Abstract
Functionally and physiologically active peptides are produced from several food proteins during gastrointestinal digestion and fermentation of food materials with lactic acid bacteria. Once bioactive peptides (BPs) are liberated, they exhibit a wide variety of physiological functions in the human body such as gastrointestinal, cardiovascular, immune, endocrine, and nervous systems. These functionalities of the peptides in human health and physiology include antihypertensive, antimicrobial, antioxidative, antithrombotic, opioid, anti-appetizing, immunomodulatory and mineral-binding activities.
Most of the bioactivities of milk proteins are latent, being absent or incomplete in the original native protein, but full activities are manifested upon proteolytic digestion to release and activate encrypted bioactive peptides from the original protein. Bioactive peptides have been identified within the amino acid sequences of native milk proteins. Due to their physiological and physico-chemical versatility, milk peptides are regarded as greatly important components for health promoting foods or pharmaceutical applications.
Milk and colostrum of bovine and other dairy species are considered as the most important source of natural bioactive components. Over the past a few decades, major advances and developments have been achieved on the science, technology and commercial applications of bioactive components which are present naturally in the milk. Although the majority of published works are associated with the search of bioactive peptides in bovine milk samples, some of them are involved in the investigation of ovine or caprine milk. The advent of functional foods has been facilitated by increasing scientific knowledge about the metabolic and genomic effects of diet and specific dietary components on human health.
Keywords: bioactive peptide, milk, proteins, functional foods, human health
Introduction
Milk of all mammalian species contains a heterogeneous mixture of lacteal secretion which contains numerous components which exhibit a wide variety of chemical and functional activities. These diversified composition and functionality of milk can be found in even protein components alone. Proteins constitute a myriad of serum and glandular-derived compounds which are different in molecular size, concentration and functionality (Regester et al., 1997). The traditional and contemporary view of the role of milk has been markedly expanded beyond the horizon of nutritional subsistence of infants (Gobbetti et al., 2007; Park, 2009a). Milk has been more than a source of nutrients to any neonate of mammalian species, as well as for growth of children and nourishment of adult humans (Park, 2009b). It contains a wide range of proteins that provide protection against enteropathogens or are essential for the manufacture and characteristic nature of certain dairy products (Korhonen and Pihlanto-Leppälä, 2004).
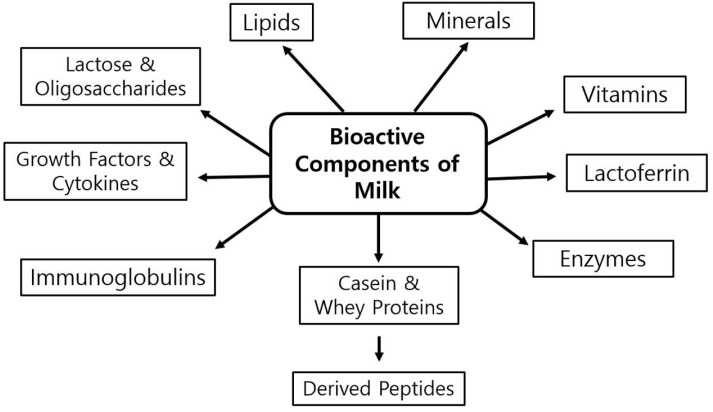
Recent studies indicate that milk furnishes a broad range of biologically active compounds that guard neonates and adults against pathogens and illnesses, such as immunoglobulins, antibacterial peptides, antimicrobial proteins, oligosaccharides, lipids, besides many other components at low concentrations, so-called “minor” components, but with considerable potential benefits, illustrated in Fig. 1 (Park, 2009b). Thus, beyond nutritional values of milk, milk-born biologically active compounds such as caseins, whey proteins and other minor constituents exhibit important physiological and biochemical functions that have crucial impacts on human metabolism and health (Gobbetti et al., 2007; Korhonen and Pihlanto-Leppala, 2004; Park, 2009a; Schanbacher et al., 1998). Bioactivity of milk components have been categorized as four major areas: (1) gastrointestinal development, activity, and function; (2) immunological development and function; (3) infant development; and (4) microbial activity, including antibiotic and probiotic action (Gobbetti et al., 2007).
Fig. 1. Major bioactive functional compounds derived from milk (Park, 2009b).
Recently, fractionation and marketing of bioactive milk ingredients have emerged as a new lucrative sector for dairy industries and specialized bio-industries. Many of these components are being exploited for both dairy and non-dairy food formulations and even pharmaceuticals (Korhonen and Pihlanto, 2007a; Krissansen, 2007; Playne et al., 2003; Shah, 2000). The dairy industry has played a leading role in the development of functional foods and also has already commercialized these products. Those products have enhanced bioactive functions in human health including the immune system, reduce elevated blood pressure, combat gastrointestinal infections, help control body weight and prevent osteoporosis (FitzGerald et al., 2004; Hartmann and Meisel, 2007; Korhonen and Marnila, 2006).
The objectives of this paper are to: (1) review the current knowledge on bioactive peptides derived from milk of major species concerning various forms of naturally occurring bioactive compounds, their physiological, biochemical and nutritional functionalities in human health, and (2) elucidate some recent studies on potential applications and development of functional foods using bioactive peptides and components in bovine milk as well as in other species milk.
Functionalities of Bioactive Peptides in Milk and Dairy Products
Numerous studies have been reported on functional properties of bioactive components in milk and dairy products especially in human and bovine milk, although more people drink the milk of goats than that of any other single species worldwide (Haenlein and Caccese, 1984; Park, 1990, 2006). On the other hand, the study of these bioactive components in milks has been difficult, because of the low concentrations of certain very potent agents in milks, their biochemical complexities, the need to develop specific methods to quantify certain factors due to their particular forms in milks, the compartmentalization of some of the agents, and the dynamic effects of lactation length and other maternal factors on concentrations or functions of the components of the systems (Goldman and Goldblum, 1995; Park, 2009a).
