Abstract
The study aimed to evaluate the effect of kefir combination from goat milk and soy milk on lipid profile, plasma glucose, glutathione peroxidase (GPx) activity and the improvement of pancreatic β-cell in diabetic rats. Male rats were divided into five treatments: normal control, diabetic control, goat milk kefir, combination of goat milk-soy milk kefir and soy milk kefir. All rats were induced by streptooztocin-nicotinamide (STZ-NA), except for normal control. After 35 d experiment, the rats were sampled for blood, sacrificed and sampled for pancreatic tissues. Results showed that diabetic rats fed kefir combination had higher (p<0.05) triglyceride than the rats fed goat milk or soy milk kefir. Decreasing of plasma glucose in diabetic rats fed kefir combination was higher (p<0.05) than rats fed goat millk kefir. The activity of GPx in diabetic rats fed three kinds of kefir were higher (p<0.01) than untreated diabetic rats. The average number of Langerhans and β-cells in diabetic rats fed kefir combination was the same as the normal control, but it was higher than diabetic control. It was concluded that kefir combination can be used as antidiabetic through maintaining in serum triglyceride, decreasing in plasma glucose, increasing in GPx activity and improving in pancreatic β-cells.
Keywords: kefir combination, lipid profile, plasma glucose, glutathione peroxidase, β-cells
Introduction
The prevalence and incidence of type 2 diabetes continues to increase in the world, especially in developing countries are much associated with increased in obesity and lifestyle changes. Diabetes mellitus describes a metabolic disorder of multiple aetiology characterize by chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both. Diabetes mellitus may present with characteristic symptoms such as thirst, polyuria, blurring of vision, and weight loss (WHO, 1999). It is classified into types 1 (insulin-dependent) and type 2 (non-insulin dependent). Diabetes mellitus type 1 cases which is primarily due to pancreatic islet-β-cell destruction and is prone ketoacidosis. Type 2 DM is heterogenous disorder characterize by a progressive decline in insulin activity (insulin resistance), followed by the inability of β-cells to compensate for insulin resistance (pancreatic β-cell disfunction) (Srinivasan et al., 2005; WHO, 1999).
Streptozotocin (STZ) can damage pancreatic β-cells, if animals injected with NA after administered STZ will protect β-cell. The mechanism of β-cells damage by STZ through transporting STZ into β-cells via glucose transporter GLUT2, so that causes DNA damage leading to increased activity of poly (ADP-ribose) polymerase (PARP-1) to repair DNA. The excessive activity of this polymerase cause diminution of intracellular NAD(+) and ATP, and finally β-cells will be necrosis. The role of NA through the protective action is by inhibition of PARP-1 activity, so that it prevent diminution of NAD+ and ATP in cells. In addition, NA is also a precursor of NAD that can increase intracellular NAD(+) levels (Szkudelski, 2012).
Type 2 diabetes and dyslipidemia is very clossely related, which is characterized by elevated levels of LDL, triglycerides and decreased HDL levels (Jeppesen et al., 1997). Induced diabetic dyslipidemia is a common character and the most prevalence is on type 2 diabetes that causes cardiovascular disease in the diabetic population (Bertoni et al., 2004). Individually, increased triglycerides and decreased HDL are factors that increase the risk of cardiovascular disease, and the combination of these two factors is as strong as the high levels of LDL cholesterol. Thus as a strong predictor of ischemic heart disease (IHD) is obtained when high LDL levels (Jeppesen et al., 1997).
Strategy for combat the occuring oxidative stress and inflammation in diabetic can be done with pharmacological and functional food therapy. Plants and animal products are a rich source of various functional food, which have health benefits. Kefir is a fermented milk product containing microorganisms such as lactic acid and acetic acid bacteria and also yeast. It is produced and used in Middle Asian countries, Russia and Caucasia for many years. In these countries, kefir has been widely considered as a beverage and a medication for treatment of various illnesses (Kýlýç et al., 1999). Kefir can be produced by cow milk or goat milk and other milk such as soy milk and rice milk. The symbiotic relationship between bacteria and yeast during kefir fermentation produces bioactive compounds that benefit for health.
Soy is a low-cost source of protein that has been consumed in Asian nations for many centuries. This food contains fiber, minerals, and isoflavones (a type of flavonoid), all beneficial nutrients that may contribute to a reduction in chronic disease risk. Regular consumption of soy protein may help to reduce symptoms associated with type 2 diabetes. Soy has been shown to decrease postprandial hyperglycemia, to improve glucose tolerance, and to decrease amounts of glycosylated hemoglobin (Heneman et al., 2007).
So far, some people avoid consumption of goat milk because it is considered a negative effect on health. This is due to the fact physiological and biochemical uniqueness of goat milk quality are just barely known and little exploited (Haenlein, 2004). Therefore it is necessary to prove that the goat milk and its products are healthy. In addition, production of goat milk is lower than cow milk causing it expensive, whereas soy milk is another high-quality source of protein with a cheap in price, so that it can be used as replacer of goat milk partially in kefir processing. Thus, the potency of goat milk kefir subtituted with soy milk as antidiabetic need to be evaluated.
Maintenance integrity of pancreatic β-cells is important in diabetic management, which is strongly influenced by diet. Therefore, it needs a proper strategy through the consumption of functional foods which has potentially maintain the β-cells. The purposes of this study were to investigate the effect of kefir combination from goat milk and soy milk on lipid profile, blood glucose levels, glutathione peroxidase activity and on improving of pancreatic β-cells in STZ-NA- induced diabetic rats.
Materials and Methods
Soy milk preparation
Preparation of soy milk according to Kasenkas et al. (2011) with slight modifications. Briefly, whole soybeans were washed and soaked overnight in distilled water. After decanting the water, the soaked soybeans were mixed with 3 times their weight of distilled water for 3 min, blended and filtered.
Kefir preparation
Goat milk and soy milk were pasteurized for 10 min at 90℃. The pasteurized milk were devided into 5 groups of kefir combination from goat and soy milk (100:0; 75:25; 50:50; 25:75 and 0:100) and cooled at room temperature. Kefir grain was inoculated into pasteurized milk as much as 2% and incubated at room temperature for 18 h (Kesenkas et al., 2011). After separating the grain and stirring, kefir samples were analyzed for antioxidant activity.
Antioxidant activity (as DPPH scavenging)
The effect of kefirs upon 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radicals was estimated according to Liu et al. (2005a). The kefir sample (200 mg) was diluted with 5 mL methanol, mixed well and centrifuged. Methanol (3.0 mL) was added into 1.0 mL supernatant and 1.0 mL of 0.16 mM DPPH. The mixture was shaken vigorously and left to stand for 30 min in the dark, and the absorbance was measured at 517 nm.
