Abstract
AIM: To study the expression of vascular endothelial growth factor C (VEGF-C) and chemokine receptor CCR7 in gastric carcinoma and to investigate their associations with lymph node metastasis of gastric carcinoma and their values in predicting lymph node metastasis.
METHODS: The expression of VEGF-C and CCR7 in gastric carcinoma tissues obtained from 118 patients who underwent curative gastrectomy was examined by immunohistochemistry. Among these patients, 39 patients underwent multi-slice spiral CT (MSCT) examination.
RESULTS: VEGF-C and CCR7 were positively expressed in 52.5 and 53.4% of patients. VEGF-C expression was more frequently found in tumors with lymph node metastasis than those without it (P < 0.001). VEGF-C expression was also closely related to lymphatic invasion (P < 0.001), vascular invasion (P < 0.01), and TNM stage (P < 0.001). However, there was no significant correlation between VEGF-C expression and age at surgery, gender, tumor size, tumor location, Lauren classification, and depth of invasion. CCR7 expression was significantly higher in patients with lymph node metastasis compared with those without lymph node metastasis (P < 0.001) and was also associated with tumor size (P < 0.01), depth of invasion (P < 0.001), lymphatic invasion (P < 0.001), and TNM stage (P < 0.001). However, the presence of CCR7 had no correlation to age at surgery, gender, tumor location, Lauren classification, and vascular invasion. Among the 39 patients who underwent MSCT examination, only CCR7 expression was related to lymph node metastasis determined by MSCT (P < 0.05). In the current retrospective study, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of VEGF-C and CCR7 expression in the diagnosis of lymph node metastasis for patients with gastric carcinoma were 73.8%, 70.2%, 72.6%, 71.4% and 72.0%, and 82.0%, 77.2%, 79.4%, 80.0% and 79.7%, respectively. After subdivision according to the combination of VEGF-C and CCR7 expression, receiver operating characteristic (ROC) analysis showed that the accuracy of the combined examination of VEGF-C and CCR7 expression in predicting lymph node metastasis was relatively high (area under ROC curve [Az] = 0.83).
CONCLUSION: The expression of VEGF-C and CCR7 is related to lymph node metastasis of gastric carcinoma and both of them may become new targets for the treatment of gastric carcinoma. Furthermore, the combined examination of VEGF-C and CCR7 expression in endoscopic biopsy specimens may be useful in predicting lymph node metastasis of gastric carcinoma and deciding the extent of surgical lymph node resection.
INTRODUCTION
Lymph node metastasis is an important prognostic factor for patients with gastric carcinoma[1-4]. In spite of this, the function of intratumor lymphatic vessels in lymph node metastasis of gastric carcinoma is unclear. Recently, the molecular pathway that signals for lymphangiogenesis and relatively specific markers for lymphatic endothelium have been described allowing analyses of tumor lymphangiogenesis to be performed[5-8]. Many experimental studies indicated that lymphangiogenic factors (VEGF-C and VEGF-D) could stimulate lymphangiogenesis in tumors by binding to their receptors (VEGFR-3) on the lymphatic endothelial cells and inducing proliferation and growth of new lymphatic capillaries, then enhance the incidence of lymph node metastasis in animal models[9-12]. A recent study by Orlandini et al[13] demonstrated that β-catenin was a negative regulator of VEGF-D mRNA stability, therefore VEGF-D expression might only be responsible for the nodal metastatic behavior in tumors that have lost or reduced expression of β-catenin. In addition, there was apparent discrepancy of VEGF-D expression in tumors reported by different authors[14-18]. Thus, here we only investigate the role of VEGF-C in promoting lymph node metastasis in gastric carcinoma.
It has long been unclear as to why particular cancers preferentially metastasize to certain sites. However, a recent report by Muller et al[19] provided evidence for preferential homing of breast cancer to metastatic sites. The findings indicated that the chemokine receptors CXCR4 and CCR7 were found on breast cancer cells and their ligands were highly expressed at sites associated with breast cancer metastases. In addition, both CCL19 and CCL21 (chemokines, ligands of CCR7) are highly expressed in lymph nodes, therefore the migration of tumor cells positive for CCR7 towards lymph nodes may share many similarities with leukocyte trafficking, which is critically regulated by chemokines and their receptors[20]. A recent study by Takanami et al[20] has demonstrated that CCR7 was related to the development of lymph node metastasis in nonsmall cell lung cancers. Here, we aim to explore the association of CCR7 expression with lymph node metastasis in gastric carcinoma. Furthermore, activation of lymphatics by VEGF-C may promote production of chemoattractants such as CCL21 by lymphatic endothelial cells and thereby facilitate tumor cell positive for CCR7 expression entry into lymphatics[21], so the other aim of this study is to investigate the value of the combined examination of VEGF-C and CCR7 expression in predicting lymph node metastasis of gastric carcinoma.
MATERIALS AND METHODS
Patients and tumor samples
One hundred and eighteen patients, who had undergone curative gastrectomy for gastric carcinoma between 2001 and 2002 in the Department of General Surgery, Ruijin Hospital affiliated to Shanghai Second Medical University, Shanghai, China, were enrolled in this study. All of the resected primary tumors and regional lymph nodes were histologically examined by hematoxylin and eosin staining according to the TNM classification. Among these patients, 39 cases preoperatively underwent MSCT examination by using the method previously reported[22].
