Abstract
Isolated cortical vein thrombosis (ICoVT) is a rare form of cerebral venous thrombosis and is easily misdiagnosed as brain tumor due to its atypical clinical presentations and radiological findings similar to brain tumors. The present study focused on 4 patients with ICoVT, 2 men and 2 women. In the 2 male patients, the onset of disease was insidious, with progressive numbness and weakness in limbs as the major symptoms. By contrast, 2 female patients suffered from acute onset of symptoms, presenting with headache and seizures. Brain computed tomography and magnetic resonance imaging showed large hemorrhagic lesions surrounded by massive edemas in the 4 patients. Brain biopsies were performed in the 4 patients due to concern for brain tumors. However, the pathological results supported the diagnosis of ICoVT, and the subsequent anticoagulant treatment administered was effective for all 4 patients. In conclusion, ICoVT can be easily misdiagnosed as brain tumor because of the atypical clinical and imaging features. The results suggested that the possibility of ICoVT always be considered in patients with neuroimaging showing cortical hemorrhagic lesions with massive edemas.
Keywords: isolated cortical vein thrombosis, cerebral venous thrombosis, pathologic features
Introduction
Isolated cortical vein thrombosis (ICoVT) is a rare form of cerebral venous thrombosis (CVT). CVT is usually diagnosed based on clinical manifestations, and magnetic resonance venography (MRV) showing the vessel(s) with non-visualization thrombosis (1). However, ICoVT is difficult to diagnose because of the variation in numbers and locations of cortical veins. Until recently, only a few cases or small series with ICoVT have been reported (2–5), suggesting the common misdiagnosis (2).
In the present study, 4 patients who were initially diagnosed with glioma based on clinical presentations and neuroimaging are reported. However, the pathological results of subsequent biopsies supported a diagnosis of ICoVT. The clinical and neuroimaging manifestations as well as the results of pathological studies of these patients were retrospectively analyzed to further differentiate ICoVT from brain tumors clinically.
Case reports
Case 1
The patient was a 36-year-old Chinese man with symptoms of progressive numbness and weakness of the right limbs in the previous 6 months. The weakness was initially experienced in the right toes. Subsequently, the weakness progressed, and the right lower limb was inflicted 2 months later. At 5 months later after the onset, the right upper extremity was also affected with weakness and numbness. The patient did not suffer from fever, nausea, vomiting, photophobia, headache, seizure or loss of consciousness. He had no history of diabetes, hypertension or autoimmune disorders. Prior physical examinations revealed his blood pressure as 154/95 mmHg. No papilledema was observed following fundoscopic examination. Right side of central facial and lingual paralysis was experienced, as well as hypertonia and grade 4 muscle strength in the right limbs, with increased muscle stretch reflexes and inducible myoclonus as well as positive Babinski's and Chaddock's signs.