The capability of the test material to scavenge DPPH radicals was calculated as (%) = [1 − (absorbance of the sample at 517 nm) / (absorbance of the control at 517 nm] × 100
Animal experimental
Male Wistar rats 8-12 wk old were individually cage and housed. During 7 d the rats were fed unrestricted amounts of a standard laboratory diet AIN-93G (Reeves et al., 1993) and were then randomly assigned into five groups (n=6 per group): 1) negative control (normal rats); 2) positive control (diabetic control); 3) diabetic rats fed goat milk kefir; 4) diabetic rats fed kefir from goat milk and soy milk; and 5) diabetic rats fed soy milk kefir. In group 4, kefir was prepared from combination of goat milk and soy milk 1:1. For rat model of type 2 diabetes was done by induction with streptozotocin-nicotinamide (STZ-NA) (Ghasemi et al., 2014). Diabetic condition was prepared by injection with streptozotocin (STZ) of 60 mg/kg body weight, i.p, and after 15 min the rats were injected with nicotinamide (NA) of 120 mg/kg body weight, i.p (prepared in fresh in 0.1 M citrate buffer pH 6.3). The rats had diabetic, if 7 d after induction, the fasting blood glucose was more than 126 mg/dL (Ghasemi et al., 2014). The dose of goat milk kefir was 2 ml/200 g body weight/day orally, for 35 d experiment with force feeding. In negative control, the rats were given 2.0 ml phosphate-buffered saline (PBS). Blood was sampled before and after treatment for analysis of lipid profiles, plasma glucose level and GPx activity. Furthermore, the rats were sacrificed by being given ketamine (80 mg/kg) anesthesia, and the pancreatic tissues were sampled for histochemical analysis. All procedures related to animal experiment in this study were approved by Medical and Health Research Ethics Committee (MHREC), Faculty of Medicine Universitas Gadjah Mada, Indonesia (Approval Number: KE/FK/907/EC).
Lipid profile analysis
Lipid profile of blood serum in rats were analyzed by enzymatic-photometric methods using Kit from DiaSys (Diagnostic Systems GmbH & Co) by Photometer (Merck-Microlab 300) at 540 nm. Total cholesterol and HDL cholesterol were measured by cholesterol oxidase-peroxidase-4-aminophenazone (CHOD-PAP) method. Triglyceride was measured by glycerol-3-phosphate oxidase, peroxidase and chromogenic reaction with 4-aminophenazone (GPO-PAP) method. HDL cholesterol was determinated after lipoprotein precipitation with phosphotungstic acid and magnesium chloride, whereas LDL were calculated by formula (Domanic et al., 2006; Nikam et al., 2013).
Blood glucose analysis
Determination of glucose in plasma was done by enzymatic photometric test using Glucose Oxidase Phenol 4-Aminoantipyrine Peroxidase (GPO-PAP) method according to instructions in Kit (Dia Sys, Holzheim, Germany). Glucose is oxidized enzymatically by glucose oxidase. The colorimetric indicator is quinoneimine, which is generated from 4-aminoantipyrine and phenol by hydrogen peroxide under the catalytic action of peroxidase.
Analysis of glutathione peroxidase activity
GPx converts reduced glutathione (GSH) to oxidized glutathione (GSSG), while reducing lipid hydroperoxides to their corresponding alcohols or free hydrogen peroxide to water. GPx activity was assayed according to instructions in BioVision’s Glutathione Peroxidase Activity Assay (Catalog 762-100, Bio Vision, USA). GPx reduced Cumene Hydroperoxide while oxidizing GSH to GSSG. The generated GSSG is reduced to GSH with consumption of NADPH by Glutathion Reductase (GR). The decrease of NADPH (measured spectrophotometrically at 340 nm) is proportional to GPx activity.
Langerhans and β-cells number
Pancreatic tissues were fixed in Bouin’s fluid for 24 h, washed in 70% alcohol and embeded in paraffin. This tissues were cut of about 5 μm. Paraffin sections were putted into water and then into mordant for 24-72 h at 37℃. Sections were washed thoroughly under running tapwater until colorless. Sections were placed in the oxidation mixture for 3-5 min and washed in water. Sections were placed in 2-5% aqueous solution of sodium bisulphate for 1 min and then were washed in water. Sections were dipped in 70% alcohol for 1 min, and then were stained in staining solution at room temperature. Only beta cells stain a deep blue while other cell types remain colorless. Excess solution was washed in water. Sections were stained in 0.5% aqueous solution of phloxine for 30-120 s, and then were washed in distilled water for 1 min. Sections were mounted and observed under microscope (Kikui et al., 1977).
Statistical analysis
All the data from this study were expressed as mean±standard deviation. Data of lipids profile in blood serum pre-test, post-test and delta (the mean value of difference between pre-test and post-test) include: triglyceride, total cholesterol, LDL and HDL. One way ANOVA followed by Duncan’s Multiple Range Test (DMRT) were used for statistical analyses, and p-values of less than 0.05 (p<0.05) indicated significant differences. The difference between the mean of lipids profile in pre-test and post-test were analyzed by Paired-Samples t-Test. Statistical analyses were performed by using the SPSS version 17 Software (SPSS, 2007).
Results and Discussion
Antioxidant activity of kefir
The antioxidant properties of kefir in this study was measured in DPPH radical scavenging activity. Based on Table 1, the average scavenging activity of kefir made from goat milk and soy milk (75:25) was lower than kefir made from goat milk and soy milk (25:75). Increasing the percentage of soy milk in kefir preparation tended to increase scavenging activity. The results were consistent with a previous study by Kasenkas et al. (2011), that superoxide scavenging activity increased sharply in kefir containing at least 50% soy milk.Thus, the high superoxide anion scavenging activity in kefir samples indicated from the isoflavones present in soy milk.
Table 1. The average of antioxidant actvity (DPPH-scavenging) of milk and kefir.
| Milk and kefir | DPPH scavenging (%) |
|---|---|
| Goat milk | 1.01±1.75a |
| Soy milk | 10.71±1.13d |
| Goat milk Kefir | 5.52±0.99bc |
| Goat milk : soy milk (75:25) kefir | 3.70±2.33ab |
| Goat milk : soy milk (50:50) kefir | 6.67±2.33bc |
| Goat milk : soy milk (25:75) kefir | 7.74±3.29cd |
| Soy milk Kefir | 8.08±1.23cd |
Different letters (a, b) in the column indicated significant difference (p<0.01).
Goat milk as a raw material of kefir had lower scavenging activity than soy milk. After fermentation into kefir, scavenging activity increased significantly. This might be due to the formation of bioactive compounds during fermentation such as antioxidative substances. Results in the present study were similar to a previous study by Liu et al. (2005b), that the cow milk fermented with kefir grain showed an increase in DPPH radical scavenging activity than before being fermented. This indicates that the kefir had a greater ability to donate protons than milk. Therefore, kefir might protect against free radical proton. Radical scavenging protons are known to be an important mechanism for antioxidants.
Body weight of experimental rats
The average of body weight in the rats at the beginning of the experiment (before treatment) and at the end of the experiment (after treatment) was shown in Table 2. The statistical changes in body weight (p<0.01) during the experiment were observed in negative control group compared to other treatment groups. The mean body weight increased by 28.25% in negative control group. In goat milk kefir and combination of goat milk-soy milk kefir groups, the mean body weight of rats increased by 6.72% and 4.27%, respectively, even though they were not significant. However, the mean body weight of rats decreased by 6.32% in positive control group and by 11.64% in soy milk kefir group.