Immunohistochemical staining
Formalin-fixed and paraffin-embedded sections of tumor tissue obtained from resected stomach were cut 4 µm thick, dewaxed in xylene and rehydrated in graded alcohol, then immersed in 30 mL/L hydrogen peroxide in methanol for 30 min to inhibit endogenous peroxidase activity. The sections were boiled in EDTA (0.01 mol/L,, pH 8.0) for 20 min for antigen retrieval. After being rinsed in phosphate-buffered saline (PBS), the sections for CCR7 staining were treated sequentially with avidin and biotin (Biotin blocking system) (Dako) to block the endogenous biotin-like molecules. Then, all sections were incubated with normal goat serum for 10 min for blocking nonspecific reaction. The sections were then incubated with primary antibody at 37 °C in humid chambers for 1 h. The rabbit anti-human VEGF-C ployclonal antibody (Zymed) was at a 1:100 dilution, and the mouse anti-human CCR7 monoclonal antibody (BD PharMingen) was at a 1:300 dilution. After washing 3 times with PBS, the sections for CCR7 staining were then incubated for 10 min at room temperature with biotinylated secondary antibody (goat anti-mouse IgM) and streptavidin conjugated to horseradish peroxidase, respectively, whereas the sections for VEGF-C staining were reacted with EnVision plus (Dako) for 30 min at room temperature. After being rinsed 3 times with PBS, the sections were incubated with 3, 3’-diaminobenzidine tetrahydrochloride (DAB) (Dako), then rinsed with distilled water, counterstained with haematoxylin, dehydrated with alcohol and xylene, and mounted routinely. Negative controls were performed in all cases by omitting the first antibody.
Evaluation of immunohistochemical staining
Two pathologists evaluated and interpreted the results of immunohistochemical staining, without knowledge of the clinical data of each patient. The immunohistochemical expression of VEGF-C was defined as positive if distinct staining was observed in at least 30% of tumor cells[23]. CCR7 expression in the cancer tissue was classified as positive if more than 10% tumor cells were immunoreactive. Very faint or equivocal immunoreaction was ignored.
Evaluation of lymph node status by MSCT
Two radiologists prospectively analyzed the CT images. Lymph nodes were considered involved when the short-axis diameter was more than 6 mm for the perigastric lymph nodes and more than 8 mm for the extraperigastric lymph nodes[24]. The preoperative N staging obtained by MSCT was compared with the pathological findings according to the TNM classification (UICC).
Statistical analysis
Statistical analyses were performed using the χ2 test and ROC analysis. P < 0.05 was considered statistically significant. All the statistical analyses were performed using SAS 6.04 software.
RESULTS
Expression of VEGF-C and CCR7 in gastric carcinoma and adjacent normal mucosa
In adjacent normal gastric mucosa, no or faint cytoplasmic staining of VEGF-C was seen in the parietal cells, but almost all epitheial cells were negative. Moderate to strong staining of VEGF-C protein was identified in the cytoplasm of immunoreactive cancer cells (Figure 1). Furthermore, in a few cases, some stromal cells were also positive for VEGF-C. The percentage of patients positive for VEGF-C was 52.5%(62/118). In adjacent normal gastric mucosa, no or very weak staining of CCR7 was also found, whereas moderate to strong staining of CCR7 was found mainly in cell membrane and/or cytoplasm of immunoreactive cancer cells as well as a few lymphocytes (Figure 2). The percentage of patients positive for CCR7 was 53.4%(63/118). Among these samples, there were 2 specimens with 1 metastatic lymph node adjacent to the primary tumor. It turned out that not only the primary tumors but also the metastatic lymph nodes were positive for both VEGF-C and CCR7 expression in both cases (Figures 3 and 4).
Figure 1.
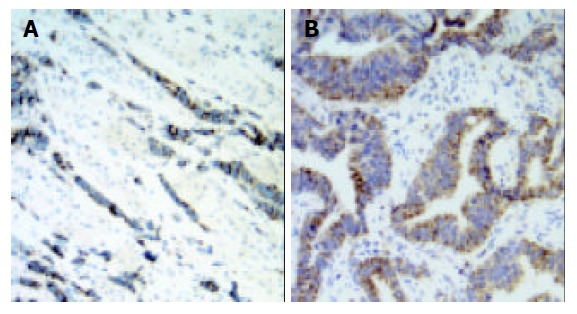
Expression of VEGF-C was observed mainly in the cytoplasm of gastric carcinoma cells (original magnification ×200). A: diffuse gastric carcinoma; B: intestinal gastric carcinoma.
Figure 2.
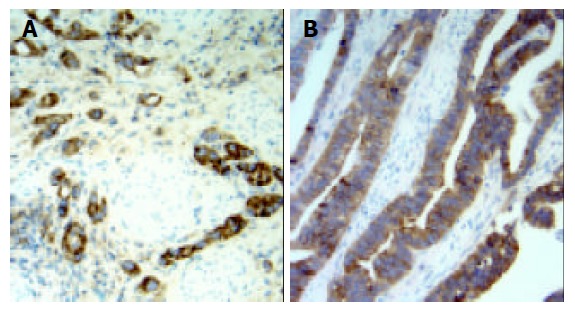
Expression of CCR7 was observed mainly in the cy-toplasm and membrane of gastric carcinoma cells (original magnification ×200). A: diffuse gastric carcinoma; B: intestinal gastric carcinoma.
Figure 3.