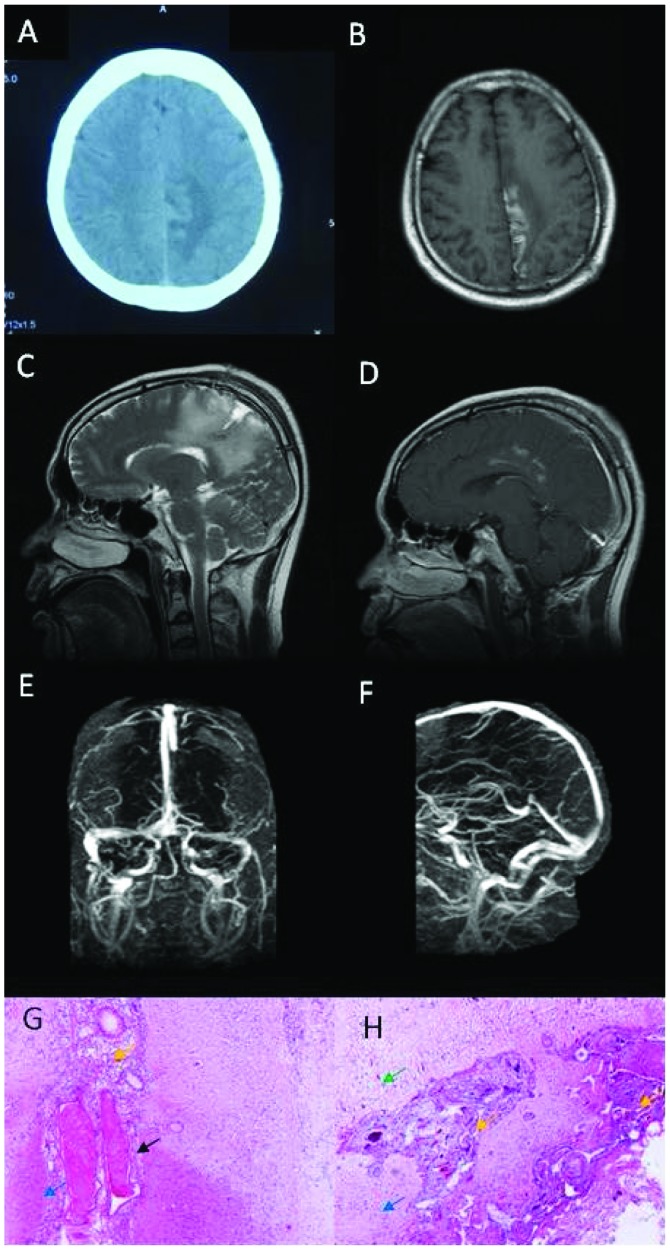
Brain computed tomography (CT) scan showed lesions of hyperdensity mixed with hypodensity in the left parietal lobe (Fig. 1A). Magnetic resonance imaging (MRI) of the brain was performed including axial T1-weighted image, T2-weighted image, fluid-attenuated inversion recovery, apparent diffusion coefficient, diffusion weighted imaging, sagittal T2-weighted image and sagittal gadolinium contrast-enhanced images and showed long T1 and T2 signals, mixed with short T1 signal (Fig. 1B and C) within the lesion, with focal enhancement in contrast-enhanced MRI (Fig. 1D). The lumbar puncture revealed cerebrospinal fluid (CSF) pressure was 143 mmH2O. Cytological and chemical studies of CSF were normal. Other routine laboratory tests including complete blood count (CBC) was normal. Folic acid was 2.77 ng/ml and the triglyceride level was 2.99 mmol/l. Biomarkers for tumors were negative. Anti-neutrophil cytoplasmic antibody (ANCA) and extractable nuclear antigen (ENA) were negative. Antithrombin III deficiency, protein C and S deficiency, and homocysteinemia were within normal limits. Activated partial thromboplastin time (APTT) and D-dimer were normal. Electroencephalography (EEG) was normal. In brain MRV, major venous sinuses were patent without abnormality (Fig. 1E and F). Results of brain digital subtraction angiography were normal. Brain biopsy was performed because of suspected glioma. However, the pathological results showed focal necrosis and degeneration of neurons, dilated small veins with congestion or thrombosis, phagocytosis and gliosis, and proliferation of small vessels and fibrous tissues, without tumor cells or characteristics of inflammatory diseases (Fig. 1G and H).
Figure 1.
Neuroimaging and pathological studies for patient 1 showed evidence of isolated cortical vein thrombosis. (A) Brain computed tomography scans show hyperdensity with some hypodensity in the left parietal lobe. (B) Brain axial magnetic resonance imaging (MRI) showed long signal with short signal in T1-weighted image in the left parietal lobe. (C) Brain sagittal MRI showed long signal in T2-weighted image in the left parietal lobe. (D) Partial focus was enhanced in contrast-enhanced MRI. (E and F) The major venous sinuses were patent and did not show any abnormal signals in brain MRV. (G and H) Histopathologic analysis revealed focal necrosis and degeneration of neurons (green arrow), dilated small veins with congestion or thrombosis (black arrow), phagocytosis and gliosis (blue arrow), proliferation of small vessels and fibrous tissue (yellow arrow). Amplification of G and H was 400 times.