Table 2. The average body weight of rats with different treatment.
| Perlakuan | Body weight (g) |
|
|---|---|---|
| Pre- treatment | Post- treatment | |
| Normal control | 279.60±18.66a | 358.60±13.97b |
| Diabetic control | 212.00±41.76a | 198.60±75.20a |
| Diabetic + goat milk kefir | 217.00±33.56a | 231.60±78.19a |
| Diabetic + goat-soy milk kefir | 257.20±33.60a | 268.20±40.11a |
| Diabetic + soy milk kefir | 221.60±47.69a | 195.80±86.25a |
Different letters (a, b) in the same row indicated significant difference (p<0.05).
The decreased body weight of rats was due to the STZ induction which lead to hyperglycemia. In the present study, the rats were induced with STZ at a dose of 60 mg/kg and NA at a dose of 120 mg/kg to induce type 2 DM. Similar with the previous study by Zafar and Naqvi (2010), that showed an association between hyperglycemia and decreased body weight of diabetic animals. According to the study, it was demonstrated that STZ was effective in producing severe hyperglycaemia in experimental animals. The STZ through its direct alkylating action could cause cellular necrosis andselective destruction of the β-cells producing hyperglycaemia at a dose of 45 mg/kg body weight. It may also be stated that STZ by producing diabetes (hyperglycemia) and hypoinsulinemia caused reduction in the body weight of diabetic animals.
Triglyceride
Diabetic rats fed a combination of goat milk and soy milk kefir with a 50:50 proportion had higher triglyceride levels than untreated diabetic rats, but there was no difference with rats fed goat milk kefir and soy milk kefir. This suggests that the breakdown of triglycerides in rats fed kefir combination less than untreated diabetic rats (positive control). Possibility of a synergistic effect between the bioactive components of goat milk and soy milk kefir could improve pancreatic beta cell damage caused by free radicals. It is known that milk contains proteins and bioactive peptides can act as immunomodulators and antioxidants (Zimecki and Kruzel, 2000). STZ in this study proved to decrease triglyceride levels in diabetic rats, so that it causes serum triglycerides decreased significantly compared to normal rats (Table 3). STZ known selective for pancreatic beta cell cytotoxicity and has been used extensively to induce type 1 diabetes in animals. However, to induce diabetes mellitus type 2, nicotinamide was injected into rats intraperitoneally to enable poly ADP ribose synthase for repair DNA damage by STZ (Oyedemi et al., 2012). Hyperglycemic conditions caused damage to pancreatic beta cells is thought to cause a deposit of triglycerides are degraded to energy for the cell, so that the triglycerides in the serum also decreased.
Table 3. Lipids profile in rats with different treatments.
| Lipid | Treatment |
||||
|---|---|---|---|---|---|
| Normal control | Diabetic control | Diabetic+Goat milk kefir | Diabetic+Goat-soy milk kefir | Diabetic+Soy milk kefir | |
| Triglyceride (mg/dL) | |||||
| Pre-test | 112.98±54.49a | 70.74±27.73a | 75.33±35.93a | 67.21±25.37a | 98.96±83.84a |
| Post-test | 174.97±7.51a | 50.80±21.78b | 66.34±37.64bc | 89.43±28.74c | 63.08±30.97bc |
| Delta* | 61.98±38.49a | −19.94±30.33b | −8.98±24.13b | 22.21±32.37ab | −35.88±67.02b |
| p pre-post** | 0.011 | 0.216 | 0.678 | 0.154 | 0.345 |
| Total Cholesterol (mg/dL) | |||||
| Pre-test | 49.25±12.09a | 57.46±12.87a | 62.61±21.01a | 43.93±6.36a | 49.58±14.37a |
| Post-test | 45.0±17.37a | 44.96±17.29ab | 61.66±11.30b | 51.00±2.90ab | 50.36±15.16ab |
| Delta* | −4.23±11.46ab | −12.50±12.26a | −0.94±27.67ab | 7.06±5.27b | 0.78±10.07ab |
| p pre-post** | 0.463 | 0.085 | 0.859 | 0.028 | 0.600 |
| LDL (mg/dL) | |||||
| Pre-test | 15.68±8.36a | 22.92±5.88ab | 25.85±8.48b | 19.70±8.69ab | 17.66±4.07ab |
| Post-test | 13.91±4.04a | 18.20±6.22ab | 23.13±8.43b | 20.23±7.71ab | 20.46±5.21ab |
| Delta* | −1.76±8.41a | −4.72±6.89a | −2.72±12.87a | 0.53±13.44a | 2.80±1.90a |
| p pre-post** | 0.629 | 0.225 | 0.544 | 0.926 | 0.016 |
| HDL (mg/dL) | |||||
| Pre-test | 37.50±8.88a | 37.48±9.37a | 38.04±10.16a | 34.01±4.57a | 34.06±16.68a |
| Post-test | 22.70±6.54a | 28.70±7.71ab | 43.64±21.17b | 25.95±2.31ab | 32.45±14.07ab |
| Delta* | −14.80±10.57a | −8.78±12.82a | 5.60±26.05a | −8.06±6.08a | −1.61±25.37a |
| p pre-post** | 0.016 | 0.200 | 0.678 | 0.023 | 0.882 |
Different letters (a, b) in the same row indicated significant difference (p<0.05).
*Delta value (difference mean between pre test-post test): positive number indicated an increase, and negative indicated a decrease.
**p<0.05 in each column indicated significant difference between pre and post-test.
Total Cholesterol
Feeding three types of kefir could not increase the total cholesterol in diabetic rats, but there was a tendency that diabetic rats fed goat milk kefir have higher cholesterol levels, even though statistically not significant (Table 3). When compared with normal rats, levels of total cholesterol in rats fed goat milk kefir was higher, although it is still below the normal range of cholesterol levels (around 100-200 mg/dL) (Poonuru et al., 2011) and still in accordance with the recommendation by Rush University Medical Center, Chiago, Illinois which it is below 200 mg/dL. The tendency of increasing cholesterol levels in diabetic rats fed goat milk kefir, might be due to goat milk contains casein which more hypercholesterolemic. Essential amino acids in casein is more effective in increasing total cholesterol than non-essential amino acids (Kurowska and Carroll, 1992). Lysine and methionine more hypercholesterolemic, while the arginine has neutralizing effect of hypercholestrolemic of other amino acid (Kurowska and Carroll, 1994). Methionine and lysine contents of goat milk were higher than soy milk (Nurliyani et al., 2014), thus the total cholesterol in diabetic rats fed goat milk kefir was higher than diabetic rats fed soy milk kefir, although there were no significant differences (Table 3).