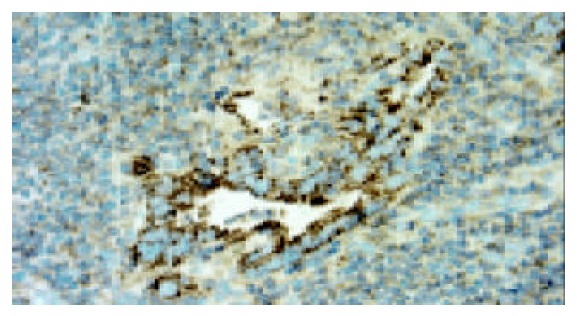
Expression of VEGF-C was observed mainly in gas-tric carcinoma cells in metastatic lymph node (original magni-fication ×400).
Figure 4.
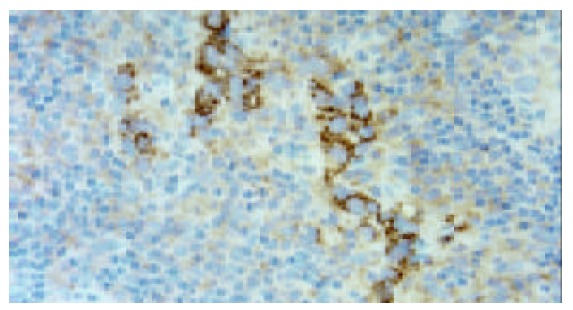
Expression of CCR7 was observed mainly in gastric carcinoma cells in metastatic lymph node (original magnifica-tion ×400).
Association of VEGF-C and CCR7 expression with clinicopathological features
The correlation between VEGF-C and CCR7 expression and clinicopathological factors is summarized in Table 1. VEGF-C expression was more frequently found in tumors with lymph node metastasis than those without it (P < 0.001). VEGF-C expression was also closely related to lymphatic invasion (P < 0.001), vascular invasion (P < 0.01), and TNM stage (P < 0.001). However, there was no significant correlation between VEGF-C expression and age at surgery, gender, tumor size, tumor location, Lauren classification, and depth of invasion. The CCR7 expression was significantly higher in patients with lymph node metastasis compared with those without lymph node metastasis (P < 0.001) and was also associated with tumor size (P < 0.01), depth of invasion (P < 0.001), lymphatic invasion (P < 0.001), and TNM stage (P < 0.001). However, the presence of CCR7 had no correlation to age at surgery, gender, tumor location, Lauren classification, and vascular invasion.
Table 1.
Relationship between expression of VEGF-C and CCR7 and clinicopathologic parameters in patients with gastric carcinoma
| No. of patients |
Expression of VEGF-C |
P value |
Expression of CCR7 |
P value | |||
| Positive | Negative | Positive | Negative | ||||
| Age | NS* | NS | |||||
| <61 years | 46 | 21 | 25 | 23 | 23 | ||
| ≥61years | 72 | 41 | 31 | 40 | 32 | ||
| Gender | NS | NS | |||||
| Male | 71 | 38 | 33 | 39 | 32 | ||
| Female | 47 | 24 | 23 | 24 | 23 | ||
| Tumor size | NS | <0.01 | |||||
| <5 cm | 53 | 23 | 30 | 21 | 32 | ||
| ≥5 cm | 65 | 39 | 26 | 42 | 23 | ||
| Tumor location | NS | NS | |||||
| Lower third (L) | 81 | 40 | 41 | 44 | 37 | ||
| Middle third (M) | 21 | 12 | 9 | 11 | 10 | ||
| Upper third (U) | 16 | 10 | 6 | 8 | 8 | ||
| Lauren classification | NS | NS | |||||
| Intestinal | 53 | 24 | 29 | 25 | 28 | ||
| Diffuse | 53 | 30 | 23 | 32 | 21 | ||
| Mixed | 12 | 8 | 4 | 6 | 6 | ||
| Depth of invasion | NS | <0.001 | |||||
| T1/2 | 24 | 9 | 15 | 5 | 19 | ||
| T3/4 | 94 | 53 | 41 | 58 | 36 | ||
| Lymph node metastasis | <0.001 | <0.001 | |||||
| Positive | 61 | 45 | 16 | 50 | 11 | ||
| Negative | 57 | 17 | 40 | 13 | 44 | ||
| Lymphatic invasion | <0.001 | <0.001 | |||||
| Positive | 68 | 48 | 20 | 51 | 17 | ||
| Negative | 50 | 14 | 36 | 12 | 38 | ||
| Vascular invasion | <0.01 | NS | |||||
| Positive | 35 | 25 | 10 | 20 | 15 | ||
| Negative | 83 | 37 | 46 | 43 | 40 | ||
| Stage | <0.001 | <0.001 | |||||
| I, II | 55 | 19 | 36 | 13 | 42 | ||
| III, IV | 63 | 43 | 20 | 50 | 13 | ||
*NS, not significant.