The patient was consulted by a pathologist and neurologist in the Navy General Hospital, and finally diagnosed with ICoVT. The patient was then admitted to the Department of Neurology for further management. The repeated lumbar puncture showed that the pressure of CSF was >330 mmH2O. The results of the cytological and chemical studies of CSF were normal. Treatment commenced with intravenous (IV) heparin for 7 days, followed by 6 days of oral Coumadin. The patient was discharged with a grade 5− muscle strength in the right upper limb and a grade 4 muscle strength in the right lower limb. Coumadin was continued following discharge. Grade 5 muscle strength in the right limbs was observed in a follow up 3 months after discharge, and repeat brain MRI revealed long T1 and T2 signals, mixed with short T1 signal in the right cingulate gyrus, with focal enhancement in contrast-enhanced MRI. Routine laboratory tests including CBC, comprehensive metabolic panel (CMP) and D-dimer were within normal limits. Oral Coumadin was continued for an additional month, and then discontinued by the patient. Full recovery was confirmed in a follow up 2 years later as an outpatient. At this time, the patient refused to undergo a brain MRI study.
Case 2
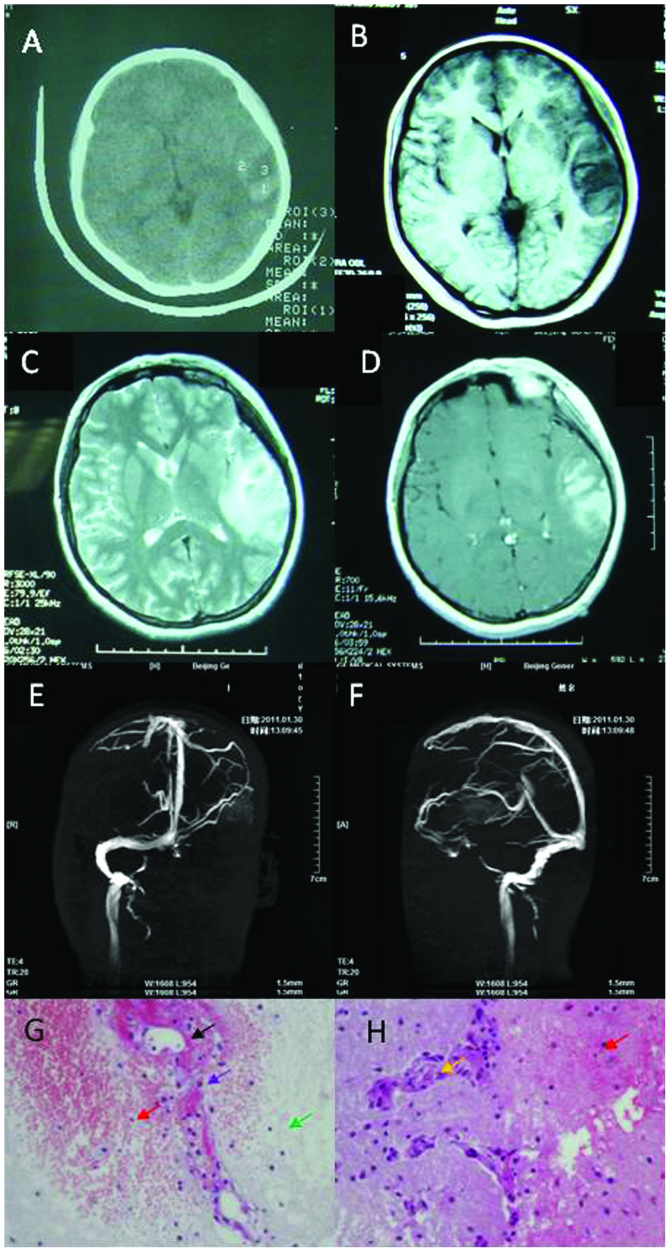
A 19-year-old Chinese woman presented with headache, dizziness for 2 days and two episodes of seizures. Her headache was constant dull pain in the left temporal region accompanied by nausea and vomiting, but without fever or photophobia. The patient experienced complex partial seizures, and each episode lasted ~1 min. She had no history of notable illness. The neurologic examination was normal. Routine laboratory workups including CBC and CMP showed mild iron deficiency anemia. Brain CT showed lesions of hypodensity mixed with hyperdensity in the left temporal lobe (Fig. 2A). Brain MRI showed long T1 and T2 signals surrounding the short T1 signal in the left temporal lobe (Fig. 2B and C). No short T1 signal or long T2 signal was identified in the sinus for venous thrombus. Some focal enhancement was evident in the contrast-enhanced MRI (Fig. 2D).