LDL and HDL
Three types of kefir given to diabetic rats had no effect on LDL cholesterol, but the diabetic rats fed goat milk kefir have higher LDL cholesterol levels compared to normal rats (Table 3). This result was similar to a previous study by Roselino et al. (2012) that administration of synbiotic product had no effect on non-HDL fraction in diabetic rats. However, diabetic rats fed synbiotic product had higher level of non-HDL fraction compared to normal rats.
Increased HDL levels in diabetic rats fed goat milk kefir by 52%, whereas in rats fed soy milk kefir increased of HDL only 13%, and in rats fed kefir combination it decreased by 9.58%. The results were consistent with a previous studies by Roselino et al. (2012) that diabetic rats fed synbiotic product from soy milk yacon extract, showed an increase HDL levels by 23.70%. Increased HDL in this study was higher than the results according to Roselino et al. (2012). This difference was caused by the fermented products were used, as in this study used goat milk kefir which different chemical and microbial composition from fermented soy milk and yacon extract (Smallanthus sonchifolius). In fermented soy milk and yacon using lactic acid bacteria, whereas fermentation in this study using kefir grain containing lactic acid bacteria and yeast. In fact goat milk kefir had positive effect on increasing serum HDL in diabetic rats compared with normal rats without kefir. It is possible that bioactive components in goat milk kefir were superior compared to soy milk kefir. According to Jascolka et al. (2013), ApoE-deficient mice also had increased HDL levels after treated with kefir filtrate prepared by fermentation of kefir grain in brown sugar solutions for 4 wk. Other previous study by Liu et al. (2006) also showed that the group of hamsters fed cow milk kefir and soy milk kefir have higher HDL levels than the group fed milk only.
Blood glucose levels
The present study demonstrated that three groups of kefir could decrease the plasma glucose level in diabetic rats. Combination of goat milk-soy milk kefir and soy milk kefir provided higher (p<0.05) levels in decreasing plasma glucose than the goat milk kefir in diabetic rats. Decreasing plasma glucose in combination of goat milk-soy milk kefir was higher than soy milk kefir group. Nevertheless, it was not significant (Table 4). This indicated the presence of a synergistic effect of the bioactive components from goat milk and soy milk in kefir, which could reduce the plasma glucose levels. This could be compared with a previous study by Hadisaputro et al. (2012), that the decreasing plasma glucosein diabetic rats fed water kefir was lower (111.00 mg/dL) than the combination of goat milk-soy milk kefir in the present study. Although this present study used a lower dose (2.0 mL/200 g body weight) of kefir, but it was more able to lower glucose more because of the synergistic effect. While a previous study, Hadisaputro et al. (2012) used water kefir only, with larger doses (3.6 mL/200 g body weight) without the addition of other materials. Other previous study (Alsayadi et al., 2014) also reported that consumption of water kefir for 5 wk had shown beneficial effect not only on blood glucose but also on body weight and lipid profiles of STZ-induced diabetic rats.
Table 4. The average of plasma glucose levels of rats with different treatment.
| Treatment groups | Plasma glucose (mg/dL) |
||
|---|---|---|---|
| Pre-treatment | Post-treatment | Delta pre-post | |
| Normal control | 73.67±12.77 | 116.86±8.85 | 43.18±4.95 |
| Diabetic control | 283.11±140.01 | 379.72±83.97 | 96.61±111.51a |
| Diabetic + goat milk kefir | 220.06±97.50 | 127.70±46.06 | −92.36±116.05a |
| Diabetic + goat-soy milk kefir | 338.41±41.74 | 112.46±11.11 | −225.95±36.71b |
| Diabetic + soy milk kefir | 391.24±146.57 | 257.38±175.16 | −133.85±172.37b |
Different letters (a, b) in the same column indicated significant difference (p<0.05).
The bioactive components from milk kefir included peptides and exopolysaccharides (EPSs), whereas bioactive components from soy milk was isoflavon. A majority of the kefir peptides was not endogenously present in the raw material milk, but they were released from milk caseins by proteases of the microbiota and were therefore specific for the product. Sixteen of the newly peptides including antioxidant and immunomodulator had been identified in kefir (Ebner et al., 2015).
Related to the immune system, EPSs acted directly or indirectly. One mechanism by which EPSs could indirectly modulate immune responses was by contributing to production of short chain fatty acids such as butyrate. One mechanism by which EPSs could indirectly modulate immune responses was by contributing to production of short chain fatty acidssuch as butyrate. Bacterial EPSs could also be scavenged and fermented by competing microbes, thereby indirectly modulating host immune responses. EPSs from Bacteroides fragilis, Bacillis subtilis, Lactobacillus kefiranofaciens, and Bifidobacterium breve could alter the function of several immune cells, including T cells, macrophages, B cells, and dendritic cells (Jones et al., 2014). The EPS induced a gut mucosal response and it was able to up and down regulate it for protective immunity, maintaining intestinal homeostasis, enhancing the IgA production at both the small and large intestine level and influencing the systemic immunity through the cytokines released to the circulating blood (Vinderola et al., 2006).
Exopolysaccharides also have been proved to have antioxidant and free radical scavenging properties. The free radical scavenging property of EPSs can also be used to inhibit oxidation of vegetable oils (Madhuri and Prabhakar, 2014). The antioxidant activity of the EPS fraction showed a correlation with the molecular properties. It might be attributed to the functional groups in the EPS fraction, which could donate electrons to reduce the radicals to a more stable form or react with the free radicals to terminate the radical chain reaction (Mao et al., 2014). In the present study (Table 1), the higher the proportion of soy milk in kefir, the higher antioxidant activity. Thus a combination of kefir with soy milk would increase the antioxidant activity which could ultimately lower the blood glucose greater than that was goat milk kefir without soy milk.
Glutathione peroxidase activity
Glutathion peroxidase activity in diabetic control rats were lower (p<0.01) than in diabetic rats fed three kinds of kefir (Table 5). Consecutively, the diabetic rats fed soy milk kefir, combination of goat milk-soy milk kefir, and goat milk kefir, had GPx activity from low to high. However, these GPx activity in rats fed these kefir were not significantly different. Goat milk kefir could increase more GPx activity compared to kefir combination from goat milk-soy milk and soy milk kefir.
Table 5. The average of glutathione peroxidase (GPx) in rats with different treatment.
| Treatment groups | GPx activity (nmol/mL/min) |
|---|---|
| Normal control | 0.133±0.00a |
| Diabetic control | 0.013±0.00b |
| Diabetic + goat milk kefir | 0.120±0.01a |
| Diabetic + goat-soy milk kefir | 0.116±0.02a |
| Diabetic + soy milk kefir | 0.094±0.03a |
Different letters (a, b) in the column indicated significant difference (p<0.01).