Among those bioactive constituents in milk and dairy products, bioactive peptides (BPs) are the most studied components in this regard. BPs have been defined as specific protein fragments that have a positive influence on physiological and metabolic functions or condition of the body and may have ultimate beneficial effects on human health (Kitts and Weiler, 2003). BPs can be delivered to the consumers in conventional foods, dietary supplements, functional foods, or medical foods. These bioactive peptides possess very important biological activities and functionalities, including antimicrobial, antihypertensive, antioxidative, anticytotoxic, immunomodulatory, opioid, and mineral-carrying activities.
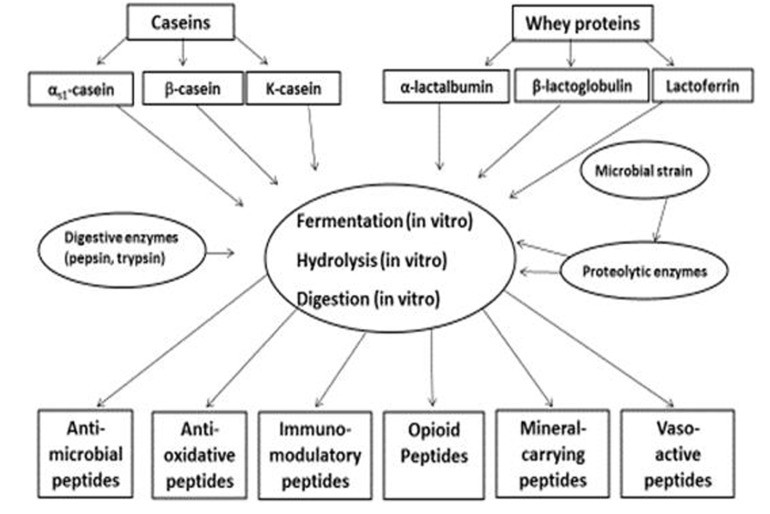
The bioactive peptides derived from a variety of dietary proteins have been reviewed by many researchers (Clare, 2003; FitzGerald and Meisel, 2003; Li et al., 2004). BPs are inactive within the sequence of the parent protein, and they can be released in three ways: (i) through hydrolysis by digestive enzymes, (ii) through hydrolysis of proteins by proteolytic microorganisms, and (iii) through the action of proteolytic enzymes derived from microorganisms or plants (Korhonen and Pihlanto, 2007b). A schematic representation of formation of bioactive peptides from major milk proteins is presented in Fig. 2.
Fig. 2. Formation of bioactive peptides from major milk proteins (Korhonen and Pihlanto, 2007b).
Many scientists have conducted a variety of research and demonstrated many biologically active compounds especially in BPs in different dairy species such as bovine, ovine and caprine milk (Table 1 and 2). The proven functionalities of milk bioactive peptides are summarized as follows:
Table 1. Major biologically active milk components and their functions1.
| Milk precursors or components | Bioactive compounds | Bioactivities observed |
|---|---|---|
| α-, β-caseins | Casomorphins | Opioid agonist (Decrease gut mobility, gastric emptying rate; increase amino acids and electrolytes uptake) |
| α-, β-caseins | Casokinins | ACE inhibitory (Increase blood flow to intestinal epithelium) |
| α-, β-caseins | Phosphopeptides | Mineral binding (Ca binding; increase mineral absorption, i.e., Ca, P, Zn) |
| α-, β-caseins | Immunopeptides | Immunomodulatory (Increase immune response and phagocytic activity) |
| Casomorphins | ||
| Casokinins | ||
| αs1-casein | Isracidin | Antimicrobial |
| αs2-casein | Casocidin | Antimicrobial |
| κ-casein | Casoxins | Opioid antagonist |
| κ-casein | Casoplatelins | Antithrombotic |
| κ-casein | ê-caseinglyco- | Probiotic (Growth of Bifidobacteria in GI tract) macropeptide |
| α-lactalbumin (α-La) | Lactorphins | Opioid agonist |
| β-lactoglobulin (β-La) | ||
| Serum albumin | Serorphin | Opioid agonist |
| α-La, β-La and Serum albumin | Lactokinins | ACE inhibitory |
| Immunoglobulins | IgG, IgA | Immunomodulatory (Passive immunity) |
| Lactoferrin | Lactoferrin | Immunomodulatory (Increase natural killer cell activity, humoral immune response, thymocyte trafficking immunological development, and interleukins-6; decrease tumor necrosis factor-α) Antimicrobial (Increase bacteriostatic inhibition of Fe-dependent bacteria; decrease viral attachment to and infections of cells) Probiotic activity (Increase growth of Bifidobacteria in GI tract) |
| Lactoferrin | Lactoferroxins | Opioid antagonist |
| Oligosaccharides | Oligosaccharides | Probiotic (Increase growth of Bifidobacteria in GI tract) |
| Glycolipids | Glycolipids | Antimicrobial (Decrease bacterial & viral attachment to intestinal epithelial cells) |
| Oligosaccharides | Oligosaccharides | |
| Prolactin | Prolactin | Immunomodulatory (Increase lymphocyte and thymocyte trafficking, and immune development) |
| Cytokines | Interleukins-1,2,6, & 10 | Immunomodulatory (Lympocyte trafficking, immune development) |
| Tumor necrosis factor-α | ||
| Interferon-γ | ||
| Transforming growth Factors-α, β;leukotriene B4 | ||
| Prostaglandin E2, Fn | ||
| Growth factors | IGF-1, TGF-α, EGF, TGF-β | Organ development and functions |
| Parathromone-P | PTHrP | Increase Ca+2 metabolism and uptake |
1Adapted from Schanbacher et al. (1998), Meisel (1998), and Clare and Swaisgood (2000), Park (2009b)
Table 2. Sequence of bioactive peptides derived from ovine and caprine milk proteins.