Figure 2.
Neuroimaging and pathological studies for patient 2 show evidence of isolated cortical vein thrombosis. (A) Brain computed tomography scans show hypodensity lesion with hyperdensity inside of the left temporal lobe. (B) Brain axial magnetic resonance imaging (MRI) shows long signal with short signal on the left temporal lobe of the T1-weighted image. (C) Brain axial MRI shows long signal on the left temporal lobe of the T2-weighted image. (D) Partial focus was enhanced in contrast-enhanced MRI. (E and F) Left sinus transverses and sigmoid sinus were not patent in the brain MRV. (G and H) Histopathologic analysis was areolar tissue with focal necrosis (green arrow), hemorrhage (red arrow), necrosis of vessel wall (black arrow), phagocytosis (purple arrow) and proliferation in small vessels (yellow arrow). Amplification of G and H was 400 times.
Lumbar puncture was completed, showing CSF pressure of 220 mmH2O, and normal cytology and chemistry. Homocysteinemia was 32.2 µmol/l, and folic acid was 3.3 nmol/l. APTT and D-dimers were within the normal limits. Protein S was decreased to 33% and protein C was normal. ANCA, ENA, antiphospholipid, anticardiolipin antibodies and antithrombin III were negative. Tumor biomarkers were also negative. EEG showed asymmetrical slow-waves in the right frontal lobe, with a mean frequency of ~1–3.5 Hz and a mean voltage of 30 mV. In the brain MRI, left transverse and sigmoid sinus were not patent (Fig. 2E and F). Brain biopsy was performed because of suspected glioma. However, the pathological analysis revealed areolar tissue with focal necrosis, hemorrhage, necrosis of vessel wall, phagocytosis, and proliferation in small vessels (Fig. 2G and H). Based on the above observations, the patient was diagnosed with ICoVT, and was treated for 3 months with anticoagulant (initially with IV heparin for 7 days followed by oral Coumadin) for thrombosis and antiepileptics for seizure. She was hospitalized for 16 days and did not report any headache, dizziness or seizure during the 3 years of follow up.
Case 3
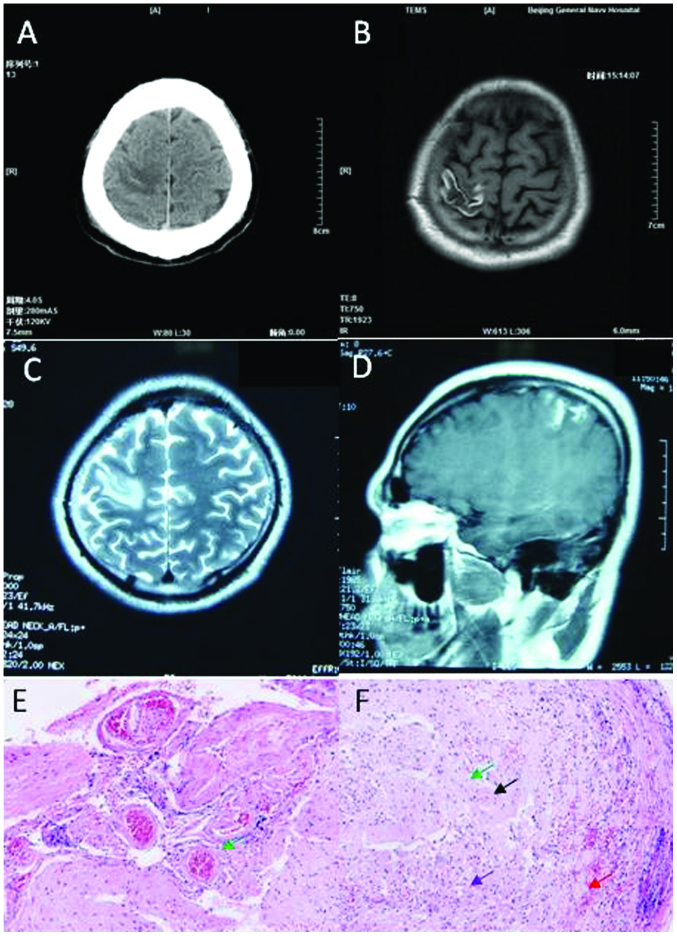