In a previous study by Hadisaputro et al. (2012), water kefir (fed 30 d) could increase GPx in STZ-induced diabetic rats. Result study by Omayma et al. (2013), showed that fermented soy milk administration was able to normalize SOD and GPx activities which reduced in the rat tumor tissue. The presence of soy isoflavone, saponin and Lactobacillus sp. which exerts potent antioxidant activity and free radical scavenging capability, exerting its activity via several anti-oxidative mechanisms: catalase, glutathione-system-related compounds and Mn-SOD, decreasing the risk of ROS accumulation also degrade the superoxide anion and hydrogen peroxide. Cells have developed a sophisticated antioxidant enzyme defense system such as SODs, GPx and CATs, where SODs convert superoxide radical (O2‧) into hydrogen peroxide (H2O2), GPx and CATs convert H2O2 into water. Unlike SOD and CAT, GPx requires several secondary enzymes (GR and G-6-PDH) and cofactors (GSH, NADPH, and glucose 6 phosphate) to function at high efficiency (Li et al., 2000).
The supply of either whey proteins or β-lactoglobulin resulted in an increase in liver GSH and prevented iron-mediated lipoprotein peroxidation. These protein effects were reproduced in rats orally administered with either GSH or its precursor, gamma-glutamylcysteine (Zommara et al., 1998). Glutathione is a tripeptide thiol which plays an important role in the stability of lysosomal and other cell membranes, and in the protection of cells from the effects of radiation and oxygen radicals. Glutathione, therefore, is crucial to the functional state or activation of many cells including both T and B lymphocytes. Unlike other edible animal and plant proteins, whey protein has substantial amounts of glutamylcysteine groups which supply the amino acid precursors necessary for the formation of glutathione, and may therefore be responsible for the immunoenhancing effect (Wong and Watson, 1995).
A previous study showed that administration of dahi containing probiotics Lactobacillus acidophilus and Lactobacillus casei in STZ-induced rats inhibited decreasing GSH level significantly. This may be due to the increasing GSH biosynthesis or decreasing oxidative stress leading to reduced GSH degradation. In addition, fermented milk containing various antioxidant compounds increases the pool of substrat for biosynthesis of GSH (Yadav et al., 2008).
Antioxidative effects ascribed to isoflavones may be partially exerted via enhancement of antioxidant enzyme activities (Yoon and Park, 2014). This effect might be due to the direct scavenging activity of the genistein phenolic ring, activation of antioxidant defense gene transcription, and modulation of ROS producing enzyme expression (Yoon and Park, 2014). However, in this study have demonstrated that an increase in GPx activity in diabetic rats was more influenced by goat milk components in kefir.
GSH has been known as cofactor for GPx (Li et al., 2000), while whey protein was precursor for GSH (Wong and Watson 1995). In the present study, goat milk kefir contained more whey protein compared to combination of goat milk-soy milk kefir or soy milk kefir. Thus, diabetic rats received goat milk kefir had higher GPx activity than the rats fed soy milk kefir or combination of goat milk-soy milk kefir, although the differences were not significant. Probably, to increase GPx activity significantly in diabetic rats is required more whey component in kefir.
Number of Langerhans islet and β-cell
The average number of Langerhans islet of diabetic rats fed combination of goat milk-soy milk kefir was higher than goat milk kefir or soy milk kefir group, even though statistically they were not significant. However, the diabetic rats fed combination of goat milk-soy milk kefir had higher (p<0.05) number of Langerhans islet compared to the untreated diabetric rats (positive control) (Table 6). The lower of Langerhans islet in untreated diabetic rats was due to the acute hyperglycemia by STZ. This condition would increase free fatty acids, insulin resistance, and also reduced of the organ of Langerhans and pancreatic β-cell volume by 60% (ADA, 2008).
Table 6. The average number of Langerhans and β-cells in rats with different treatment.
| Treatment groups | Langerhans | Beta cells |
|---|---|---|
| Normal control | 2.60±0.32a | 61.29±16.26a |
| Diabetic control | 1.16±0.28b | 2.58 ±1.23b |
| Diabetic + goat milk kefir | 2.27±1.05ab | 15.03±7.70bc |
| Diabetic + goat-soy milk kefir | 3.23±0.53a | 66.55±20.69a |
| Diabetic + soy milk kefir | 3.10±1.45a | 29.73±28.07c |
Different letter (a, b, c) in the same column indicate significant difference, for Langerhans (p<0.05), and for β-cells (p<0.01).
Kefir prepared from combination of goat milk and soy milk could increase more number of β-cells than goat milk kefir or soy milk kefir in diabetic rats. The number of β-cells in diabetic rats fed kefir combination was similar to the normal rats (negative control), whereas the number of β-cells in diabetic rats fed goat milk kefir was similar to untreated diabetic rats (Table 6).
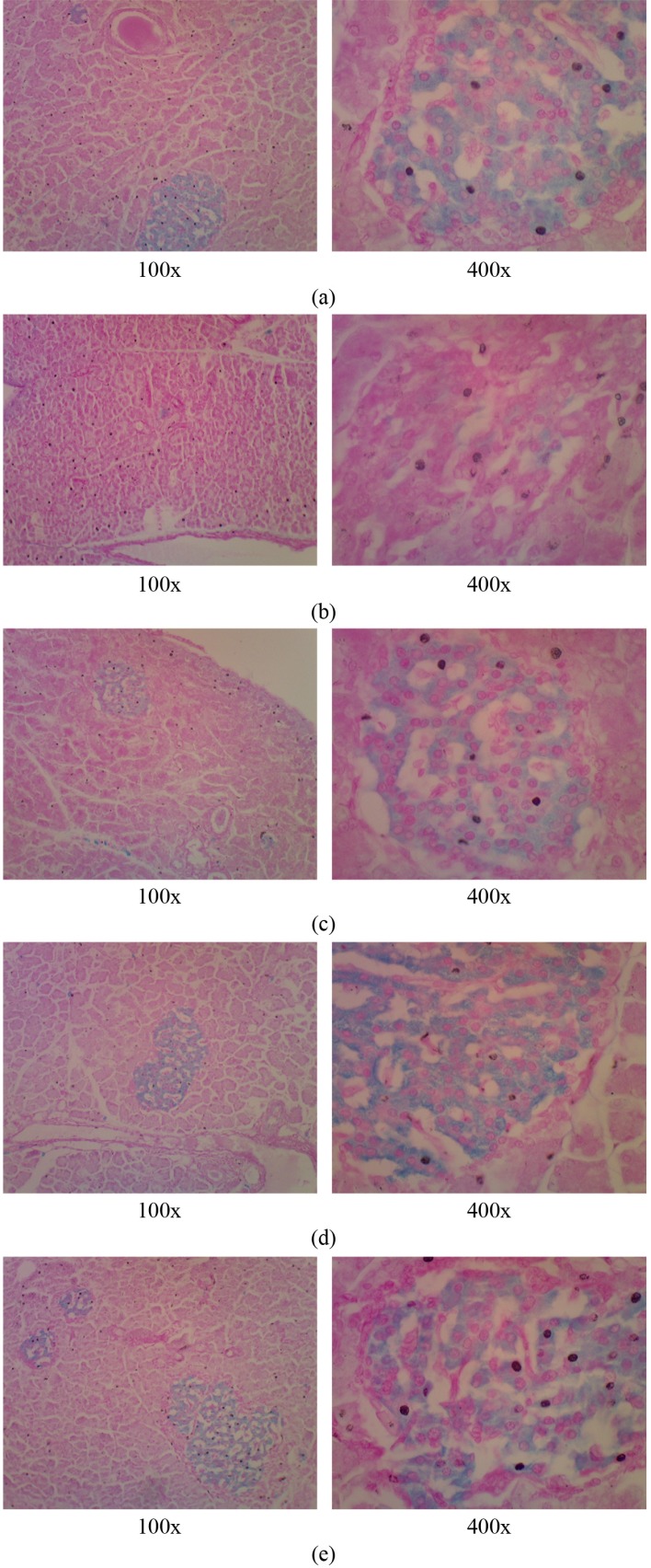
By using Victoria-blue staining, we observed the morphology of pancreatic β-cells (Fig. 1). Normal rats showed strongly stained by Victoria blue, so that it was too easy to find Langerhans islets and β-cells (Fig. 1A). There was no induction of STZ in the normal rats, so there was no change in morphological of pancreatic β-cells. Different from untreated diabetic rats, it was very difficult to find Langerhans islets and β-cells, because the cell density had been changed and damaged in β-cells. Thus, no blue colour (very rarely found) in Victoria-blue staining which was specific for beta cells cytoplasm. This result was similar to a previous study using Victoria-blue and H & E staining, that untreated diabetic rats showed shrunken islets of Langerhans displaying degenerative and necrotic changes, and decreased cellular density. The nucleus of necrotic cells indicated either pyknosis or marginal hyperchromasia. There was mostly hydropic degeneration and degranulation in the cytoplasm of the degenerative and necrotic cells, while some of the cells with a pyknotic nucleus had a dark eosinophilic cytoplasm (Kanter et al., 2004; Nugroho et al., 2014).
