Abstract
Recurrent hot-spot mutations in the telomerase reverse transcriptase (TERT) promoter have been reported in various types of tumor. In several tumor types, TERT promoter mutations are associated with poor clinical outcomes. TERT promoter mutations are reported to be rare in soft tissue tumors, with the exception of myxoid liposarcoma (MLS). Our previous study reported that TERT promoter mutations occurred in a subset of solitary fibrous tumors (SFTs) and were associated with adverse clinical outcomes in Japanese individuals. The site-specific frequency (e.g. central nervous or soft tissue origin) of TERT promoter mutations in our SFT cases appeared to be different from previously reported values in a European population. These findings prompted the present study to elucidate the potential role of ethnic background in the different frequencies of TERT promoter mutations in bone and soft tissue sarcomas. In the present study, TERT promoter mutations were examined in 180 cases of bone and soft tissue sarcomas. TERT promoter region mutations were identified in 10 cases [5 SFTs, 3 MLSs, 1 undifferentiated pleomorphic sarcoma (UPS) and 1 malignant granular cell tumor]. All mutations were C228T. The frequencies of TERT promoter mutation in MLS and UPS were 23.1 (3/13) and 5% (1/20), respectively. Only 1/5 patients with TERT-mutated tumors experienced local recurrence or distant metastasis. The present study revealed the first case of a malignant granular cell tumor with a TERT promoter mutation and revealed that the frequency of TERT promoter mutations in MLSs of Japanese patients is lower compared with that reported in German patients, providing evidence of a possible ethnic difference in the frequency of TERT promoter mutations.
Keywords: TERT, mutation, bone and soft tissue sarcoma
Introduction
Telomere activity is associated with malignant potential in neoplasia (1). The ability to maintain telomere length is a typical feature in neoplasia and previous studies have revealed the robust expression of telomerase reverse transcriptase (TERT) in numerous human malignancies (2,3). Recurrent hot-spot mutations in the TERT promoter were intitially reported in melanoma (4,5) and subsequently in various tumor types, including primary nervous system tumors (6). Two hot-spot mutations, C228T and C250T, create novel binding sites for E-twenty-six (ETS) transcription factors, resulting in a maximum 4- to 5-fold increase in the induction of the TERT gene (4,5). Previous studies also demonstrated that TERT promoter mutations occur in ~50% of SFTs of central nervous system (CNS) origin (6). However, with the exception of myxoid liposarcomas (MLSs), TERT promoter mutations are relatively rare in soft tissue sarcomas, including SFTs (7). Our previous study reported that TERT promoter mutations are associated with poor clinical outcomes in SFT in Japanese individuals (8). However, the tumor site-specific frequency of TERT promoter mutations in our SFT cases (8) appeared to be different from previously reported values (6,7). These findings prompted the present study to elucidate the potential role of ethnic background in the possibly different frequencies of TERT promoter mutations in bone and soft tissue sarcomas.
In the present study, TERT promoter mutations were examined in 180 cases of bone and soft tissue sarcomas to elucidate its frequency in Japanese patients. It was demonstrated that TERT promoter mutation rates in MLSs of Japanese patients were lower compared with the reported values in German patients.
Materials and methods
Sample preparation
The sarcoma tissue samples were collected from the pathology records at the Pathology Division of Juntendo University Hospital (Tokyo, Japan), which were surgically resected between April 1990 and March 2010 at Juntendo University Hospital. Diagnoses were made based on the standard histopathological criteria in conjunction with immunohistochemical and molecular analysis, according to the current World Health Organization classification (9). In total, 180 cases of bone and soft tissue sarcomas were included in the present study. Among these 180 cases, data regarding 40 SFT cases were from our previous study (8). In addition, 3 cases of lipoblastoma and 5 cases of granular cell tumor of soft tissue origin were also collected for comparison. The numbers and types of bone and soft tissue tumors used in the present study are summarized in Table I.
Table I.