Relationship between expression of VEGF-C and CCR7 and lymph node metastasis of gastric carcinoma determined by MSCT
As shown in Table 2, among the 39 patients who underwent MSCT examination, CCR7 expression was related to lymph node metastasis determined by MSCT (P < 0.05), whereas there was no significant correlation between VEGF-C expression and lymph node metastasis determined by MSCT (Figures 5-7). However, both VEGF-C and CCR7 expression were closely related to lymph node metastasis determined by pathological examination (P < 0.01). In addition, the accuracy of MSCT in determining the N stage was 69.2%(27/39) (Table 3). Findings at MSCT were concordant with pathological findings in 15 of 20 N0 tumors (75.0%), 5 of 10 N1 tumors (50.0%), and 7 of 9 N2 tumors (77.8%), respectively. The sensitivity, specificity, PPV, NPV, and accuracy of MSCT in predicting lymph node metastasis were 84.2%(16/19), 75.0%(15/20), 76.2%(16/21), 83.3%(15/18), and 79.5%(31/39). However, the sensitivity, specificity, PPV, NPV, and accuracy of VEGF-C and CCR7 expression in the diagnosis of lymph node metastasis for patients with gastric carcinoma were 68.4%(13/19), 75.0%(15/20), 72.2%(13/18), 71.4%(15/21) and 71.8%(28/39), and 73.7% (14/19), 75.0%(15/20), 73.7%(14/19), 75.0%(15/20) and 74.4%(29/39), respectively (Table 2).
Table 2.
Correlation between expression of VEGF-C and CCR7 and lymph node metastasis determined by MSCT and pathological examination
| No. of patients |
Expression of VEGF-C |
P value |
Expression of CCR7 |
P value | |||
| Positive | Negative | Positive | Negative | ||||
| Lymph node metastasis1 | NS3 | <0.05 | |||||
| Positive | 21 | 12 | 9 | 14 | 7 | ||
| Negative | 18 | 6 | 12 | 5 | 13 | ||
| Lymph node metastasis2 | <0.01 | <0.01 | |||||
| Positive | 19 | 13 | 6 | 14 | 5 | ||
| Negative | 20 | 5 | 15 | 5 | 15 | ||
Lymph node metastasis determined by MSCT;
Lymph node metastasis determined by pathological examination;
NS, not significant.
Figure 5.
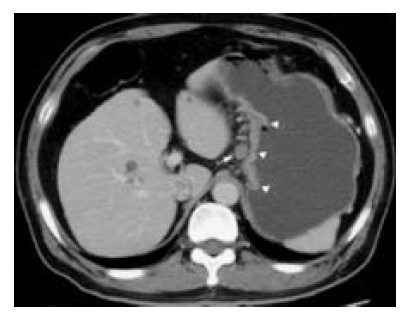
CT scan shows a metastatic lymph node (confirmed by pathologic examination) (arrow) 10 mm in short-axis diameter, which is adjacent to the primary lesion of gastric carcinoma (arrowhead).
Figure 7.
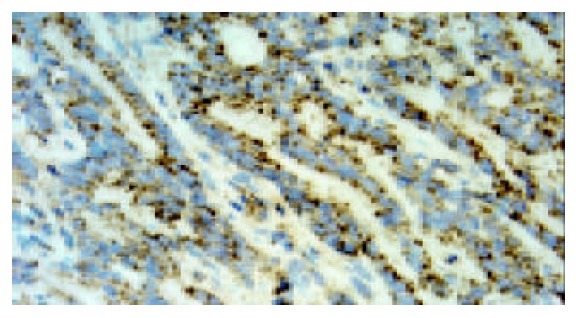
The same patient as Figure 5, expression of VEGF-C was observed in the cytoplasm of gastric carcinoma cells (original magnification ×400).
Table 3.
Comparison of MSCT and pathological diagnosis of N stage
| Pathologic N stage | No. of patients |
N stage determined by MSCT |
|||
| N0 | N1 | N2 | |||
| N0 | 20 | 151 | 2 | 3 | |
| N1 | 10 | 2 | 51 | 3 | |
| N2 | 9 | 1 | 1 | 71 | |
MSCT findings were concordant with pathological findings
Figure 6.
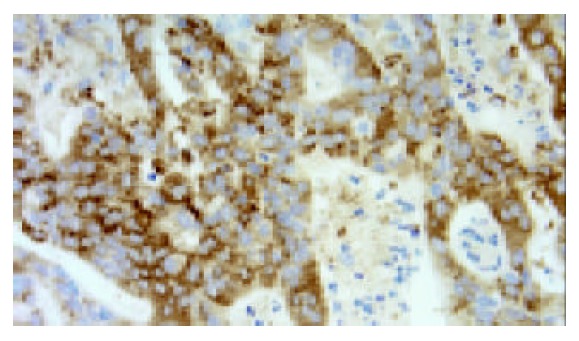
The same patient as Figure 5, expression of CCR7 was observed in the cytoplasm and membrane of gastric carci-noma cells (original magnification ×400).
Value of the combined examination of VEGF-C and CCR7 expression in predicting lymph node metastasis
As shown in Table 1, in the current retrospective study, the sensitivity, specificity, PPV, NPV, and accuracy of VEGF-C and CCR7 expression in the diagnosis of lymph node metastasis for patients with gastric carcinoma were 73.8%(45/61), 70.2%(40/57), 72.6%(45/62), 71.4%(40/56) and 72.0%(85/118), and 82.0%(50/61), 77.2%(44/57), 79.4% (50/63), 80.0%(44/55) and 79.7%(94/118), respectively. To further investigate the value of the combined examination of VEGF-C and CCR7 expression in predicting lymph node metastasis, we divided patients into four groups: group A, CCR7(+) and VEGF-C(+); group B, CCR7(+) and VEGF-C(-); group C, CCR7(-) and VEGF-C(+); group D, CCR7(-) and VEGF-C(-). In groups A, B, C, and D, the percentage of patients with lymph node metastasis was 85.4%(41/48), 60.0%(9/15), 28.6%(4/14), and 17.1%(7/41), respectively (Table 4). In addition, ROC analysis showed that the accuracy of the combined examination of VEGF-C and CCR7 expression in predicting lymph node metastasis was relatively high (area under ROC curve [Az] = 0.83) (Figure 8). When the patients with positive expression of both VEGF-C and CCR7 were regarded as those with lymph node metastasis, the PPV in the diagnosis of lymph node metastasis reached to 85.4%(41/48). In addition, when the patients with negative expression of both VEGF-C and CCR7 were regarded as those without lymph node metastasis, the NPV reached to 82.9% (34/41).