A 32-year-old Chinese man presented with numbness and weakness in the left limbs for 2 months. The patient experienced numbness in the fingers of the left hand for 2 months prior to admission. One month after admission, the patient felt weak and numb on the left side of the face and upper limbs. The weakness and numbness was not alleviated after taking oral vitamins. The patient did not exhibit fever, seizure or headache. He had no previous history of illness. The neurological examination demonstrated hypertonia in the left limbs with grade 4 muscle strength. The patient exhibited left hyperreflexia, and positive Babinski's and Chaddock's signs. No papilledema was observed following the fundoscopic examination. Routine laboratory tests including CBC, CMP were normal. APTT and D-dimer were normal. Brain CT scan revealed hypodense lesions, within which there were hyperdense lesions, in the right parietal lobe (Fig. 3A). Brain MRI revealed long signal in the T1-weighted image and T2 weighed image, with short signal in the T1-weighted image in the right parietal lobe (Fig. 3B and C), and focal enhancement was detected in the contrast-enhanced MRI (Fig. 3D).
Figure 3.
Neuroimaging and pathological studies for patient 3 show evidence of isolated cortical vein thrombosis. (A) Brain computed tomography scans show hypodense lesion with some hyperdense within the right parietal lobe. (B) Brain axial magnetic resonance imaging (MRI) shows long signal with short signal in the right parietal lobe in the T1-weighted image. (C) Brain axial MRI shows long signal in the right parietal lobe in the T2-weighted image. (D) Partial focus was enhanced in the contrast-enhanced MRI. (E and F) Histopathologic analysis was sporadic hemorrhage (red arrow), tissue structure disappearance, astrocytic reaction and microglia (purple arrow), proliferation of small vessels (black arrow) and perivascular cuffing (green arrow). Amplification of G was 400 times and H was 200 times.
Lumbar puncture revealed CSF pressure of 160 mmH2O, protein level of 0.557 g/l. ANCA and ENA were negative. Biomarkers for tumors were also negative. Antiphospholipid and anticardiolipin antibodies, antithrombin III deficiency, protein C and S deficiency, and homocysteinemia were normal. EEG was normal. Brain biopsy was performed because glioma was suspected. The pathological study showed sporadic hemorrhage, tissue structure disappearance, astrocytic reaction, and microglia, proliferation of small vessels, and perivascular cuffing (Fig. 3E and F). The patient was diagnosed with ICoVT based on the comprehensive studies, and was treated with anticoagulant, initially heparin for 7 days, followed by oral Coumadin for 3 months. The patient was fully recovered, and was able to walk normally without any numbness or weakness during the 4 years of follow up.