Fig. 1. Victoria-blue stained sections for pancreatic β-cells (showed blue-stained in cytoplasm). A) normal rats; B) untreated diabetic rats; C, D & E) diabetic rats treated with goat milk kefir, combination of goat milk-soy milk kefir and soy milk kefir, respectively. Magnification 100 and 400x.
High number of β- cells in rats fed combination of goat milk-soy milk kefir compared to soy milk or goat milk kefir showed a synergistic effect of bioactive components from goat milk and soy milk in the kefir. It is understandable that in combination of kefir fermentation can produce bioactive peptides that majority release from casein by microbial protease and exospolisaccharides (Ebner et al., 2015), which can act as an antioxidant and immunomodulator (Ebner et al., 2015; Jones et al., 2014; Madhuri and Prabhakar, 2014). In addition, isoflavones in kefir combination derived from soy milk also has a high antioxidant activity. Thus, to regenerate β-cells damaged by free radicals may be required combination of bioactive components from kefir.
It was seen on Table 1, there were no differences in antioxidant activity between goat milk kefir, kefir combination from goat milk-soy milk, and soy milk kefir. However, kefir combination showed better than soy milk kefir or goat milk kefir in improving β-cells (Table 6). This indicated that combination of bioactive component in kefir made from goat milk and soy milk played an important role in the repair of β-cells. It is known that diabetes mellitus is characterized by hyperglycemia results in generation of free radical that can reduce antioxidant defences thus leading to the disruption of cellular functions, oxidative damage to membranes, and increased to lipid peroxidation (ADA, 2004; Sharma et al., 2010). Free radicals have an unpaired electron, which makes them very reactive (Isfahlan et al., 2010). DPPH is often used to determine the antioxidant activity is a stable free radical that accepts an electron or hydrogen radical and becomes a stable diamagnetic molecule (Loo et al., 2007), and principally the antioxdant compounds are able to donate the hydrogen atom to radicals (Isfahlan et al., 2010). According to Bolanho and Beleia (2011), the antioxidant activity correlated with total phenolics and avonoids. While, soy milk rich in phenolic compounds such as phenolic acid (hydroxybenzoic acids, hydroxycinnamic acid), flavonoids (flavones, flavonols, flavanols), and isoflavones: glucosides (daidzin, genistin, glycitin), aglycones (daidzein, genistein, glycitein) (Rodríguez-Roque et al., 2013). Phenolic compounds are a high potent antioxidant due to the hydroxyl groups in phenolic structure that able to chelate metals, inhibit lipoxygenase and scavenge free radicals such as DPPH and hydroxyl radicals, thus they act as free radical terminators (Rani and Pradeep, 2015; Yin et al., 2008). The DPPH radical scavenging activity of the fermented soy milk extracts with Lactobacillus paracasei showed effective than that of standard ascorbic acid (Rani and Pradeep, 2015). In addition, due to their phenolic structure, is known to be involved in the healing process of free radical-mediated diseases, including diabetes. In a previous study that ethanol and aqueous extracts of Stephania hernandifolia were found have a strong antioxidant activity, due to the presence of phenols and flavonoids, which may have a major role in reducing oxidative stress associated with diabetes (Sharma et al., 2010).
For the improvement in β-cells, not only due to the antioxidant effect but also due to the immunomodulator of the milk was very important, since inflammation linked to β-cells damage. This inflammation was caused by proinflammatory cytokines secreted by immune cells that infiltrated the pancreas as mediator β-cells destruction. This cytokines were inducted by mitochondrial stress (Cerf, 2013).
In a previous study, plain kefir can reduce pro-inflammatory cytokines tumor necrosis factor α (TNF-α) in diabetic rats (Hadisaputro et al., 2012), so that it contribute to the improvement of β-cell damage by free radicals. Synergistic effect has also been reported by Huang et al. (2013), that the therapeutic effects L.plantarum K68 isolated from Taiwanese traditional food fu-tsai and FVF products, were due to increased antioxidant status and decreased proinflammatory cytokines. The increasing the antioxidant status and decreasing proinflammatory cytokine were as a result from synergistic effect between L. plantarum and FVF product that promising antidiabetic, anti-inflammatory, and antioxidant in high fat-fructose-fed rats
Soy milk kefir may also contribute to improve β-cells, through the inhibitory effect on pro-inflammatory cytokines such as reported by Liao et al. (2010), that TNF-, interleukin (IL)-6, IL-1 and prostaglandin E2 (PGE2) produced by RAW 264.7 macrophages induced by LPS could be inhibited by fermented soy milk.
In conclusion, the antidiabetic effect of kefir could be enhanced by a combination of goat milk and soy milk (1:1) in kefir fermentation due to the synergistic effect between goat milk and soy milk compounds. Kefir combination from goat milk and soy milk, had no negative effect on the lipid profile of blood serum in diabetic rats, even goat milk kefir could increase the highest percentage in HDL by 52% in diabetic rats compared to normal rats. The kefir combination also could maintain triglyceride level in diabetic rats. Specifically, to increase GPx activity in diabetic rats is required more level component from goat milk kefir. Furthermore, the role of pancreatic β-cell was important in type 2 diabetic management, which histologically in Victoria-blue staining was already demonstrated in this study. An increase number of β-cells was clearly showed in diabetic rats treated with this kefir combination. The increasing activity of antioxidant enzymes such as GPx will improve the damage of β-cell. Overall the kefir combination prepared from goat milk and soy milk as antidiabetic was through maintaining in triglyceride level, decreasing in plasma glucose levels, increasing in GPx activity and improving in pancreatic β-cells. Thus, the kefir has the potential to be used as a functional food for type 2 diabetes therapy.