Table 4.
Relationship between expression of VEGF-C and CCR7 after the subdivision and lymph node metastasis
| Lymph node metastasis | No. of patients |
Expression of VEGF-C and CCR7 |
|||
| CCR7(+) and VEGF-C(+) | CCR7(+) and VEGF-C(-) | CCR7(+) and VEGF-C(+) | CCR7(+) and VEGF-C(-) | ||
| Positive | 61 | 41 | 9 | 4 | 7 |
| Negative | 57 | 7 | 6 | 10 | 34 |
Figure 8.
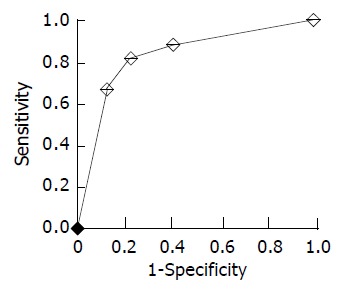
ROC curve generated from the combination of VEGF-C and CCR7 expression shows area under curve to be 0.83.
DISCUSSION
Tumor cells can disseminate within the body by a number of mechanisms, such as direct invasion of surrounding tissues, lymphatic spread and hematogenous spread. The role of angiogenesis involving blood vessels in facilitating the growth and hematogenic spread of solid tumors has been well established. The ability of tumor cells to induce angiogenesis is considered a prerequisite for tumor growth, invasion, and successful metastasis, and the angiogenic switch is recognized as one of the key events in tumorigenesis[25-30]. In contrast, studies of the lymphatic system have been hindered by the lack of specific markers for tumor-associated lymphatic vessels and growth factors that control lymphatic development and lymphatic vessel growth (lymphangiogenesis), although the importance of the lymphatic system as a pathway for lymph node metastasis has been well recognized. In the past few years, with the identification of proteins such as hyaluronan receptor LYVE-1[31], prox-1[7], podoplanin [32], and desmoplakin[33] specifically expressed on lymphatic vessels and the discovery of molecules (VEGF-C and VEGF-D) that can drive lymphatic vessel growth, occurrence of tumor lymphaniogenesis and its correlation with lymph node metastasis has been proved in animal models and clinicopathological studies.
Nine recently reported animal studies have provided the first direct in vivo evidence that tumours are able not only to induce lymphangiogenesis but also to enhance lymph node metastasis[8-12,34-37]. Yanai et al[37] constructed a VEGF-C transfectant (AZ-VEGF-C) from the AZ521 human gastric carcinoma cell line, which ordinarily shows little nodal metastatic potential and little VEGF-C expression. They orthotopically implanted transfected tumor cells into the stomachs of nude mice. The number of mice developing lymph node metastases and the number of lymph node metastases per mouse with nodal metastases were higher than with implants of mock-transfected control cells. In addition, the number of vessels stained for VEGFR-3 in tumors and surrounding tissues was higher for AZ-VEGF-C than for controls. Furthermore, recent studies in human head and neck cancer[38,39] and melanoma[40] have demonstrated the existence of proliferating intratumoral lymphatic vessels. Clinical studies using the RT-PCR, in situ hybridization, and/or immunohistochemistry have revealed significant associations between lymph node metastasis and VEGF-C or VEGF-D expression in human primary tumors of the breast, colon, rectum, prostate, testis, esophagus, stomach, thyroid, lung, pleura, head-and-neck squamous cells, uterine cervix, and endometrium[41]. Because of the reasons above-mentioned, we only investigated the VEGF-C expression in gastric carcinoma.
The findings of the current study demonstrated a positive correlation of VEGF-C expression with both lymph node metastasis and lymphatic invasion in gastric carcinoma. Our results were consistent with those of previous reports[23,42-47]. A study by Yanai et al[37] indicated that VEGF-C was a specific lymphangiogenic growth factor with an important role in lymph node metastasis of gastric carcinoma in an animal model. In addition, the studies by Yonemura et al[42,43] showed that VEGF-C mRNA level was closely related to VEGFR-3 mRNA level and the number of VEGFR-3-positive lymphatic vessels in human gastric carcinoma. Thus, VEGF-C may stimulate lymphangiogenesis in gastric carcinoma by binding to their receptors (VEGFR-3) on the lymphatic endothelial cells and inducing proliferation and growth of new lymphatic capillaries, then enhance the incidence of lymph node metastasis in gastric carcinoma. However, our present study showed that VEGF-C expression was closely related to lymph node metastasis determined by pathological examination, but not by MSCT examination, although the accuracy of MSCT in the diagnosis of the lymph node metastasis for 39 patients with gastric carcinoma reached to 79.5%. Nevertheless, Hashimoto et al[48] demonstrated that VEGF-C mRNA expression was correlated with lymph node metastasis diagnosed by magnetic resonance (MR) imaging in cervical cancer. So further study must be made. Furthermore, our present study showed that the VEGF-C expression was correlated with vascular invasion in gastric carcinoma. Other investigators[42,45] also found a positive correlation between VEGF-C expression and vascular invasion in gastric carcinoma. In addition, a study by Amioka et al[23] showed that VEGF-C expression in tumor cells was closely related to the microvessel density (MVD) in gastric carcinoma. As we all know, VEGF-C is a ligand for VEGFR-2 and VEGFR-3. VEGFR-2 is mainly expressed on vascular endothelial cells. However, VEGF-C displays greater affinity for VEGFR-3 than VEGFR-2, and only the mature 21-kDa form of VEGF-C can bind to VEGFR-2[36]. As a result, VEGF-C may also be correlated with angiogenesis in gastric carcinoma, but the activity of tumor angiogenesis by VEGF-C may be weak. Further study is needed to investigate the biological effect of VEGF-C on angiogenesis in gastric carcinoma.
