, in particular water absorption and retention by the protein structures of muscular tissue as well as water retention during heat processing (Huff-Lonergan and Lonergan, 2005). In general freezing, frozen storage and thawing all contribute to a decrease in the water-holding capacity of meat (Vieira et al., 2009) agreed to the result of the current study. An initial increase and a subsequent decrease in WHC has been reported by Joo et al. (1999), Kristensen and Purslow (2001), Straadt et al. (2007) and Rahman et al. (2014) agreed with current study. Except cycle three the result of WHC in this study is satisfactory which agreed to Nasreen et al. (2012).
pH
The increase or decrease of meat pH from the initial value influences the rate of oxidation as well as the microbial shelf life and drip loss and vice versa. This result might be due to loss of minerals and small protein compounds as exudates from beef samples, causing ionic imbalance in the beef muscle which resulted in a decreased pH (Vieira et al., 2009) which agreed with present study. The pH value also might be decreased with repeated freeze-thaw cycles in the current study due to increase in glycolysis with subsequent thawing and phosphorylase activation (Elkhalifa et al., 1984). This result was also in agreement with Jun et al. (2012) who stated that within the first 10 freeze-thaw cycles pH decreased (p<0.05) but increased (p>0.05) after 5 further cycles. The accelerating effect of repeated freeze-thaw cycles on beef pH is required further examination concerned with different thawing methods and thawing rate.
However, the influence of meat exudates on meat quality during cold storage has not been identified. Therefore, we hypothesized that the presence of exudates affects the physicochemical quality of beef muscle as drip loss, cooking loss, WHC and muscle pH.
Lipid oxidation
In current study peroxide, TBA and acid value were chosen as representative for primary, secondary lipid oxidation and lipid hydrolysis respectively. POVs of beef muscle were increased (p<0.05) with repeated freeze-thaw cycles and affected by thawing methods in contrast to the TBARS and FFA value, which is in agreement with Rahman et al. (2014). In this study, the highest POV was 0.166 mg meq/kg thawing at 40℃ warm water after three freeze-thaw cycles, which is accepted by Narasimhan et al. (1986) reported that acceptability of beef and beef products will be limited when the POV will be more than 25 mg meq/kg. However rate of production and the amount of peroxide in frozen storage of beef muscle might be reduced than refrigerated storage (Javanmard et al., 2006; Lee and Chae, 2000; Novelli et al., 1998). Peroxide formation is low at the beginning of frozen storage, after which the rate of formation increased rapidly but induction period depends on the nature of lipid and the presence of antioxidants in beef muscle. Peroxide values are not static and it is difficult to provide a specific guideline relating peroxide value to rancidity. Moderate peroxide values might be the result of depletion of peroxides after reaching high concentrations but high level of peroxide definitely indicates of a rancid fat. The amount of peroxide remains low in highly unsaturated fats, even after extensive oxidation, because initially peroxides formed from unsaturated fats are highly unstable and react quickly to form secondary oxidation products (Gotoh and Wada, 2006; Levermore, 2004).
Free fatty acids are not only the products of enzymatic degradation but also microbial degradation of lipids. FFA gives an idea about stability of lipid during preservation. The significant increase in FFA levels of beef muscle during 80 d of frozen storage (control) might be due to growth inhibition of lipolytic microbes, total myofibrillar protein solubility, and intramuscular free fatty acids concentration decreased (p<0.05) in frozen storage which is in agreement with Qi et al. (2012) and Rahman et al. (2014). However, similar trend but higher value of FFA was found in buffalo meat nuggets during refrigerated storage (Sahoo and Anjaneyulu, 1997). Modi et al. (2007) also found similar result from freshly prepared dehydrated chicken kebab mix had FFA values of 0.99±0.205, and gradually (p<0.05) increased to 1.74±0.073 during 6 mon of storage. Lipolytic enzymes have been considered as an accelerator for lipid oxidation (Morcuende et al., 2003) due to accumulation of FFAs those were prone to lipid peroxidation, particularly long chain unsaturated FFAs. The possible lipolytic enzymes present in beef are microbial lipases, phospholipases, acid lipases, neutral lipases, phospholipase A and lysophospholipase reported by Cilla et al. (2006). The activity of lipolytic enzymes and autooxidation of lipids might be partially inhibited during frozen storage, resulting in reduced FFA values.