Examination TERT mutations of bone and soft tissue tumors.
| Tumor type | TERT mutations (no. cases) |
|---|---|
| Soft tissue sarcomas | |
| Myxoid liposarcoma | 3 (13) |
| Well differentiated liposarcoma | 0 (18) |
| Myxofibrosarcoma | 0 (6) |
| Pleomorphic undifferentiated sarcoma | 1 (20) |
| Leiomyosarcoma | 0 (19) |
| Pleomorphic leiomyosarcoma | 0 (5) |
| Rhabdomyosarcoma | 0 (5) |
| Synovial sarcoma | 0 (7) |
| Dermatofibrosarcoma protuberans | 0 (6) |
| Ewing/primitive neuroectodermal tumor | 0 (6) |
| Alveolar soft part sarcoma | 0 (3) |
| Malignant peripheral nerve sheath tumor | 0 (1) |
| Extraskeletal myxoid chondrosarcoma | 0 (1) |
| Clear cell sarcoma | 0 (1) |
| Endometrial stromal sarcoma | 0 (1) |
| Malignant granular cell tumor | 1 (2) |
| Solitary fibrous tumor | 5 (40) |
| Total | 10 (154) |
| Bone sarcomas | |
| Osteosarcoma | 0 (14) |
| Chondrosarcoma | 0 (10) |
| Malignant fibrous histiocytoma of bone | 0 (2) |
| Total | 0 (26) |
| Benign tumors (control) | |
| Lipoblastoma | 0 (3) |
| Granular cell tumor | 0 (5) |
TERT, telomerase reverse transcriptase.
Ethics approval
This study was approved by the research Ethics Committee of Juntendo University, School of Medicine (Tokyo, Japan). Written informed consent was obtained from the patients.
Mutational analysis of the TERT promoter
The genomic DNA was extracted from each formalin-fixed, paraffin-embedded tissue block. When isolating DNA, the most representative tissue blocks, containing the maximum percentage of tumor tissue were selected. The surrounding non-tumoral tissues were manually removed by dissection to enrich the percentage of tumor cells. The TERT promoter region mutations were examined using polymerase chain reaction (PCR), followed by direct sequencing with previously described primer pairs (6). The AccuPrime™ GC-rich DNA polymerase kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used for PCR. The PCR products were electrophoresed in a 2% agarose gel and were recovered using the QIAquick Gel Extraction kit (Qiagen, Hilden, Germany). Isolated PCR products were subsequently sequenced using a capillary sequencing machine 202 (3730xl Genetic Analyzer; Applied Biosystems) in the sense and antisense directions, and were analyzed by Sequencing Analysis V3.5.1 software (Applied Biosystems; Thermo Fisher Scientific, Inc.). Once mutations were detected, the corresponding non-tumoral DNA were also extracted to confirm the obtained mutations as tumor-specific mutations.
Results
TERT promoter region mutations were identified in 10 cases [5 SFTs, 3 MLSs (Fig. 1), 1 pleomorphic sarcoma and 1 malignant granular cell tumor]. These mutations were confirmed as tumor-specific mutations. The clinicopathological data of cases with TERT promoter mutations are summarized in Table II. All mutations were C228T. The frequencies of TERT promoter mutation in MLS and pleomorphic undifferentiated sarcoma were 23.1 (3/13) and 5% (1/20), respectively. It was demonstrated that 2/3 MLSs with a TERT promoter mutation contained areas with a round-cell component. It was recently reported that TERT promoter mutations were associated with an adverse clinical course in SFTs (8), therefore, the prognostic impact of TERT promoter mutations in these tumors was also assessed. However, 4/5 patients with TERT-mutated tumors experienced no local recurrence or distant metastasis. Only 1 patient with TERT-mutated pleomorphic sarcoma experienced lung metastasis 46 months following the wide resection of the tumor and subsequently underwent a resection of the metastasized tumor. This patient survived and currently exhibits no evidence of the disease. Furthermore, TERT promoter mutations were detected in 1/2 patients with malignant granular cell tumor, although it was not observed in any of 5 granular cell tumors.
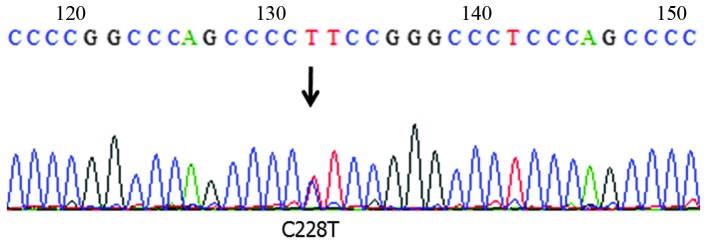
Figure 1.
Telomerase reverse transcriptase gene promoter mutation in a case of myxoid liposarcoma (Case 96). The representative chromatogram shows a heterozygous C228T mutation.
Table II.