Acknowledgments
The authors thank Prof. Soeparno, Ph.D for reading the manuscript. This research was supported by a grant from “Peneltian Strategis Nasional”, Directorate of General Higher Education, The Ministry of Education and Culture, The Republic of Indonesia (Number: 089/SP2H/PL/DIT.LITABMAS/V/2013, 13 Mei 2013).
References
- 1.ADA (American Diabetes Association) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.ADA (American Diabetes Association) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:S55–S60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 3.Alsayadi M., Al- Jawfi Y., Belarbi M., Soualem-Mami Z., Merzouk H., Sari D. C., Sabri F., Ghalim M. Evaluation of anti-hyperglycemic and anti-hyperlipidemic activities of water kefir as probiotic on streptozotocin-induced diabetic wistar rats. J. Diabetes Mellitus. 2014;4:85–95. [Google Scholar]
- 4.Bertoni A. G., Hundley W. G., Massing M. W., Bonds D. E., Burke G. L., Goff D. C. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 5.Bolanho B. C., Beléia A. D. P. Bioactive compounds and antioxidant potential of soy products. Alim. Nutr. 2011;22:539–546. [Google Scholar]
- 6.Cerf M. E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013;4:1–12. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domaniç N., Gelisgen R., Civelek S., Demir A. S., Ural D., Andican G. G., Vural V. A., Burçak G. Homocysteine and nitric oxide in patients undergoing diagnostic coronary angiography. Acta Med. Okayama. 2006;60:35–41. doi: 10.18926/AMO/30756. [DOI] [PubMed] [Google Scholar]
- 8.Ebner J., Arslan A. A., Fedorova M., Hoffmann R., Küçükçetin A., Pischetsrieder M. Peptide profiling of bovine kefir reveals 236 unique peptides released from caseins during its production by starter culture or kefir grains. J. Proteomics. 2015;18:41–57. doi: 10.1016/j.jprot.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Ghasemi A., Khalifi S., Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. Acta Physiol. Hung. 2014;101:408–420. doi: 10.1556/APhysiol.101.2014.4.2. [DOI] [PubMed] [Google Scholar]
- 10.Hadisaputro S., Djokomoeljanto R. R., Soesatyo M. H. The effects of oral plain kefir supplementation on proinflammatory cytokine properties of the hyperglycemia Wistar rats induced by streptozotocin. Acta Med. Indones. 2012;44:100–104. [PubMed] [Google Scholar]
- 11.Haenlein G. F. W. Goat milk in human nutrition. Small Ruminant Res. 2004;51:155–163. [Google Scholar]
- 12.Heneman K., Steinberg F., Zidenberg-Cherr S. Some fact about soy. Nutrition and Health Info-Sheet. UC Cooperative Extension Center for Health and Nutrition Research. Department of Nutrition, University of California; 2007. pp. 1–4. [Google Scholar]
- 13.Huang H.Y., Korivi M., Tsai C. H., Yang J. H., Ying-Chieh T. Supplementation of lactobacillus plantarum k68 and fruit-vegetable ferment along with high fat-fructose diet attenuates metabolic syndrome in rats with insulin resistance. Evid. Based Complement. Alternat. Med. 2013:1–12. doi: 10.1155/2013/943020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isfahlan A. J., Mahmoodzadeh A., Hassanzadeh A., Heidari R., Jamei R. Antioxidant and antiradical activities of phenolic extracts from Iranian almond (Prunus amygdalus L.) hulls and shells. Turk. J. Biol. 2010;34:165–173. [Google Scholar]
- 15.Jascolka T. L., Aguilar E.C., Teixeira L. G., Lages P. C., de Cássia Raimundo I., Beltrão N. R. M., de Oliveira Matoso R., Carneiro R. P., Nicoli J. R., Alvarez-Leite J. I. Kefir Supplementation Improves lipid profile and oxidative stress but does not reduce atherosclerotic lesion in apoE deficient mice. J. Food Nutr. Disor. 2013;2:1–7. [Google Scholar]
- 16.Jeppesen J., Hein H. O., Suadicani P., Gyntelberg F. Relation of high TG-low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen Male Study. Arterioscler. Thromb. Vasc. Biol. 1997;17:1114–1120. doi: 10.1161/01.atv.17.6.1114. [DOI] [PubMed] [Google Scholar]
- 17.Jones S. E., Paynich M. L., Knight K. L. Exopolysaccharides: Sweet success with probiotic therapeutics. Inflamm. Cell Signal. 2014;1:2–8. [Google Scholar]
- 18.Kanter M., Coskun O., Korkmaz A., Oter S. Effects of Nigella sativa on oxidative stress and -cell damage in streptozotocin-induceddiabeticrats. Anat. Rec. A. 2014;279A:685–691. doi: 10.1002/ar.a.20056. [DOI] [PubMed] [Google Scholar]
- 19.Kesenkas H., Dinkci N., Seckin K., Kinik O., Gonc S. Antioxidant properties of kefir produced from different cow and soy milk mixtures. J. Agric. Sci. 2011;17:253–259. [Google Scholar]
- 20.Kikui Y., Seguchi H., Mizoguti H. A differential staining method for A- and B- cells in the pancreatic islets of Langerhans. Acta Histochem. Cytochem. 1977;10:10–13. [Google Scholar]
- 21.Kýlýc S., Uysal H., Akbulut N., Kavas G., Kesenkas H. Chemical, microbiological and sensory changes in ripening kefirs produced from starters and grains. Ege University Journal of Agricultural Faculty. 1999;36:111–118. [Google Scholar]
- 22.Kurowska E. M., Carroll K. K. Effect of high levels of selected dietary essential amino acids on hypercholesterolemia and down-regulation of hepatic LDL receptors in rabbits. Biochim. Biophys. Acta. 1992;1126:185–191. doi: 10.1016/0005-2760(92)90289-8. [DOI] [PubMed] [Google Scholar]
- 23.Kurowska E. M., Carroll K. K. Hypercholesterolemic responses in rabbits to selected groups of dietary essential amino acids. J. Nutr. 1994;124:364–370. doi: 10.1093/jn/124.3.364. [DOI] [PubMed] [Google Scholar]
- 24.Li S., Yan T., Yang J. Q., Oberley T. D., Oberley L. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 25.Liao C. L., Huang H. Y., Sheen L. Y., Chou C. C. Anti-inflammatory activity of soymilk and fermented soymilk prepared with lactic acid bacterium and bifidobacterium. J. Food Drug Anal. 2010;18:202–210. [Google Scholar]
- 26.Liu J. R., Lin Y. Y., Chen M. J., Chen L. J., Lin C. W. Antioxidative activities of kefir. Asian Australas. J. Anim. Sci. 2005a;18:567–573. [Google Scholar]