Chemokines are chemoattractant cytokines that bind to specific G-protein-coupled receptors on target cells; these target cells follow chemokine concentration gradients into selected tissues. Through their interactions with target cells, these small proteins induce cytoskeletal rearrangement and directional migration. Chemokines are involved in physiologic and pathologic regulation of leukocyte trafficking[41]. Recent studies showed that chemokine receptor CCR7 was expressed in the tumor cells of breast cancer[19], nonsmall cell lung cancer[20], esophageal squmous cell carcinoma[49], and gastric carcinoma[50]. In addition, both CCL21 and CCL19 (chemokines, liagands of CCR7) show most abundant expression in lymph nodes. CCL21 is expressed in the high endothelial venules of lymph nodes and in the T-cell zone of lymph nodes. CCL19 is expressed predominantly by stromal cells within the T-cell zones of lymph nodes[19]. Previous studies showed that lymphatic endothelial cells also produced CCL21[51,52]. In vitro experiments showed that actin polymerization and pseudopodia formation, which were needed for the invasion of malignant cells into tissues and for efficient metastasis, could be induced by CCL21 and CCL19 in tumor cells with CCR7 expression[19,49,50]. Thus, the migration of tumor cells positive for CCR7 towards lymph nodes may share many similarities with leukocyte trafficking. In addition, CCR7 may help retain tumor cells in the lymph nodes where the CCR7 ligands are rich.
Wiley et al[53] examined the role of CCR7 in regional lymph node metastasis in a murine melanoma model. They transduced a murine melanoma cell line, B16, with CCR7 and, surprisingly, transduction with this single gene was sufficient to substantially increase the metastasis to draining lymph nodes of B16 cells, which otherwise have a low propensity to spread, and metastasis was completely blocked by adding neutralizing anti-CCL21 antibodies. In the model used by Wiley et al transduced cells were injected subcutaneously, thus mimicking lymphgenic metastasis of melanoma cells from the skin. Of interest, intravenous application of CCR7-transduced B16 melanoma cells did not show increased spread of the tumor cells to lung or peripheral lymph nodes. In clinicopathologic studies, a positive correlation of CCR7 expression with lymphatic invasion and lymph node metastasis has been found in nonsmall cell lung cancer[20], esophageal squmous cell carcinoma[49], and gastric carcinoma[50]. Here we investigate the role of chemokine receptor CCR7 in directional migration of gastric carcinoma cells towards lymph nodes.
The findings of the current study demonstrated that the CCR7 expression in the cytoplasm and / or member of gastric carcinoma cells was closely correlated with lymphatic invasion, lymph node metastasis, and TNM stage, but was not correlated with vascular invasion. Our findings were consistent with those reported by Mashino et al[50]. In addition, the study by Mashino et al[50] showed that CCR7 expression was closely related to histological classification, whereas the current study showed that there was no significant correlation between CCR7 expression and Lauren classification. The main reason for this discrepancy was that there was no significant correlation between Lauren classification and lymph node metastasis among the 118 patients, although the incidence of lymph node metastasis in diffuse gastric carcinoma (50.9%) was higher than that in intestinal gastric carcinoma (45.3%). Furthermore, the current study showed that the CCR7 expression was closely correlated with lymph node metastasis determined not only by pathological examination but also by MSCT examination.
Many factors affect prognosis in patients with gastric carcinoma, including tumor size, lymph node metastasis, depth of tumor invasion, distant metastasis, histological classification, and surgery performed. Of these, lymph node metastasis is one of the most important factors. Therefore, preoperative evaluation of lymph node status is essential. With the development of endoscopic or laparoscopic resection of small, localized gastric carcinoma, the pretherapeutic evaluation of lymph node metastasis has become more important. For preoperative N staging, endoscopic ultrasonography (EUS), CT, and MR imaging have been used[4,54-58]. However, the paraaortal and celiacal regions are often beyond the scope of the EUS[55], and comparative studies demonstrated that the accuracy of CT in N staging was higher than that of MR imaging[57,58]. Therefore, among these three methods, CT may be superior to EUS and MR imaging in predicting lymph node status for patients with gastric carcinoma. In the current study, the accuracy of MSCT in N staging was 69.2%. Notably, the accuracy of MSCT in predicting lymph node metastasis reached to 79.5%, and the sensitivity and specificity was 84.2% and 75.0%, respectively. However, although spiral CT could detect lymph nodes small than 5 mm and the sensitivity for detecting metastasis-positive nodes was remarkably higher than that for detecting metastasis-negative nodes[59], both overstaging and downstaging still existed because lymph node size was not a reliable indicator for lymph node metastasis of gastric carcinoma[60]. Furthermore, Maruyama computer program can also be used to predict lymph node metastasis for patients with gastric carcinoma. Analysis of eight prognostic parameters such as gender, age, macroscopic type, location, position, diameter, histological classification, and depth of invasion using this computer program can gain the information on the expected frequency of metastasis in the 16 lymph node stations of each patient with gastric carcinoma[61,62]. However, to date, the prospective study for evaluating the accuracy of this computer program has not been reported. Two retrospective studies[61,62] showed that the accuracy of Maruyama computer program in predicting lymph node metastasis was relatively high, but the specificity was too low. In addition, only clinicopatologic parameters were included in this computer program, whereas the molecular biologic features of gastric carcinoma were neglected.