| Peptide fragment | Sequence | Biological activity | Reference |
|---|---|---|---|
| Ovine αs1-CN f(86-92) | VPSERYL | ACE-inhibitory | Gómez-Ruiz et al. (2002) |
| Ovine αs1-CN f(102-109) | KKYNVPQL | ACE-inhibitory | Ómez-Ruiz et al. (2002) |
| Caprineαs1-CN f(143-146) | AYFY | ACE-inhibitory | Lee et al. (2005) |
| Ovine αs2-CN f(165-170) | LKKISQ | Antibacterial | López-Expósito et al. (2006) |
| Ovine αs2-CN f(165-181) | LKKISQYYQKFAWPQYL | Antibacterial | López-Expósito et al. (2006) |
| Caprineαs2-CN f(174-179) | KFAWPQ | ACE-inhibitory | Quiróset al. (2005) |
| Ovine αs2-CN f(184-208) | VDQHQAMKPWTQPKTKAIPYVRYL | Antibacterial | López-Expósito et al. (2006) |
| Ovine αs2-CN f(202-204) | IPY | ACE-inhibitory | Gómez-Ruiz et al. (2002) |
| Ovine and caprineαs2-CN f(203-208) | PYVRYL | Antibacterial | López-Expósito et al. (2006) |
| ACE-inhibitory | Quirós et al. (2005) | ||
| Antihypertensive | Recio et al. (2005) | ||
| Ovine αs2-CN f(205-208) | VRYL | ACE-inhibitory | Gómez-Ruiz et al. (2002) |
| Ovine and caprineβ-CN f(47-51) | DKIHP | ACE-inhibitory | Gómez-Ruiz et al. (2002) |
| Ovine β-CN f(58-68) | LVYPFTGPIPN | ACE-inhibitory | Quirós et al. (2005) |
| Caprineκ-CN f(59-61) | PYY | ACE-inhibitory | Lee et al. (2005) |
| Ovine and caprineκ-CN f(106-111) | MAIPPK | ACE-inhibitory | Manso et al. (2003) |
| Ovine and caprineκ-CN f(106-112) | MAIPPKK | ACE inhibitory | Manso et al. (2003) |
| Ovine κ-CN f(112-116) | KDQDK | Antithrombotic | Qian et al. (1995) |
| Caprineβ-Lg f(46-53) | LKPTPEGD | ACE-inhibitory | Hernández-Ledesma et al. (2002) |
| Caprineβ-Lg f(58-61) | LQKW | ACE-inhibitory | Hernández-Ledesma et al. (2002) |
| Caprineβ-Lg f(103-105) | LLF | ACE-inhibitory | Hernández-Ledesma et al. (2002) |
| Caprineβ-Lg f(122-125) | LVRT | ACE-inhibitory | Hernández-Ledesma et al. (2002) |
| Ovine and caprine LF f(17-41) | ATKCFQWQRNMRKVRGPPVSCIKRD | Antibacterial | Vorland et al. (1998) |
| Ovine and caprine LF f(14-42) | QPEATKCFQWQRNMRKVRGPPVSCIKRDS | Antibacterial | Recio and Visser (2000) |
Antihypertensive peptides
The angiotensin is one of two polypeptide hormones and a powerful vasoconstrictor that functions in the body by controlling arterial blood pressure through the contraction of smooth muscles of the blood vessel (Park, 2009a). Angiotensin converting enzyme (ACE)-inhibitory peptide blocks the conversion of angiotensin I to angiotensin II. The ACE causes elevation of blood pressure by converting angiotensin-I to the potent vasoconstrictor, angiotensin-II, and by degrading bradykinin, a vasodilatory peptide, and enkephalins (Petrillo and Ondetti, 1982).
As shown in Table 1, several ACE-inhibitory peptides were identified by in vitro enzymatic digestion of milk proteins or chemical synthesis of peptide analogs (Gobbetti et al., 2004). The ACE-inhibitors derived from milk proteins are attributed to different fragments of casein, named casokinins (Meisel and Schlimme, 1994), or whey proteins, named lactokinins (FitzGerald and Meisel, 2000).
Antioxidative peptides
Peptides derived from αs-casein have free radical-scavenging activity and inhibit enzymatic and nonenzymatic lipid peroxidation (Rival et al., 2001; Suetsuna et al., 2000). Proteolytic enzymes can release antioxidative peptides from caseins, soybean and gelatine by enzymatic hydrolysis (Korhonen and Pihlanto, 2003).
Low temperature-processed whey protein contained high levels of specific dipeptides (glutamylcysteine) (Bounous and Gold, 1991). These dipeptides can promote the synthesis of glutathione, which is an important antioxidant for cellular protection and repair processes.
Apart from milk peptides, Byun et al. (2009) analyzed fish protein hydrolysates from the rotifer Brachionus rotundiformis, using different proteases (Alcalase, α-chymotrypsin, Neutrase, papain, pepsin and trypsin), and found antioxidant peptides from the hydrolysates. Antioxidant activities of hydrolysates were evaluated using DPPH radical scavenging activity.
Antithrombotic peptides
These peptides reduce or inhibit the formation of blood clots. Caseinomacropeptide (CMP) is a peptide split from k-casein when the milk protein is coagulated by rennin enzyme. This CMP has functions of inhibiting the aggregation of blood platelets and binding of the human fibrinogen γ-chain to platelet surface fibrinogen receptors (Fiat et al., 1993). There are two reported antithrombotic peptides, derived from human and bovine k-caseinoglycopeptides, which were identified in the plasma of 5-d-old newborns after breast-feeding and feeding cow milk based formula (Chabance et al., 1998).
Casoplatelin, peptide derived from κ-CN (Table 1), exhibited influence on platelet function and inhibited both the aggregation of ADP-activated platelets and the binding of human fibrinogen γ-chain to its receptor region on the platelets surface (Jolles et al., 1986). Sheep caseins derived κ-caseinoglycopeptide (106-171) reduced thrombinand collagen-induced platelet aggregation in a dose dependent manner (Qian et al., 1995).
Hypocholesterolemic peptides
Iwami et al. (1986) reported that a peptide having high bile acid-binding capacity can inhibit the reabsorption of bile acid in the ileum, whereby it can decrease the blood cholesterol level. A novel peptide (Ile-Ile-Ala-Glu-Lys) from tryptichydrolysate of β-lactoglobulin showed hypocholesterolemic effect (Nagoaka et al., 2001).
The serum cholesterol-lowering activity is directly influenced by the degree of fecal steroid excretion (Nagata et al., 1982). Cholesterol is become soluble in bile salt-mixed micelles and then absorbed (Wilson and Rudel, 1994). Cholesterol absorption was suppressed by this peptide in Caco-2 cells in vitro, and also the peptide elicited hypocholesterolemic activity in vivo in rats after oral administration of the peptide solution. The mechanism of the hypocholesterolemic effect by these peptides has not been delineated (Korhonen and Pihlanto, 2007b), while four bioactive peptides were identified in the hydrolysate which corresponded to β-lactoglobulin f9-14, f41-60, f71-75, and f142-146.