Clinical information of tumors with TERT mutations.
| Case | Age/sex | Location | Diagnosis | Mutation | Treatment | Prognosis |
|---|---|---|---|---|---|---|
| 96 | 47/F | R. lower leg | Myxoid liposarcoma with RC | C228T | CTx+WR+CTx | NED (58 mos) |
| 276 | 58/M | R. thigh | Malignant granular cell tumor | C228T | WR | NED (72 mos) |
| 278 | 60/M | R. thigh | Pleomorphic sarcoma | C228T | WR | Lung metastasis (46 mos) |
| Alive with NED (103 mos) | ||||||
| 280 | 56/M | R. thigh | Myxoid liposarcoma | C228T | WR+RTx | NED (61 mos) |
| 370 | 43/M | L. thigh | Myxoid liposarcoma with RC | C228T | WR+CTx | NED (43 mos) |
TERT, telomerase reverse transcriptase; F, female; M, male; R, right; L, left; RC, round cell; CTx, chemotherapy; WR, wide resection; RTx, radiation therapy NED; no evidence of disease.
Other mutations neighboring the hot-spots were also noted in 3 cases, C229T in a case of synovial sarcoma, C230T in a case of myxofibrosarcoma and C232T in a case of Ewing/primitive neuroectodermal tumor, however, these mutations generated no consensus binding sites for ETS transcription factors within the TERT promoter region (10).
Discussion
Telomeres are extended by the protein complex, telomerase, in which the enzyme TERT exerts a pivotal role (11). TERT promoter hot-spot mutations recently emerged as an underlying mechanism of TERT upregulation in certain human cancer types. In SFTs of the CNS, TERT promoter mutations were identified in 50% of cases (6). However, in our previous study, TERT promoter mutations were detected in 5/40 SFTs (12.5%, 0/6 of CNS origin, 2/25 of pleural/lung origin and 3/9 of soft tissue origin (8). However, another previous study reported that TERT promoter mutations were observed in 4/31 (13%) SFTs of soft tissue origin (7). These findings appear to be at odds and prompted the present study to investigate if a difference in ethnic background may contribute to this discrepancy. In the present study, a TERT promoter mutation in MLS was detected in 3/13 cases (23.1%), which is lower compared with a previously reported value of 74% in this tumor type (7). The sensitivity of the examination may affect the lower frequency of TERT promoter mutation, however, the present study attempted to enrich the quantities of tumor cells during DNA isolation. These findings supported the present hypothesis that ethnic differences may affect the frequency of TERT promoter mutations.
In the present study, 2/3 MLSs with TERT promoter mutations contained a round-cell component, however, there was no association between TERT promoter mutation and the presence of a round-cell component, consistent with previous findings (7).
Lipoblastoma is a benign lipogenic tumor arising in infants and younger children. The histology of lipoblastoma overlaps with other lipomatous tumors, including MLSs, therefore, the frequency of TERT promoter mutations was assessed in lipoblastomas. However, 0/3 cases of this tumor type harbored a TERT promoter mutation.
Granular cell tumor is a benign Schwann cell lesion and typically occurs in the skin and subcutis. The clinical and morphological criteria for malignant granular cell tumor is well described (12). In the present study, 2 cases of malignant granular cell tumors were included. The clinical course in one was previously reported in detail (13), although it was the other case, which harbored the TERT promoter mutation. Genetic alterations in malignant granular cell tumors remain to be described in detail, however, it has been reported that a malignant granular cell tumor is characterized by a gain of chromosome 10 and a loss of p16 (14). Another previous report shows that malignant granular cell tumors share certain cytogenetic abnormalities with malignant peripheral nerve sheath tumors (MPNSTs), leading to the hypothesis that they may represent histogenetically associated lesions (15). The present study identified a TERT promoter mutation in 1/2 malignant granular cell tumor cases, however, not in a sporadic MPNST case. A previous study demonstrated that the TERT promoter mutation is also rare in sporadic MPNSTs and absent in neurofibromatosis type 1-associated MPNSTs (16), although another previous study revealed that TERT promoter hot-spot mutations were observed in 6% of MPNSTs (7). The present study cannot comment on the hypothesis of histogenetic similarity between malignant granular cell tumors and MPNSTs, since the present study included only a few cases of these tumor types. However, although malignant granular cell tumors are relatively rare, it is of interest to further investigate the frequency of TERT promoter mutations in malignant granular cell tumors to elucidate the association between TERT promoter mutations and the malignant behavior of this tumor. TERT promoter mutations in this tumor type may be in part driven by its presence in the dermal or subcutaneous localization, since this type of C to T alteration is a ultraviolet signature mutation, and TERT promoter mutations are frequently observed in atypical fibroxanthomas and pleomorphic dermal sarcomas (17).