Recently, a study using the microarray technology showed that lymph node metastasis of gastric carcinoma could be precisely predicted from the view of molecular biologic features. In this study, investigators developed an equation to achieve a scoring parameter for the prediction of lymph node metastasis on the basis of 12 genes with statistically significant differences in expression between node-positive and node-negative tumors. In the prospective study, the lymph node status of all nine test cases was correctly predicted[63]. In the current retrospective study, the sensitivity, specificity, PPV, NPV, and accuracy of VEGF-C and CCR7 expression in the diagnosis of lymph node metastasis for patients with gastric carcinoma were 73.8%, 70.2%, 72.6%, 71.4% and 72.0%, and 82.0%, 77.2%, 79.4%, 80.0% and 79.7%, respectively. Especially when the combined examination of VEGF-C and CCR7 expression was performed, the PPV and NPV reached to 85.4% and 82.9%, respectively. However, the PPV and NPV of MSCT in predicting lymph node metastasis was only 76.2% and 83.3%, respectively, among 39 patients. In addition, successful lymph node metastasis requires a complex series of related steps, such as detachment of cancer cells, invasion into the lymphatic vessels, transfer within the lymphatic vessels, embedding in the lymph nodes and proliferation in the lymph nodes. Therefore, lymphangiogenesis and directional migration of gastric carcinoma cells towards lymph nodes may only be two main steps involved in lymph node metastasis. Thus, we hypothesize that an equation established on the basis of many factors involved in the metastasis-associated steps can predict the lymph node status for patients with gastric carcinoma more accurately, especially when it is combined with Maruyama computer program and MSCT.
In conclusion, the current study demonstrates that expression of VEGF-C and CCR7 is related to lymph node metastasis of gastric carcinoma and both of them may become new targets for the treatment of gastric carcinoma. Furthermore, the combined examination of VEGF-C and CCR7 expression in endoscopic biopsy specimens may be useful in predicting lymph node metastasis of gastric carcinoma and deciding the extent of surgical lymph node resection.
Footnotes
Edited by Zhang JZ Proofread by Xu FM
References
- 1.Zheng HC, Li YL, Sun JM, Yang XF, Li XH, Jiang WG, Zhang YC, Xin Y. Growth, invasion, metastasis, differentiation, angiogenesis and apoptosis of gastric cancer regulated by expression of PTEN encoding products. World J Gastroenterol. 2003;9:1662–1666. doi: 10.3748/wjg.v9.i8.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2003;9:1409–1414. doi: 10.3748/wjg.v9.i7.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987–993. doi: 10.3748/wjg.v8.i6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan C, Zhu ZG, Zhu Q, Yan M, Chen J, Liu BY, Yin HR, Lin YZ. [A preliminary study of endoscopic ultrasonography in the preoperative staging of early gastric carcinoma] Zhonghua Zhong Liu Za Zhi. 2003;25:390–393. [PubMed] [Google Scholar]
- 5.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 6.Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 9.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 10.Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Härkönen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946–951. doi: 10.1002/ijc.10283. [DOI] [PubMed] [Google Scholar]
- 11.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 12.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 13.Orlandini M, Semboloni S, Oliviero S. Beta-catenin inversely regulates vascular endothelial growth factor-D mRNA stability. J Biol Chem. 2003;278:44650–44656. doi: 10.1074/jbc.M304255200. [DOI] [PubMed] [Google Scholar]
- 14.Funaki H, Nishimura G, Harada S, Ninomiya I, Terada I, Fushida S, Tani T, Fujimura T, Kayahara M, Shimizu K, et al. Expression of vascular endothelial growth factor D is associated with lymph node metastasis in human colorectal carcinoma. Oncology. 2003;64:416–422. doi: 10.1159/000070301. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami M, Furuhata T, Kimura Y, Yamaguchi K, Hata F, Sasaki K, Hirata K. Expression analysis of vascular endothelial growth factors and their relationships to lymph node metastasis in human colorectal cancer. J Exp Clin Cancer Res. 2003;22:229–237. [PubMed] [Google Scholar]
- 16.White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, Murray JC. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002;62:1669–1675. [PubMed] [Google Scholar]
- 17.Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200:183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Sakamoto A, Sakamoto T, Maruyama H, et al. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9:1361–1369. [PubMed] [Google Scholar]
- 19.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 20.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105:186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 21.Cassella M, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2002;979:120–130. doi: 10.1111/j.1749-6632.2002.tb04873.x. [DOI] [PubMed] [Google Scholar]