Opioid peptides
An opioid is any chemical such as morphine that resembles opiates in its pharmacological effects. Opioids are defined as peptides (i.e., enkephalins) which have an affinity for an opiate receptor and opiate-like effects, inhibited by naloxone (Gobbetti et al., 2007). As shown in Table 1, many opioid peptides have been identified. Opioid peptides are opioid receptor ligands with agonistic or antagonistic activities (Park, 2009a).
The αs1-casein-exorphin (αs1-CN f90-96), β-casomorphins-7 and -5 (β-CN f60-66 and f60-64, respectively), and lactorphins (α-lactalbumin f50-53 and β-lactoglobulin f102-105) act as opioid agonists, whereas casoxins (i.e., k-CN f35-42, f58-61, and f25-34) act as opioid antagonists (Gobbetti et al., 2007; Meisel and FitzGerald, 2000). β-Casomorphins were found in the analoguous position of the natural proteins in cow, sheep, water buffalo, and human β-CN (Meisel and Schlimme, 1996).
In all endogenous and exogenous opioid peptides, the common structural feature of these peptides is the presence of a Tyr residue at the amino terminal end (except for αs1-CN-exorphin, casoxin 6, and lactoferroxin B and C) and of another aromatic residue, Phe or Tyr, in 3rd or 4th position (Gobbetti et al., 2007). The hydrolysis of Lactobacillus GG fermented UHT milk by the pepsin/trypsin has shown to release several opioid peptides from αs1- and β-CN, and α-lactalbumin (Rokka et al., 1997).
Antiappetizing peptides
These peptides have functions of suppressing the appetite, whereby they prevent gaining weight and obesity. Zhang and Beynen (1993) reported that the total whey protein in the diet has been associated with a lowering of LDL cholesterol, and also related to the increased release of an appetite-suppressing hormone, cholecystokinin.
The bioactive functions of total whey protein may arise from the combinations of active whey protein fractions or amino acid sequences. Regester et al. (1997) suggested that this physiological role of total whey protein has a great potential for processed whey products in development of new and lucrative health food markets as functional food ingredients.
Antimicrobial peptides
These peptides have bacterial membrane-lytic activities which disrupt normal membrane permeability. The total antibacterial effect in milk is greater than the sum of individual immunoglobulin and nonimmunoglobulin such as lactoferrin, lactoferricins, lactoperoxidase, lysozyme, lactenin, casecudubs, etc. (Gobbetti et al., 2007; Park, 2009a).
Peptides exhibiting antimicrobial activities have been isolated and purified from several
Bovine milk protein hydrolysates, edible plants, fish and eggs (Clare et al., 2003; Gobbetti et al., 2004). Among antimicrobial peptides, the lactoferricins are studied the most, which are derived from bovine and human lactoferrin (Kitts and Weiler 2003; Wakabayashi et al., 2003). Lactoferricins have been shown to have antimicrobial activity against various Gram-positive and -negative bacteria, yeasts and filamentous fungi (Korhonen and Pihlanto, 2007b).
Lactoferricin is an amphipathic, cationic peptide with anti-microbial (Wakabayashi et al., 2003) and anti-cancer (Eliassen et al., 2002) properties. Lactoferricin can be generated by the pepsin-mediated digestion of lactoferrin. The MilkAMP database contains a total of 111 peptides (natural, synthetic and modified) comprising or derived from the complete lactoferricin (Théolier et al., 2013), which displays anti-microbial and anti-carcinogenic functions.
Lactenin may have been the first antibacterial factor found in milk, which has been released from rennet hydrolysis of milk (Jones and Simms, 1930). Casecidins are a group of basic, glycosylated and high molecular weight (about 5 kDa) polypeptides, which possess bactericidal properties against lactobacilli and also against various pathogenic bacteria such as Staphylococcus aureus. Isracidin is another antibacterial peptide derived from αs1-CN, which is hydrolyzed with chymosin (Hill et al., 1974).
Immunomodulatory peptides
Peptides and protein hydrolysates generated from milk caseins and major whey proteins exert immunomodulatory effects [possess immune cell functions], including lymphocyte proliferation, antibody synthesis, and cytokine regulation (Gill et al., 2000). Casein derived peptides are produced during fermentation of milk by lactic acid bacteria. These peptides have become special interest to food researchers and food processing industry due to their immune cell functions. These immunomodulatory peptides have been shown to modulate the proliferation of human lymphocytes, to stimulate the phagocytic activities of macrophages, and to down-regulate the production of certain cytokines (Korhonen and Pihlanto, 2003, 2007; Matar et al., 2003). Immunomodulatory peptides generated from milk include αs1-CN f194-199 (αs1-immunocasokinin) and β-CN f193-202, f63-68, f191-193 (immunopeptides), which are synthesized by hydrolysis with pepsin-chymosin.
The proliferation of human colonic lamina propria lymphocytes was inhibited by immunomodulatory effect of β-casomorphin-7, where the antiproliferative effect of micromolar concentrations was reversed by the opiate receptor antagonist naloxone (Elitsur and Luk, 1991). Free amino acid glutamine can be substituted by glutamine-containing peptides, where glutamine is required for lymphocyte proliferation, and it is also utilized at a high rate by immunocompetent cells for the immunomodulatory effect (Calder, 1994).
Cytomodulatory peptides
Peptides derived from caseins can modulate cell viability such as proliferation and apoptosis in different human cell cultures, inhibit cancer cell growth or stimulate the activity of immunocompetent cells and neonatal intestinal cells (Hartmann et al., 2000). Peptides derived from milk act as specific signals that may trigger viability of cancer cells (Gobbettti et al., 2007).
Casein hydrolysis by bacteria using commercial yogurt starter cultures can yield bioactive peptides which affect colon cell Caco-2 kinetics in vitro. Roy et al. (1999) also found that skim cow milk digested with cell-free extract of the yeast Saccharomyces cerevisiae showed antiproliferative activity towards leukemia cells.