- 27.Liu J. R., Chen M. J., Lin C. W. Antimutagenic and antioxidant properties of milk-kefir and soymilk-kefir. J Agr. Food Chem. 2005b;53:2467–2474. doi: 10.1021/jf048934k. [DOI] [PubMed] [Google Scholar]
- 28.Liu J. R., Wang S. Y., Chen M.J., Chen H. L., Yueh P. Y., Lin C. W. Hypocholesterolaemic effects of milk-kefir and soyamilk-kefir in cholesterol-fed hamsters. Br. J. Nutr. 2006;95:939–946. doi: 10.1079/bjn20061752. [DOI] [PubMed] [Google Scholar]
- 29.Loo A. Y, Jain K., Darah I. Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculata. Food Chem. 2007;104:300–307. [Google Scholar]
- 30.Madhuri K. V., Prabhakar K. V. Microbial exopolysaccharides: biosynthesis and potential applications. Orient J. Chem. 2014;30:1401–1410. [Google Scholar]
- 31.Mao D. B., Shi C. W., Wu J. W., Xu C. P. Optimization of exopolysaccharide production in submerged culture of Daedalea dickinsii and its antioxidant activity. Bioproc. Biosyst. Eng. 2014;37:1401–1409. doi: 10.1007/s00449-013-1111-3. [DOI] [PubMed] [Google Scholar]
- 32.Nikam S., Nikam P., Joshi A., Viveki R. G., Halappanavar A. B., Hungund B. Effect of regular physical exercise (among circus athlets) on lipid profile, lipid peroxidation and enzymatic antioxidants. Int. J. Biochem. Res. Rev. 2013;3:414–420. [Google Scholar]
- 33.Nugroho A. E., Rais I. R., Setiawan I., Pratiwi P. Y., Hadibarata T., Tegar M., Pramono S. Pancreatic effect of andrographolide isolated from Andrographis paniculata (Burm. F.) nees. Pak. J. Biol. Sci. 2014;17:22–31. doi: 10.3923/pjbs.2014.22.31. [DOI] [PubMed] [Google Scholar]
- 34.Harmayani E. Microbiological quality, fatty acid and amino acid profiles of kefir produced from combination of goat and soy milk. Pak. J. Nutr. 2014;13:107–115. [Google Scholar]
- 35.Omayma A. R., El-Sonbaty S. M., Aziza S. A., Aboelftouh A. E. Effect of probiotic fermented soy milk and gamma radiation on nitrosourea-induced mammary carcinogenesis. Nature Sci. 2013;11:35–42. [Google Scholar]
- 36.Oyedemi S., Bradley G., Afolayan A. Antidiabetic activities of aqueous stem bark extract of Strychnoshenningsii Gilg in streptozotocin-nicotinamide type 2 diabetic rats. Iran. J. Pharm. Res. 2012;11:221–228. [PMC free article] [PubMed] [Google Scholar]
- 37.Poonuru S., Pathak S. R., Vats H. S., Pathak R. D. Rapid reduction of severely elevated serum triglycerides with insulin infusion, gemfibrozil and niacin. J. Clin. Med. Res. 2011;9:38–41. doi: 10.3121/cmr.2010.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rani V. U., Pradeep B. V. Antioxidant properties of soy milk fermented with Lactobacillus paracasei KUMB B005. Int. J. Pharm. Sci. Rev. Res. 2015;30:39–42. [Google Scholar]
- 39.Reeves P. G., Neilsen F. H., Fahey G. C. AIN-93 purified diets for laboratory rodents: Final report of the american institute of nutrition Ad Hoc writing committee on the formulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez-Roque M. J., Rojas-Graü M. A., Elez-Martínez P., Martín-Belloso O. Soymilk phenolic compounds, isoavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013;136:206–212. doi: 10.1016/j.foodchem.2012.07.115. [DOI] [PubMed] [Google Scholar]
- 41.Roselino M. N., Pauly-Silveira N. D., Cavallini D. C. U., Celiberto L. S., Pinto R. A., Vendramini R. C., Rossi E. A. A potential synbiotic product improves the lipid profile of diabetic rats. Lipids Health Di. 2012;11:114. doi: 10.1186/1476-511X-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Normal Range for Common Laboratory Test. Chiago, Illinois: [Google Scholar]
- 43.Sharma U., Sahu R. K, Roy A., Golwala D. K. In vivo antidiabetic and antioxidant potential of Stephania hernandifolia in streptozotocin-induced-diabetic rats. J. Young Pharm. 2010;2:255–260. doi: 10.4103/0975-1483.66803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SPSS. SPSS Statistics Base 17.0 User’s Guide. SPSS Inc.; Chicago, IL: 2007. pp. 60606–6412. 233 South Wacker Drive, 11th Floor. [Google Scholar]
- 45.Srinivasan K., Viswanad B., Asrat L., Kaul C. L., Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012;237:481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- 47.Vinderola G., Perdigón G., Duarte J., Farnworth E., Matar C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine. 2006;36:254–260. doi: 10.1016/j.cyto.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 48.WHO. Definition, diagnosis and classification of diabetes mellitus and its complications report of a WHO consultation Part 1: Diagnosis and classification of diabetes mellitus. World Health Organization, Department of Non communicable Disease Surveillance; Geneva: 1999. [Google Scholar]
- 49.Wong C. W., Watson D. L. Immunomodulatory effects of dietary whey proteins in mice. J. Dairy Res. 1995;62:359–368. doi: 10.1017/s0022029900031058. [DOI] [PubMed] [Google Scholar]
- 50.Yadav H., Jain S., Sinha P. R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy Res. 2008;75:189–195. doi: 10.1017/S0022029908003129. [DOI] [PubMed] [Google Scholar]
- 51.Yin J., Heo S. I., Wang M. H. Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots. Nutr. Res. Pract. 2008;2:247–251. doi: 10.4162/nrp.2008.2.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon G. A., Park S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014;8:618–624. doi: 10.4162/nrp.2014.8.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zafar M., Naqvi S. N. H. Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in Albino rats: A comparative study. Int. J. Morphol. 2010;28:135–142. [Google Scholar]
- 54.Zimecki M., Kruzel M. L. Systemic or local coadministeration of lactoferrin with sensitizing dose of antigen enhanches delayed type hypersensitivity in mice. Immunol. Lett. 2000;74:183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 55.Zommara M., Toubo H., Sakono M., Imaizumi K. Prevention of peroxidative stress in rats fed on a low vitamin e-containing diet by supplementing with a fermented bovine milk whey preparation: effect of lactic acid and β-lactoglobulin on the antiperoxidative action. Biosci. Biotechnol. Biochem. 1998;62:710–717. doi: 10.1271/bbb.62.710. [DOI] [PubMed] [Google Scholar]