Fresh beef undergoes many undesirable changes during preservation at both refrigeration and freezing temperatures. Lipid peroxidation is one of the primary mechanisms of beef quality deterioration in terms of flavor, color, texture, and nutritive value and the production of toxic compounds (Pearson et al., 1983). TBARS is a secondary oxidation product commonly used as a measurement of lipid oxidation. The secondary by-products of lipid oxidation like aldehydes, possessing cytotoxic and genotoxic properties due to their high reactivity. TBA and peroxide values correlated positively with each other and increased significantly during storage time. Lipid oxidation is an important quality deteriorating determinant for meat and meat products, as it may lead to rancidity of lipid (Jin et al., 2009; Nolsøe and Undeland 2009). During frozen state microbial spoilage of beef and beef products may be delayed but beef quality deterioration continued by oxidation of lipid. An irrevocable natural tendency of increasing of TBARS value during refrigerated and frozen states of meat and meat products has been reported by many researchers (Devatkal et al., 2004; Rajkumar et al., 2004). Fernindez et al. (1997) and Borneo et al. (2009) stated that compounds produced from lipid oxidation like malonaldehyde and other short-chain carbon products are very much unstable and probably decompose to organic alcohols and acids, which are not detected by the TBA test. This is because of secondary oxidation products particularly low molecular weight volatile compounds were formed and lost during storage. TBARS value increased slowly within the cycles and among the cycles by thawing methods in present study. According to Tan and Shelef (2002), lipid oxidation rate was slower in frozen meats than in refrigerated meat and the TBARS values showed very small differences during preservation at −20℃ for 69 d. The findings of the current study were in agreement with previous report (Tan and Shelef, 2002). In this experiment TBARS values are ranged within 0.272 to 0.542 that are lower than the acceptance limit of TBARS for rancidity (1.0 mg/kg). Beef is a rich source of polyunsaturated fatty acids, cholesterol, proteins and pigments etc. The oxidative stability of beef depends upon the balance of anti and pro-oxidants and the composition of these oxidation substrates (Bertelsen et al., 2000).
Thawing at 40℃ showed the highest TBARS value in every cycle in this experiment because heating could affect many factors involved in lipid oxidation. Disruption of muscle cell structure, inactivation of antioxidative enzymes and releases of oxygen from oxymyoglobin all are the results of heat. The activation energy for oxidation is decreased by high temperature resulting breaks down hydroperoxides to free radicals, which propagate lipid peroxidation. Heating seemed to be very pro-oxidative for the pre-frozen meat samples as measured by the high TBARS values (Igene et al., 1979). This statement was in agreement with the results of present study. Li et al. (2012) and Rahman et al. (2014) stated that TBARS value increased significantly (p<0.05), as the number of freeze-thaw cycles increased which is similar to current study. As a result, freeze-thaw cycles affected the physicochemical quality and lipid degradation of beef muscle, causing the deterioration of beef quality. The finding of the current study was also in agreement with previous report of Li et al. (2012).
Conclusion
The freeze-thaw process significantly affected drip loss, cooking loss, WHC, pH, POV, FFA and TBARS value of beef muscle. Oxidation processes in beef lead to a degradation of lipid has a deleterious effect on the physicochemical properties of beef. The physicochemical deterioration of beef muscle accelerated significantly as the number of repeated freeze-thaw cycles increased, and causing the deterioration of beef quality. The repeated freeze and thaw should be minimized in terms of beef quality to avoid physicochemical deterioration. From the findings of the current study, it may be concluded that refrigerator thawing (4℃) is more suitable than running tap water or warm water (40℃) thawing and avoiding repeated freezing and thawing of beef whenever possible is the best approach.
Acknowledgments
This study was supported by Higher Education Quality Enhancement Project (HEQEP).