Case 4
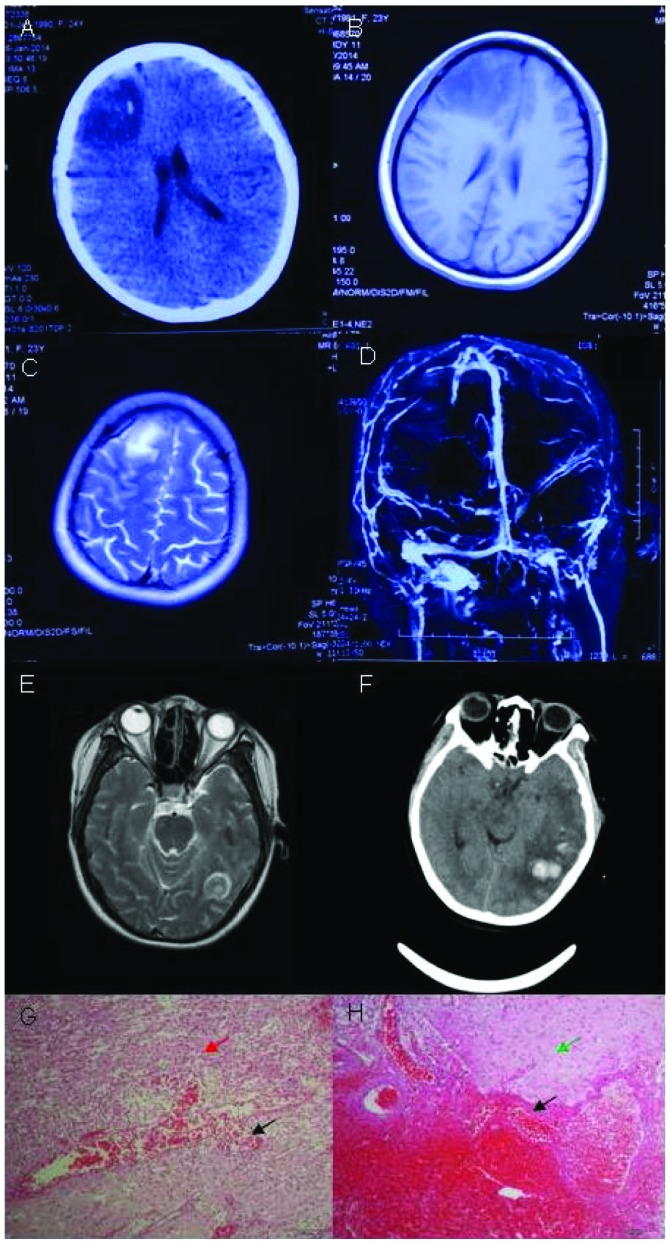
A 23-year-old Chinese parturient visited clinic following headache for 6 days and 1 episode of seizure. She exhibited headache, which was persistent dull pain in the right frontal lobe accompanied with vomiting and dizziness. She had a cold without fever prior to the delivery of the baby. The one-time seizure lasted for several minutes, with loss of consciousness, lockjaw, tonic and clonic in limbs, and urinary and fecal incontinence. The patient did not have a history of major illness. The neurologic examination was normal. Her D-dimer was normal. The brain CT scan revealed hypodense mixed with hyperdense lesions in the right frontal lobe (Fig. 4A). The brain MRI revealed long signal in the T1-weighted image and the T2-weighted image, mixed with short T1 signal in the right frontal lobe (Fig. 4B and C). Contrast-enhanced MRI showed focal enhancement within the lesion. In the brain MRV, the major venous sinuses were patent without abnormalities (Fig. 4D). The pressure CSF was 240 mmH2O, and red blood cell (RBC) count was 290×106/l in CSF. CBC, CMP and APTT were normal. D-dimer was 1,949 ng/ml (<429 ng/ml). Protein S activity was 53% and protein C activity was 52%. ANCA, ENA, antiphospholipid, anticardiolipin antibodies and antithrombin III were negative. The tumor biomarkers were also negative. Brain tumor was suspected and the patient was hospitalized. Right frontal brain biopsy was performed. Her conditions deteriorated and she became comatose 2 days after the surgery. Bilateral papilledemas were observed with blurry border of discus opticus. Her left limbs had grade 2 muscle strength and her right limbs had grade 4 muscle strength.
Figure 4.