Caseinophosphopeptides (CPPs) have also been reported to exhibit cytomodulatory effects. Cytomodulatory peptides obtained from casein fractions can inhibit cancer cell growth or stimulate the activity of immunocompetent cells and neonatal intestinal cells (Meisel and FitzGerald, 2003). Gobbetti et al. (2007) reported that peptides released from a lyophilized extract of Gouda cheese inhibited proliferation of leukemia cells.
Mineral binding peptides
Mineral-binding phosphopeptides or caseinophosphopeptides (CCPs) have the function of carriers for different minerals by forming soluble organophosphate salts, especially Ca++ ion; About 1 mol of CPP can bind 40 mol of Ca2+(Meisel and Olieman, 1998; Schlimme and Meisel, 1995). The αs1-, αs2- and β-CN of cow milk contain phosphorylated regions which can be released by digestive enzymes. Specific CPPs can form soluble organophosphate salt and increase Ca absorption by limiting Ca precipitation in the ileum (Korhonen and Pihlanto, 2007b).
Most CPPs contain a common motif, such as a sequence of three phosphoseryl followed by two glutamic acid residues (Gobbetti et al., 2007). The negatively charged side chains, particularly the phosphate groups, of these amino acids of CPPs become the specific binding sites for minerals (Gobbetti et al., 2007). Chemical phosphorylation of αs1- and β-CN increased the binding capacity and the stability of these proteins in the presence of Ca2+ (Yoshikawa et al., 1981).
Growth factors
Milk growth factor [MGF] is a peptide having the complete N-terminal sequence homologous to bovine TGF-β2.MGF suppresses in vitro proliferation of human T-cells, which includes proliferation induced by mitogen, IL-2 and exposure of primed cells to tetanus toxoid antigen (Stoeck et al., 1989). Many growth factors in milk have been identified, such as insulin-like growth factor, platelet-derived growth factor, epidermal growth factor, and transforming growth factor; lactulose from lactose, nucleotides, somatotropin for bifidus growth (Grosnvenor et al., 1992; Park, 2009a).
Human milk contains physiologically active levels of growth factor, whereas bovine milk has much lower levels of growth factor activity (Grosvenor et al., 1992; Wu and Elsasser, 1995). Colostrum of most mammals usually contains high concentrations of growth factors and others bioactive compounds, while the high levels of these growth factor compounds drop rapidly during the first 3 d postpartum (Brown and Blakeley, 1984; Denhard et al., 2000). Goat milk is shown to be a great source of physiologically active growth factors (Wu and Elsasser, 1995).
Unlike bovine milk, human milk contains a growth-promoting activity for Lactobacillus bifidus var. Pennsylvanicus (Gyorgy et al., 1974), which is responsible for the predominance of Lactobacillus in the bacterial flora of large intestines of breast-fed infants. Caprine milk has yet to be studied in this premise. The bifidus growth-factor activity is attributed to N-containing oligosaccharides (Gyorgy et al., 1974) and glycopeptides and glycoproteins (Bezkorovainy et al., 1979). The oligosaccharide moiety of those molecules may possess the bifidobacterium growth-promoter activity which is associated with caseins (Bezkorvainy and Topouzian, 1981).
Bioactive Peptides Uniquely Derived from Whey Proteins
There are many bioactive peptides derived from whey proteins. Some of the known bioactive peptides obtained from whey proteins include α-lactorphin, β-lactorphin, β-lactotensin, serorphin, albutensin A and lactoferricin (Table 3). Some whey proteins are known to contain bioactive peptides with weak opioid activity, including serorphin, albutensin from serum albumin fraction, lactoferroxin from lactoferrin and lactotensin from β-lactoglobulin (Shah, 2000; Tani et al., 1994).
Table 3. Bioactive peptides derived from whey proteins.
| Precursor protein | Fragment | Peptide sequence | Name | Function |
|---|---|---|---|---|
| α-Lactalbumin | 50-53 | Tyr-Gly-Leu-Phe | α-Lactorphin | Opioid agonist ACE inhibition |
| α-Lactoglobulin | 102-105 | Tyr-Leu-Leu-Phe | β-Lactorphin | Non-opioid stimulatory effect on ileum |
| 142-148 | Ala-Leu-Pro-Met-His-Ile-Arg | - | ACE inhibition | |
| 146-149 | His-Ile-Arg-Leu | β-Lactotensin | Ileum contraction | |
| Bovine serum albumin | 399-404 | Tyr-Gly-Phe-Gln-Asp-Ala | Serorphin | Opioid |
| 208-216 | Ala-Leu-Lys-Ala-Trp-Tyr-Gly-Phe-Gln-Asp-Ala | Albutensin A | Ileum contraction, ACE inhibition | |
| Lactoferrin | 17-42 | Lys-Cys-Arg-Arg-Trp-Glu-Trp-Arg-Met-Lys-Lys-Leu-Gly-Ala-Pro-Ser-Ile-Thr-Cys-Val-Arg-Arg-Ala-Phe | Lactoferricin | Antimicobial |
Adapted from Korhonen et al. (1998).
It was found that minor whey proteins such as lactoferrin, lysozyme, lactoperoxidase and immunoglobulins are believed to be antimicrobial proteins. These whey proteins generate bioactive peptides. Lactoferrin is a dominant whey protein in human milk and plays an important role in iron uptake in the intestine (Hutchens et al., 1994; Vilgoen, 1995). Bovine lactoferrin is homologous to human lactoferrin. Bovine colostrum and milk contain about 1.5-5 mg/mL and 0.1 mg/mL, respectively.
Lactoferricin is a simple peptide consisting of 25 amino acid residues. A similar active peptide consisting of 47 amino acid residues has been obtained from human lactoferrin. The lactoferrin molecule is folded into two globular units, where each one is capable of binding one ferric (Fe+3) ion (Shah, 2000).
Conclusions
Bovine milk and colostrum have been shown to be highly important source of natural bioactive components for human nutrition and health. Bioactive peptides are liberated during gastrointestinal digestion and fermentation of food materials by lactic acid bacteria. Research have proven that these peptides exhibit a wide variety of physiological functionalities, including antimicrobial, antihypertensive, antithrombotic, antioxidative, opioid, anti-appetizing, immunomodulatory, mineral-binding and growth promoting activities.