References
- 1.Ambrosiadis I., Theodorakakos N., Georgakis S., Lekas S. Influence of thawing methods on the quality of frozen meat and drip loss. Fleishwirtsch. 1994;74:284–286. [Google Scholar]
- 2.Bertelsen G., Jakobsen M., Juncher D., Moller J., KrogerOhlsen M., Weber C., Skibsted L. H. Oxidation, shelf-life and stability of meat and meat products; Proceedings of the 46th International Congress of Meat science and Technology; 2000. pp. 516–524. [Google Scholar]
- 3.Borneo R., Leon A. E., Aguirre A., Ribotta P., Cantero J. J. Antioxidant capacity of medicinal plants from the Province of Cordoba (Argentina) and their in vitro testing in a model food system. J. Food Chem. 2009;112:664–670. [Google Scholar]
- 4.Breidenstein B. C. Nutrient composition: Nutrient value of meat. Food Nutr. News. 1987;59:43–58. [Google Scholar]
- 5.Cilla I., Martinez L., Beltran J. A., Roncales P. Effect of low-temperature preservation on the quality of vacuum-packaged dry-cured ham: refrigerated boneless ham and frozen ham cuts. Meat Sci. 2006;73:12–21. doi: 10.1016/j.meatsci.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Devatkal S., Mendiratta S. K., Kondaiah N. Quality characteristics of loaves from buffalo meat, liver and vegetables. Meat Sci. 2004;67:377–383. doi: 10.1016/j.meatsci.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Eastridge J. S., Bowker B. C. Effect of rapid thawing on the meat quality attributes of USDA select beef strip loins steaks. J. Food Sci. 2011;76:156–162. doi: 10.1111/j.1750-3841.2010.02037.x. [DOI] [PubMed] [Google Scholar]
- 8.Elkhalifa E. A., Anglemier A. F., Kennick W. H., Elgasim E. A. Influence of pre-rigor pressurization on postmortem beef muscle creatine phosphokinase activity and degradation of creatine phosphate and adenosine triphosphate. J. Food Sci. 1984;49:595–597. [Google Scholar]
- 9.Estévez M. Protein carbonyls in meat systems: A review. Meat Sci. 2011;89:259–279. doi: 10.1016/j.meatsci.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 10.FAO. FAO (Food and Agriculture Organization of the United Nations), Production Year Book. Rome, Italy: 2013. [Google Scholar]
- 11.Farouk M. M., Wieliczko K. J., Merts I. Ultra-fast freezing and low storage temperatures are not necessary to maintain the functional properties of manufacturing beef. Meat Sci. 2004;66:171–179. doi: 10.1016/S0309-1740(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 12.Fernindez J., Perez-Alvarez J. A., Fernandez-Lopez J. A. Thiobarbituric acid test for monitoring lipid oxidation in meat. J. Food Chem. 1997;59:345–353. [Google Scholar]
- 13.Gotoh N., Wada S. The importance of peroxide value in assessing food quality and food safety. J. Am. Oil Chem. Soc. 2006;83:473–474. [Google Scholar]
- 14.Grau R., Hamm G. Eine Einfache Methode zur Bestimmung der Wasserbindung in Muskel. Die Naturwissenschaften. 1953;40:29–30. [Google Scholar]
- 15.Huff-Lonergan E., Lonergan S. M. Mechanism of water- holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Igene J. O., Pearson A. M., Merkel R. A., Coleman T. H. Effect of frozen storage time, cooking and holding temperature upon extractable lipids and TBA values of beef and chicken. J. Anim. Sci. 1979;49:701–707. [Google Scholar]
- 17.Insani E. M., Eyherabide A., Grigoni G., Sancho A. M., Pencel N. A., Descalzo A. M. Oxidative stability and its relationship with natural antioxidants during refrigerated retail display of beef produced in Argentina. Meat Sci. 2008;79:444–452. doi: 10.1016/j.meatsci.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Javanmard M., Rokni N., Bokaie S., Shahhosseini G. Effects of gamma irradiation and frozen storage on microbial, chemical and sensory quality of chicken meat in Iran. Food Control. 2006;17:469–473. [Google Scholar]