Neuroimaging and pathological studies for patient 4 show evidence of isolated cortical vein thrombosis. (A) Brain computed tomography (CT) scan shows hypodense lesion with hyperdensity within the right frontal lobe. (B) Brain magnetic resonance imaging (MRI) shows long signal with short T1 signal in the right frontal lobe. (C) Brain MRI shows long T2-weighted image in the right frontal lobe. (D) In the brain MRV, the major venous sinuses were patent and did not show any abnormal signals. (E) Brain MRI shows long T2-weighted image in the left temporal lobe. (F) Brain CT scan shows hypodense lesion with hyperdensity within the left temporal lobe. (G and H) Histopathologic analysis revealed focal necrosis, and hemorrhage with lots of gitter cells (red arrow). Histopathologic analysis revealed astrocytic reaction, degeneration of neurons and endothelial proliferation in small vessels (green arrow). Histopathologic analysis revealed focal vascular mass with vasodilatation and congestion (black arrow). Amplification of G and H was 200 times.
Repeat brain MRI revealed large cerebral hemorrhage in the left temporal and occipital lobes (Fig. 4E). Pathological results showed focal necrosis, hemorrhage with lots of gitter cells, astrocytic reaction, degeneration of neurons and endothelial proliferation in small vessels, and a focal vascular mass with vasodilatation and congestion (Fig. 4G and H). The pathological study did not support the diagnosis of brain tumor and ICoVT was considered the final diagnosis. The patient was administered an anticoagulant and antiepileptic. On the first day of anticoagulant treatment with IV heparin, her conditions did not improve and she remained in the coma. The left limbs had grade 1 muscle strength and the right limbs had grade 3 muscle strength. Another brain CT was performed, which showed cerebral hemorrhage in the left temporal and occipital lobes with mass effect (Fig. 4F). The treatment of anticoagulant was suspended for two days, and then restarted. During the anticoagulant treatment for the following 2 days, her conditions improved and the patient regained consciousness. The left limbs showed grade 3 muscle strength, and the right limbs had grade 4 muscle strength.
After 12 days of treatment with heparin followed by 15 days of oral Coumadin, the patient was able to walk independently. At 20 days after admission, the patient received a repeat brain MRI which showed that cerebral hemorrhage in the left temporal and occipital lobes was reduced. At 21 days after admission, lumbar puncture showed that the pressure of CSF was 270 mmH2O, and RBC Count was 3×106/l. CBC and CMP were normal. The patient remained in the hospital for 46 days, and was then discharged with grade 5− muscle strength in the left extremities and normal strength in the right limbs. She was able to speak fluently and clearly, and open-close eyes freely when leaving the hospital. Repeat brain CT scan showed mixed density of lesions in the left temporal and occipital lobes, and hypodense lesions in the right frontal lobe. The patient continued oral Coumadin and was under rehabilitaion for mild paralysis.
Discussion
CVT is a relatively uncommon but potentially life-threatening condition, accounting for 1–2% of strokes in young adults (6). CVT includes dural sinus thrombosis, deep venous thrombosis and ICoVT. As previously reported, the mortality rate of CVT ranged between 5 and 30% (7). ICoVT is even less common, accounting for 17% of CVT (8), and its prognosis is good with early diagnosis and appropriate management. However, early diagnosis of ICoVT is difficult both clinically and radiologically (5). Patients undergo brain biopsy as they are initially diagnosed with brain tumors. and are then diagnosed with ICoVT following comprehensive workups including pathological examinations.
It has been reported that usage of oral contraceptive, pregnancy or puerperium, infection, genetic thrombophilia, lumbar puncture, malignancy, sinusitis, trauma and surgery, intracranial hypotension, history of lumbar puncture, and medications constitute risk factors for ICoVT (8). Of the 4 patients presented in the current study, decreased protein S activity was detected in 1 patient, and another patient was postpartum.