The myriad of innate bioactive peptides and biologically and physiologically active milk compounds from casein, whey proteins and other components in milk have been discovered. They present an excellent source of natural ingredients for different applications in functional foods. Industrial or semi-industrial scale processing techniques are available for fractionation and isolation of major proteins from colostrum and milk. In the near future, several break-through products based on these ingredients will be launched on worldwide markets. These bioactive peptides and milk components could be targeted to the development of functional food products for infants, elderly and immune-compromised people as well as to improve performance and prevent diet-related chronic diseases.
References
- 1.Bezkorovainy A., Grohlich D., Nichols J. H. Isolation of a glycopeptide fraction with Lactobacillus bifidus subspecies Pennsylvanicus growth-promoting activity from whole human milk casein. Am. J. Clin. Nutr. 1979;32:1428–1432. doi: 10.1093/ajcn/32.7.1428. [DOI] [PubMed] [Google Scholar]
- 2.Bezkorovainy A., Topouzian N. Bifidobacteriumbifidus var. Pennsylvanicus growth promoting activity of human milk casein and its derivatives. Int. J. Biochem. 1981;13:585–590. doi: 10.1016/0020-711x(81)90184-1. [DOI] [PubMed] [Google Scholar]
- 3.Bounous G., Gold P. The biological activity of undenatured dietary whey proteins: Role of glutathione. Clin. Invest. Med. 1991;14:296–309. [PubMed] [Google Scholar]
- 4.Brown K. D., Blakeley D. M. Partial purification and characterization of a growth factor present in goat’s colostrum. Biochem. J. 1984;219:609–617. doi: 10.1042/bj2190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byun H.-G., Lee J. K., Park H. G., Jeon J.-K., Kim S.-K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009;44:842–846. [Google Scholar]
- 6.Calder P. C. Glutamine and the immune system. Clin. Nutr. 1994;13:2–8. doi: 10.1016/0261-5614(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 7.Chabance B., Marteau P., Rambaud J. C., Migliore-Samour D., Jolles P., Boynard M., Perrotin P., Buillet R., Fiat A. M. Casein peptided release and passage to the blood in humans during digestion of milk or yogurt. Biochimie. 1998;80:155–165. doi: 10.1016/s0300-9084(98)80022-9. [DOI] [PubMed] [Google Scholar]
- 8.Clare D. A., Catignani G. L., Swaisgood H. E. Biodefense properties of milk: the role of antimicrobial proteins and peptides. Curr. Pharm. Des. 2003;9:1239–1255. doi: 10.2174/1381612033454874. [DOI] [PubMed] [Google Scholar]
- 9.Clare D. A., Swaisgood H. E. Bioactive milk peptides: A prospectus. J. Dairy Sci. 2000;83:1187–1195. doi: 10.3168/jds.S0022-0302(00)74983-6. [DOI] [PubMed] [Google Scholar]
- 10.Denhard M., Claus R., Munz O., Weiler U. Course of epidermal growth factor (EGF) and insulin-like growth factor (IFG-I) in mammary secretions of the goat during endpregnancy and early lactation. J. Vet. Med. Ser. A. 2000;47:533–540. doi: 10.1046/j.1439-0442.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 11.Eliassen L. T., Berge G., Sveinbjornsson B., Svendsen J. S., Vorland L. H., Rekdal O. Evidence for a direct antitumor mechanism of action of Bovine lactoferricin. Anticancer Res. 2002;22:2703–2710. [PubMed] [Google Scholar]
- 12.Elitsur Y., Luk G. D. β-casomorphin (BCM) and human colonic lamina proprialymphocyte proliferation. Clin. Exp. Immunol. 1991;85:493–497. doi: 10.1111/j.1365-2249.1991.tb05755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiat A. M., Miglilore-Samour D., Jolles P., Crouet L., Collier C., Caen J. Biologicallyactive peptides from milk proteins with emphasis on two example concerning antithrombotic and immuno-modulating activities. J. Dairy Sci. 1993;76:301–310. doi: 10.3168/jds.s0022-0302(93)77351-8. [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald R. J., Meisel H. Milk protein derived peptide inhibitors of angiotensin-I converting enzyme. Brit. J. Nutr. 2000;84:S33–S37. doi: 10.1017/s0007114500002221. [DOI] [PubMed] [Google Scholar]
- 15.FitzGerald R. J., Meisel H. Milk protein hydrolysates and bioactive peptides In: Fox P. F., McSweeney P. L. H., editors. Advances in Dairy Chemistry. 3rd ed. Kluwer Academic/Plenum Publishers; NY: 2003. pp. 675–698. [Google Scholar]
- 16.FitzGerald R. J., Murray B. A., Walsh D. J. Hypotensive peptides from milk proteins. J. Nutr. 2004;134:980S–988S. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- 17.Gill H. S., Coull F., Rutherfurd K. J., Cross M. L. Immunoregulatory peptides in bovine milk. Br. J. Nutr. 2000;84:S111–S117. doi: 10.1017/s0007114500002336. [DOI] [PubMed] [Google Scholar]
- 18.Gobbetti M., Minervini F., Rizzello C. G. Angiotensin I-converting enzyme inhibitory and antimicrobial bioactive peptides. Int. J. Dairy Technol. 2004;57:173–188. [Google Scholar]
- 19.Gobbetti M., Minervini F., Rizzello C. G. Bioactive peptides in dairy products In: Hui Y. H., editor. Handbook of food products manufacturing. John Wiley & Sons, Inc.; 2007. pp. 489–517. [Google Scholar]
- 20.Goldman A. S., Goldblum R. M. Defense agents in milk: A defense agents in human milk In: Jensen R., editor. Handbook of Milk Composition. Academic Press; NY: 1995. pp. 727–748. [Google Scholar]
- 21.Grosvenor C. E., Picciano M. F., Baumrucker C. R. Hormones and growth factors in milk. Endocr. Rev. 1992;14:710–728. doi: 10.1210/edrv-14-6-710. [DOI] [PubMed] [Google Scholar]