- 19.Jeong J. Y., Kim G. D., Yan H. S., Joo S. T. Effect of freeze-thaw cycles on physicochemical properties and color stability of beef semimembranosus muscle. Food Res. Int. 2011;44:3222–3228. [Google Scholar]
- 20.Jin S. K., Kim I. S., Choi Y. J., Kim B. G., Hur S. J. The development of imitation crab sticks containing chicken breast surimi. LWT-Food Sci. Technol. 2009;42:150–156. [Google Scholar]
- 21.Johnson A. R. The nutrient composition of Australian meats and poultry: A preface. Food Technol. Australia. 1987;39:183–184. [Google Scholar]
- 22.Joo S. T., Kauffman R. G., Kim B. C., Park G. B. The relationship of sarcoplasmic and myofibrillar protein solubility to color and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999;52:291–297. doi: 10.1016/s0309-1740(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 23.Joo S. T., Kim G. D. Meat quality traits and control technologies In: Joo ST, editor. Control of Meat Quality. Research Signpost; Kerala: 2011. pp. 1–20. [Google Scholar]
- 24.Kristensen L., Purslow P. P. The effect of ageing on the water-holding capacity of pork: Role of cytoskeletal proteins. Meat Sci. 2001;58:17–23. doi: 10.1016/s0309-1740(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 25.Lawrie R. A. Lawrie’s meat science. 6th ed. Technomic Publishing Inc.; Lancaster, PA: 1998. [Google Scholar]
- 26.Lee S., Phillips A. L., Liebler D. C., Faustman C. Porcine oxymyoglobin and lipid oxidation in vitro. Meat Sci. 2003;63:241–247. doi: 10.1016/s0309-1740(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee S. K., Chae B. J. Influence of dietary rancid rice bran on the oxidative and color stability of ground chicken and pork during frozen storage. Korean J. Food Sci. Anim. Resour. 2000;20:296–302. [Google Scholar]
- 28.Levermore R. Rancidity in fresh and stored pork products. Meat Int. 2004;14:16–18. [Google Scholar]
- 29.Leygonie C., Britz T. J., Hoffman L. C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012a;91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Leygonie C., Britz T. J., Hoffman L. C. Meat quality comparison between fresh and frozen/thawed ostrich M. iliofibularis. Meat Sci. 2012b;91:364–368. doi: 10.1016/j.meatsci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Xu X., Zhou G. Effect of freeze-thaw cycle on meat quality of beef striploin. Jiangsu J. Agri. Sci. 2012;95:230–237. [Google Scholar]
- 32.Modi V. K., Sachindra N. M., Nagegowda P., Mahendrakar N. S., Rao D. N. Quality changes during the storage of dehydrated chicken kebab mix. Int. J. Food Sci. Technol. 2007;42:827–835. [Google Scholar]
- 33.Morcuende D., Estevez M., Ruiz J., Cava R. Oxidative and lipolytic deterioration of different muscles from free-range reared Iberian pigs under refrigerated storage. Meat Sci. 2003;65:1157–1164. doi: 10.1016/S0309-1740(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 34.Narasimhan S., Raghuver K. G., Arumngham C., Bhat K. K., Sen D. P. Oxidative rancidity of ground nut oil evaluation by sensory and chemical indices and their correlation. J. Food Sci. Technol. 1986;23:273–277. [Google Scholar]
- 35.Nasreen M. A., Ayad B. A., Hozan J. H. Study of some chemical, quality, sensory and bacteriology characteristics of frozen beef meat imported to Sulaimani as compared to local meat. J. Tikrit University for Agril. Sci. 2012;12:29–39. [Google Scholar]
- 36.Nolsøe H., Undeland I. The acid and alkaline solubilization process for the isolation of muscle proteins: State of the art. Food Biop. Technol. 2009;2:1–27. [Google Scholar]
- 37.Novelli E., Zanardi E., Ghiretti G. P., Campanini G., Dazzi G., Madarena G., Chizzolini R. Lipid and cholesterol oxidation in frozen stored pork, Salame Milano and Mortadella. Meat Sci. 1998;48:29–40. doi: 10.1016/s0309-1740(97)00072-7. [DOI] [PubMed] [Google Scholar]