In conclusion, the present study revealed the first case, to the best of our knowledge, of malignant granular cell tumor with a TERT promoter mutation and demonstrated that the frequency of TERT promoter mutations in MLSs of Japanese patients is lower compared with that reported in German patients.
Acknowledgements
The present study was supported, in part, by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Science, Sports and Culture, Tokyo, Japan (grant no. 26670286).
References
- 1.Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, Ghossein RA, Fagin JA. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98:E1562–E1566. doi: 10.1210/jc.2013-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 3.Ulaner GA, Hoffman AR, Otero J, Huang HY, Zhao Z, Mazumdar M, Gorlick R, Meyers P, Healey JH, Ladanyi M. Divergent patterns of telomere maintenance mechanisms among human sarcomas: Sharply contrasting prevalence of the alternative lengthening of telomeres mechanism in Ewing's sarcomas and osteosarcomas. Genes Chromosomes Cancer. 2004;41:155–162. doi: 10.1002/gcc.20074. [DOI] [PubMed] [Google Scholar]
- 4.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 5.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koelsche C, Sahm F, Capper D, Reuss D, Sturm D, Jones DT, Kool M, Northcott PA, Wiestler B, Böhmer K, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126:907–915. doi: 10.1007/s00401-013-1195-5. [DOI] [PubMed] [Google Scholar]
- 7.Koelsche C, Renner M, Hartmann W, Brandt R, Lehner B, Waldburger N, Alldinger I, Schmitt T, Egerer G, Penzel R, et al. TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J Exp Clin Cancer Res. 2014;33:33. doi: 10.1186/1756-9966-33-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akaike K, Kurisaki-Arakawa A, Hara K, Suehara Y, Takagi T, Mitani K, Kaneko K, Yao T, Saito T. Distinct clinicopathological features of NAB2-STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Hum Pathol. 2015;46:347–356. doi: 10.1016/j.humpath.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. World Health Organization International Agency for Research on Cancer. IARC Press; 2013. World Health Organization (WHO) Classification of Tumours of Soft Tissue. [Google Scholar]
- 10.Borah S, Xi L, Zaug AJ, Powell NM, Dancik GM, Cohen SB, Costello JC, Theodorescu D, Cech TR. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347:1006–1010. doi: 10.1126/science.1260200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Li S, Stohr BA. The role of telomere biology in cancer. Annu Rev Pathol. 2013;8:49–78. doi: 10.1146/annurev-pathol-020712-164030. [DOI] [PubMed] [Google Scholar]
- 12.Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: Diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22:779–794. doi: 10.1097/00000478-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Saito T, Mitomi H, Torigoe T, Takagi T, Suehara Y, Okubo T, Kaneko K, Yao T. Malignant granular cell tumor with an unusually long clinical course: An autopsy case with review of literature. J Cancer Sci Ther. 2012;4:260–263. doi: 10.4172/1948-5956.1000152. [DOI] [Google Scholar]
- 14.Papachristou DJ, Palekar A, Surti U, Cieply K, McGough RL, Rao UN. Malignant granular cell tumor of the ulnar nerve with novel cytogenetic and molecular genetic findings. Cancer Genet Cytogenet. 2009;191:46–50. doi: 10.1016/j.cancergencyto.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Di Tommaso L, Magrini E, Consales A, Poppi M, Pasquinelli G, Dorji T, Benedetti G, Baccarini P. Malignant granular cell tumor of the lateral femoral cutaneous nerve: Report of a case with cytogenetic analysis. Hum Pathol. 2002;33:1237–1240. doi: 10.1053/hupa.2002.129207. [DOI] [PubMed] [Google Scholar]
- 16.Dubbink HJ, Bakels H, Post E, Zwarthoff EC, Verdijk RM. TERT promoter mutations and BRAF mutations are rare in sporadic, and TERT promoter mutations are absent in NF1-related malignant peripheral nerve sheath tumors. J Neurooncol. 2104;120:267–272. doi: 10.1007/s11060-014-1553-8. [DOI] [PubMed] [Google Scholar]
- 17.Griewank KG, Schilling B, Murali R, Bielefeld N, Schwamborn M, Sucker A, Zimmer L, Hillen U, Schaller J, Brenn T, et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod Pathol. 2014;27:502–508. doi: 10.1038/modpathol.2013.168. [DOI] [PubMed] [Google Scholar]