- 22.Gyorgy P., Jeanloz R. W., Von Nicolai H., Zilliken F. Undialyzable growth factors for Lactobacillus bifidus var. Pennsylvanicus. Eur. J. Biochem. 1974;43:29–33. doi: 10.1111/j.1432-1033.1974.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 23.Haenlein G. F. W., Caccese R. Goat milk versus cow milk In: Haenlein G. F. W., Ace D. L., editors. Extension Goat Handbook. USDA Publication; Washington, D.C., E-1: 1984. pp. 1–4. [Google Scholar]
- 24.Hartmann R., Gunther S., Martin D., Meisel H., Pentzien A. K., Schlimme E., Scholz N. Cytochemical model systems for the detection and characterization of potentially bioactive milk components. Kieler Milchwirtschaftliche Forschungsberichte. 2000;52:61–85. [Google Scholar]
- 25.Hartmann R., Meisel H. Food-derivedpeptides with biological activity: from research to food applications. Curr. Opin. Biotech. 2007;18:1–7. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Hill R. D., Lahov E., Givol D. A rennin-sensitive bond in alpha and beta casein. J. Dairy Res. 1974;41:147–153. doi: 10.1017/s0022029900015028. [DOI] [PubMed] [Google Scholar]
- 27.Hutchens T. W., Rumball S. V., Lonnerdal B. Lactoferrin: structure and function. Adv. Exp. Med. Biol. 1994;357:1–298. [Google Scholar]
- 28.Iwami K., Sakakibara K., Ibuki F. Involvement of post-digestion hydrophobic peptides in plasma cholesterol-lowering effect of dietary plant protein. Agri. Bio. Chem. 1986;50:1217–1222. [Google Scholar]
- 29.Jolles P., Levy-Toledano S., Fiat A. M., Soria C., Gillesen D., Thomaidis A., Dunn F. W., Caen J. Analogy between fibrinogen and casein: effect of an undecapeptide isolated from k-casein on platelet function. Eur. J. Biochem. 1986;158:379–382. doi: 10.1111/j.1432-1033.1986.tb09764.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones F. S., Simms H. S. The bacterial growth inhibitor (lactenin) of milk. J. Exp. Med. 1930;51:327–339. doi: 10.1084/jem.51.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitts D. D., Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003;9:1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- 32.Korhonen H., Marnila P. Bovine milk antibodies for protection against microbial human diseases In: Mine Y., Shahidi S., editors. Nutraceutical Proteins and Peptides in Health and Disease. Taylor & Francis Group; Boca Raton, F. L., USA: 2006. pp. 137–159. [Google Scholar]
- 33.Korhonen H., Pihlanto-Leppala A. Milk-derived bioactive peptides: formation and prospects for health promotion In: Shortt C., O’Brien J., editors. Handbook of Functional Dairy Products. CRC Press; Boca Raton, F. L., USA: 2004. pp. 109–124. [Google Scholar]
- 34.Korhonen H., Pihlanto-Leppala A., Rantamaki P., Tupasela T. The functional and biological properties of whey proteins: prospects for the development of functional foods. Agri. Food Sci. Finland. 1998;7:283–296. [Google Scholar]
- 35.Korhonen H., Pihlanto A. Food-derived bioactive peptides - opportunities for designing future foods. Curr. Pharm. Des. 2007a;9:1297–1308. doi: 10.2174/1381612033454892. [DOI] [PubMed] [Google Scholar]
- 36.Korhonen H., Pihlanto A. Bioactive peptides from food proteins In: Hui Y. H., editor. Handbook of food products manufacturing. John Wiley & Sons, Inc.; 2007b. pp. 5–37. [Google Scholar]
- 37.Korhonen H., Pihlanto-Leppala A. Milk-derived bioactive peptides: Formation and prospects for health promotion In: Shortt C., O’Brien J., editors. Handbook of functional dairy products. CRC Press; Boca Raton, F. L., USA: 2004. pp. 109–124. [Google Scholar]
- 38.Krissansen G. W. Emerging health properties of whey proteins and their clinical implications. J. Amer. College Nutr. 2007;26:713S–723S. doi: 10.1080/07315724.2007.10719652. [DOI] [PubMed] [Google Scholar]
- 39.Li G., Le G., Shi Y., Shrestha S. Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004;24:469–486. [Google Scholar]
- 40.Matar C., LeBlanc J. G., Martin L., Perdigon G. Active peptides released in fermented milk: role and functions In: Farnworth ER, editor. Handbook of Fermented Functional Foods. Functional Foods and Nutraceuticals series. CRC Press; Boca Raton, F. L., USA: 2003. pp. 177–201. [Google Scholar]
- 41.Meisel H. Overview on milk protein-derived peptides. Inter. Dairy J. 1998;8:363–373. [Google Scholar]
- 42.Meisel H., FitzGerald R. J. Opioid peptides encrypted in intact milk protein sequences. Br. J. Nutr. 2000;84:27–31. doi: 10.1017/s000711450000221x. [DOI] [PubMed] [Google Scholar]
- 43.Meisel H., FitzGerald R. J. Biofunctional peptides From milk proteins: mineral binding andcytomodulatory effects. Curr. Pharm. Des. 2003;9:1289–1295. doi: 10.2174/1381612033454847. [DOI] [PubMed] [Google Scholar]
- 44.Meisel H., Olieman C. Estimation of calcium-binding constants of casein phosphopeptides by capillary zone electrophoresis. Analytica Chimica Acta. 1998;372:291–297. [Google Scholar]
- 45.Meisel H., Schlimme E. Inhibitors of angiotensin-converting enzyme derived from bovine casein (casokinins) In: Brantl V., Teschemacher H., editors. κ-casomorphins and related peptides: recent developments. VCH; Weinheim: 1994. pp. 27–33. [Google Scholar]
- 46.Meisel H., Schlimme E. Bioacive peptides derived from milk proteins: ingredients for functional foods. Kieler Milchwirtschaftliche Forschungsberichte. 1996;48:343–357. [Google Scholar]