The neuroimaging features of ICoVT include the primary changes of the affected superficial cortical vein, which can be directly visualized and the secondary changes of venous outflow obstruction in brain MRI. Venous congestion was evident as swollen gyri (5). Imaging of ICoVT revealed focal hemorrhage venous infarction and edema. Parenchymal changes were usually subcortical (2). Intracranial vascular malformations, hemorrhage transformation in ischemic infarctions, aneurysm rupture and bleeding within the brain due to a tumor were among the deferential diagnoses.
For our patients, the primary pathological findings were focal necrosis, hemorrhage, and dilated small vein with congestion or thrombosis. Degeneration of neuron, phagocytosis, gliosis and endothelial proliferation in small vessels were also observed. Although a pathological study is not recommended routinely for ICoVT, it is valuable for some patients when they do not present with typical T2* imagings, or especially when the differentiation with brain tumor is difficult. Early diagnosis is important for patients with ICoVT since early management is associated with favorable outcome.
In the present study, the 4 cases were initially misdiagnosed as brain tumors because of atypical clinical presentations and neuroimaging studies. However, the final diagnoses of ICoVT were made based on the comprehensive workups including the pathological studies. According to the guidelines of ‘Diagnosis and management of cerebral venous thrombosis’ (8), patients who exhibit CVT are required to receive neuroimaging studies including T2-weighted-gradient-echo image, which may have high accuracy of diagnosis. However, whether T2-weighted-gradient-echo image was useful for the patients of the present study was unclear. The accuracy of the misdiagnosis of ICoVT with brain tumors in the patients who underwent brain biopsy also remains unclear. For difficult patients, a pathological examination would be useful in determining the diagnosis, and the subsequent treatment such as anticoagulation.
In summary, ICoVT is an uncommon disease that is often difficult to diagnose because of non-specific clinical presentations and atypical CT and MRI imaging findings. Brain biopsy is probably the last resort for making the diagnosis. For difficult cases, such as those examined in the present study, earlier diagnosis even with brain biopsy is important for improved prognosis and treatment.
References
- 1.Hinman JM, Provenzale JM. Hypointense thrombus on T2-weighted MR imaging: a potential pitfall in the diagnosis of dural sinus thrombosis. Eur J Radiol. 2002;41:147–152. doi: 10.1016/S0720-048X(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho JM, Gerritsma JJ, Zuurbier SM, Stam J. Isolated cortical vein thrombosis: systematic review of case reports and case series. Stroke. 2014;45:1836–1838. doi: 10.1161/STROKEAHA.113.004414. [DOI] [PubMed] [Google Scholar]
- 3.Rathakrishnan R, Sharma VK, Luen TH, Chan BP. The clinico-radiological spectrum of isolated cortical vein thrombosis. J Clin Neurosci. 2011;18:1408–1411. doi: 10.1016/j.jocn.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Xue SF, Ma QF, Ma X, Jia JP. Isolated cortical vein thrombosis: a widely variable clinicoradiological spectrum. Eur Neurol. 2013;69:331–335. doi: 10.1159/000346813. [DOI] [PubMed] [Google Scholar]
- 5.Sharma VK, Teoh HL. Isolated cortical vein thrombosis - the cord sign. J Radiol Case Rep. 2009;3:21–24. doi: 10.3941/jrcr.v3i3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen JE, Duck M, Gomori JM, Itshayek E, Leker RR. Isolated cortical vein thrombosis: a rare cause of venous stroke with good prognosis after timely diagnosis and treatment. Neurol Res. 2013;35:127–130. doi: 10.1179/1743132812Y.0000000148. [DOI] [PubMed] [Google Scholar]
- 7.Khealani BA, Wasay M, Saadah M, Sultana E, Mustafa S, Khan FS, Kamal AK. Cerebral venous thrombosis: a descriptive multicenter study of patients in Pakistan and Middle East. Stroke. 2008;39:2707–2711. doi: 10.1161/STROKEAHA.107.512814. [DOI] [PubMed] [Google Scholar]
- 8.Saposnik G, Barinagarrementeria F, Brown RDJ, Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY. American Heart Association Stroke Council and the Council on Epidemiology and Prevention: Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]