- 38.Park S. Y., Yoo S. S., Eun J. B., Lee H. C., Kim Y. J., Chin K. B. Evaluation of lipid oxidation and oxidative products as affected by pork meat cut, packaging method, and storage time during frozen storage (−10℃) J. Food Sci. 2007;72:C114–C119. doi: 10.1111/j.1750-3841.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 39.Pearson A. M., Gray J. I., Wolzak Arlene M., Horenstein N. A. Safety implications of oxidized lipids in muscle food. Food Technol. 1983;37:121–129. [Google Scholar]
- 40.Qi J., Li C., Chen Y., Gao F., Xu X., Zhou G. H. Changes in meat quality of ovine longissimus dorsi muscle in response to repeated freeze and thaw. Meat Sci. 2012;92:619–626. doi: 10.1016/j.meatsci.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Rahman M. H., Hossain M. M., Rahman S. M., Hashem M. A., Oh D. H. Effect of repeated freeze-thaw cycles on beef quality and safety. Korean J. Food Sci. Anim. Resour. 2014;34:482–495. doi: 10.5851/kosfa.2014.34.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajkumar V., Agnihotri M. K., Sharma N. Quality and shelf life of vacuumed and aerobic packed chevon patties under refrigeration. Asian-Aust. J. Anim. Sci. 2004;17:548–553. [Google Scholar]
- 43.Robinson F. The nutritional contribution of meat to the British diet: Recent trends and analyses. Nutr. Bull. 2001;26:283–293. [Google Scholar]
- 44.Rukunudin I. H., White P. J., Bern C. J., Bailey T. B. A modified method for determining free fatty acids from small soybean sample sizes. J. Am. Oil Chem. Soc. 1998;75:563–568. [Google Scholar]
- 45.Sahoo J., Anjaneyulu A. S. R. Effect of natural antioxidants and vacuum packaging on the quality of buffalo meat nuggets during refrigerated storage. Meat Sci. 1997;47:223–230. doi: 10.1016/s0309-1740(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 46.Sallam K. I., Ishioroshi M., Samejima K. Antioxidants and antimicrobial effects of garlic in chicken sausage. Lebensm.-Wiss. Technol. 2004;37:849–855. doi: 10.1016/j.lwt.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmedes A., Homer G. A new thiobarbituricacid (TBA) method for determining freemalondialdehyde (MDA) and hydroperoxides selectively as a measure of lipidperoxidation. J. Am. Oil Chem. Soc. 1989;66:813–817. [Google Scholar]
- 48.Soyer A., Özalp B., Dalmýþ Ü., Bilgin V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. J. Food Chem. 2010;120:1025–1030. [Google Scholar]
- 49.Straadt I. K., Rasmussen M., Andersen M. J., Bertram H. C. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss - A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007;75:687–695. doi: 10.1016/j.meatsci.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Sultana A., Nakanishi A., Roy B. C., Mizunoya W., Tatsumi R., Ito T., Tabata S., Rashid H., Katayama S., Ikeuchi Y. Quality improvement of frozen and chilled beef biceps femoris with the application of salt-bicarbonate solution. Asian-Aust. J. Anim. Sci. 2008;21:903–911. [Google Scholar]
- 51.Tan W., Shelef L. A. Effect of sodium chloride and lactates on chemical and microbial changes in refrigerated and frozen fresh ground pork. Meat Sci. 2002;62:27–32. doi: 10.1016/s0309-1740(01)00223-6. [DOI] [PubMed] [Google Scholar]
- 52.Traore S., Aubry L., Gatellier P., Przybylski W., Jaworska D., Kajak-Siemaszko K., Santé-Lhoutellier V. Higher drip loss is associated with protein oxidation. Meat Sci. 2012;90:917–924. doi: 10.1016/j.meatsci.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 53.Whitney E. N., Rolfes S. R. Understanding nutrition. 10th Edition. Wadsworth; Australia: 2008. [Google Scholar]
- 54.Xia X., Kong B., Liu Q., Liu J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009;83:239–245. doi: 10.1016/j.meatsci.2009.05.003. [DOI] [PubMed] [Google Scholar]