- 47.Nagaoka S., Futamura Y., Miwa K., Takako A., Yamauchi K., Kanamaru Y., Tadashi K., Kuwata T. Identification of novel hypocholesterolemic peptides derived from bovine milk β-lactoglobulin. Biochem. Biophys Res. Commun. 2001;281:11–17. doi: 10.1006/bbrc.2001.4298. [DOI] [PubMed] [Google Scholar]
- 48.Park Y. W. Nutrient profiles of commercial goat milk cheeses manufactured in the United States. J. Dairy Sci. 1990;73:3059–3067. doi: 10.3168/jds.S0022-0302(90)78993-X. [DOI] [PubMed] [Google Scholar]
- 49.Park Y. W. Goat milk - Chemistry and Nutrition In: Park Y. W., Haenlein G. F. W., editors. Handbook of Milk of Non-Bovine Mammals. Blackwell Publishers; Ames, Iowa and Oxford, England: 2006. pp. 34–58. [Google Scholar]
- 50.Park Y. W. Bioactive components of goat milk In: Park Y. W., editor. Bioactive Components in Milk and Dairy Products. Wiley-Blackwell Publishers; Ames, Iowa and Oxford, England: 2009a. pp. 43–82. [Google Scholar]
- 51.Park Y. W. Overview of bioactive components in milk and dairy products In: Park Y. W., editor. Bioactive Components in Milk and Dairy Products. Wiley-Blackwell Publishers; Ames, Iowa and Oxford, England: 2009b. pp. 3–14. [Google Scholar]
- 52.Park Y. W., Juárez M., Ramos M., Haenlein G. F. W. Physicochemical characteristics of goat and sheep milk. Special Issue book on Goat milk and Sheep milk. Small Ruminant Res. J. 2007;68:88–113. [Google Scholar]
- 53.Petrillo E. W., Ondetti M. A. Angiotensin converting enzyme inhibitors: Medicinal chemistry and biological actions. Med. Res. Rev. 1982;2:1–41. doi: 10.1002/med.2610020103. [DOI] [PubMed] [Google Scholar]
- 54.Playne M. J., Bennett L. E., Smithers G. W. Functional dairy foods and ingredients. Australian J. Dairy Technol. 2003;58:242–264. [Google Scholar]
- 55.Qian Z. Y., Jolles P., Migliore-Samour D., Schoentgen F., Fiat A. M. Sheep kappa-casein peptides inhibit platelet aggregation. Biochim Biophys Acta. 1995;1244:411–417. doi: 10.1016/0304-4165(95)00047-f. [DOI] [PubMed] [Google Scholar]
- 56.Regester G. O., Smithers G. W., Mitchell I. R., McIntosh G. H., Dionysius D. A. Bioactive factors in milk: Natural and induced In: Welch R., Burns D., Davis S., Popay A., Prosser C., editors. Milk composition, production and biotechnology. CAB International; 1997. pp. 119–132. [Google Scholar]
- 57.Rival S. G., Boeriu C. G., Wichers H. J. Caseins and casein hydrolysates. 2. Antioxidativeproperties and relevance to lipoxygenase inhibition. J. Agr. Food Chem. 2001;4:295–302. doi: 10.1021/jf0003911. [DOI] [PubMed] [Google Scholar]
- 58.Rokka T., Syvoja E. L., Tuominen J., Korhonen H. Release of bioactive peptides by enzymatic proteolysis of Lactobacillus GG fermented UHT milk. Milchwissenschaft. 1997;52:675–678. [Google Scholar]
- 59.Roy M. K., Watanabe Y., Tamai Y. Induction of apoptosis in HL-60 cells by skimmed milk digested with a proteolytic enzyme from the yeast Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999;88:426–432. doi: 10.1016/s1389-1723(99)80221-7. [DOI] [PubMed] [Google Scholar]
- 60.Schanbacher F. L., Talhouk R. S., Murray F. A., Gherman L. I., Willet L. B. Milk-born bioactive peptides. Int. Dairy J. 1998;8:393–403. [Google Scholar]
- 61.Schlimme E., Meisel H. Bioactive peptides derived from milk proteins. Structural, physiological, and analytical aspects. Die Nahrung. 1995;39:1–20. doi: 10.1002/food.19950390102. [DOI] [PubMed] [Google Scholar]
- 62.Shah N. P. Effects of milk-derived bioactives: an overview. Brit. J. Nutr. 2000;84:S3–S10. doi: 10.1017/s000711450000218x. [DOI] [PubMed] [Google Scholar]
- 63.Stoeck M., Ruegg C., Miescher S., Carrel S., Cox D., Von Fliedner V., Alkan S. Comparison of the immunosuppressive properties of milk growth factor and transforming growth factors beta 1 and beta 2. J. Immunol. 1989;143:3258–3265. [PubMed] [Google Scholar]
- 64.Suetsuna R., Ukeda H., Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000;11:128–131. doi: 10.1016/s0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 65.Tani F., Shiiota A., Chiba H., Yoshikawa M. Saerorphin, and opioid peptide derived from bovine serum albumin In: Brandtl V., Teschemacher H., editors. β-Casomorphins and Related Peptides: Recent Developments. VCH; Weinheim, Germany: 1994. [Google Scholar]
- 66.Théolier J., Fliss I., Jean J., Hammami R. Milk AMP: a comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci. Technol. 2013;94:181–193. [Google Scholar]
- 67.Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 68.Wakabayashi H., Takase M., Tomita M. Lactoferricin derived from milk proteinlactoferrin. Curr. Pharm. Des. 2003;9:1277–1287. doi: 10.2174/1381612033454829. [DOI] [PubMed] [Google Scholar]
- 69.Wilson Md., Rudel L. L. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J. Lipid Res. 1994;35:943–955. [PubMed] [Google Scholar]
- 70.Wu F. Y., Elsasser T. H. Studies on cell growth promoting activity in goat milk. J. Chinese Agric. Chem. Soc. 1995;33:326–332. [Google Scholar]
- 71.Yoshikawa M., Sasaki R., Chiba H. Effect of chemical phosphorylation of bovine casein components on the properties related to casein micelle formation. Agr. Bio. Chem. 1981;45:909–914. [Google Scholar]
- 72.Zhang X., Beynen A. Lowering effect of dietary milk-whey protein v. casein on plasmaand liver cholesterol concentrations in rats. Brit. J. Nutr. 1993;70:139–146. doi: 10.1079/bjn19930111. [DOI] [PubMed] [Google Scholar]