- 22.Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr. 1998;22:288–294. doi: 10.1097/00004728-199803000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, Chayama K. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002;38:1413–1419. doi: 10.1016/s0959-8049(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 24.D'Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol. 2000;10:1877–1885. doi: 10.1007/s003300000537. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZQ, Li JS, Lu GM, Zhang XH, Chen ZQ, Meng K. Correlation of CT enhancement, tumor angiogenesis and pathologic grading of pancreatic carcinoma. World J Gastroenterol. 2003;9:2100–2104. doi: 10.3748/wjg.v9.i9.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604–1606. doi: 10.3748/wjg.v9.i7.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421–1426. doi: 10.3748/wjg.v9.i7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong B, Sun TJ, Yuan HY, Hu MB, Hu WD, Cheng FL. Cyclooxygenase-2 expression and angiogenesis in colorectal cancer. World J Gastroenterol. 2003;9:1237–1240. doi: 10.3748/wjg.v9.i6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng S, Han MY, Xiao ZX, Peng JP, Dong Q. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J Gastroenterol. 2003;9:1227–1230. doi: 10.3748/wjg.v9.i6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144–1155. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sedivy R, Beck-Mannagetta J, Haverkampf C, Battistutti W, Hönigschnabl S. Expression of vascular endothelial growth factor-C correlates with the lymphatic microvessel density and the nodal status in oral squamous cell cancer. J Oral Pathol Med. 2003;32:455–460. doi: 10.1034/j.1600-0714.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 33.Ebata N, Nodasaka Y, Sawa Y, Yamaoka Y, Makino S, Totsuka Y, Yoshida S. Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res. 2001;61:40–48. doi: 10.1006/mvre.2000.2280. [DOI] [PubMed] [Google Scholar]
- 34.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 36.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanai Y, Furuhata T, Kimura Y, Yamaguchi K, Yasoshima T, Mitaka T, Mochizuki Y, Hirata K. Vascular endothelial growth factor C promotes human gastric carcinoma lymph node metastasis in mice. J Exp Clin Cancer Res. 2001;20:419–428. [PubMed] [Google Scholar]
- 38.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. [PubMed] [Google Scholar]
- 39.Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–1926. [PubMed] [Google Scholar]
- 40.Straume O, Jackson DG, Akslen LA. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9:250–256. [PubMed] [Google Scholar]
- 41.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98:413–423. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 42.Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]
- 43.Yonemura Y, Fushida S, Bando E, Kinoshita K, Miwa K, Endo Y, Sugiyama K, Partanen T, Yamamoto H, Sasaki T. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 2001;37:918–923. doi: 10.1016/s0959-8049(01)00015-6. [DOI] [PubMed] [Google Scholar]
- 44.Kabashima A, Maehara Y, Kakeji Y, Sugimachi K. Overexpression of vascular endothelial growth factor C is related to lymphogenous metastasis in early gastric carcinoma. Oncology. 2001;60:146–150. doi: 10.1159/000055312. [DOI] [PubMed] [Google Scholar]
- 45.Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol. 2001;78:132–137. doi: 10.1002/jso.1133. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi A, Kono K, Itakura J, Amemiya H, Feng Tang R, Iizuka H, Fujii H, Matsumoto Y. Correlation of vascular endothelial growth factor-C expression with tumor-infiltrating dendritic cells in gastric cancer. Oncology. 2002;62:121–127. doi: 10.1159/000048257. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa M, Kitayama J, Kazama S, Nagawa H. Expression of vascular endothelial growth factor C and D (VEGF-C and -D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol. 2003;33:21–27. doi: 10.1093/jjco/hyg008. [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto I, Kodama J, Seki N, Hongo A, Yoshinouchi M, Okuda H, Kudo T. Vascular endothelial growth factor-C expression and its relationship to pelvic lymph node status in invasive cervical cancer. Br J Cancer. 2001;85:93–97. doi: 10.1054/bjoc.2001.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H, Imamura M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9:3406–3412. [PubMed] [Google Scholar]
- 50.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 51.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 53.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 54.Xi WD, Zhao C, Ren GS. Endoscopic ultrasonography in preoperative staging of gastric cancer: determination of tumor invasion depth, nodal involvement and surgical resectability. World J Gastroenterol. 2003;9:254–257. doi: 10.3748/wjg.v9.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willis S, Truong S, Gribnitz S, Fass J, Schumpelick V. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surg Endosc. 2000;14:951–954. doi: 10.1007/s004640010040. [DOI] [PubMed] [Google Scholar]
- 56.Chen F, Ni YC, Zheng KE, Ju SH, Sun J, Ou XL, Xu MH, Zhang H, Marchal G. Spiral CT in gastric carcinoma: comparison with barium study, fiberoptic gastroscopy and histopathology. World J Gastroenterol. 2003;9:1404–1408. doi: 10.3748/wjg.v9.i7.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim AY, Han JK, Seong CK, Kim TK, Choi BI. MRI in staging advanced gastric cancer: is it useful compared with spiral CT. J Comput Assist Tomogr. 2000;24:389–394. doi: 10.1097/00004728-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM, Kim KM. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR Am J Roentgenol. 2000;174:1551–1557. doi: 10.2214/ajr.174.6.1741551. [DOI] [PubMed] [Google Scholar]
- 59.Fukuya T, Honda H, Hayashi T, Kaneko K, Tateshi Y, Ro T, Maehara Y, Tanaka M, Tsuneyoshi M, Masuda K. Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer. Radiology. 1995;197:705–711. doi: 10.1148/radiology.197.3.7480743. [DOI] [PubMed] [Google Scholar]
- 60.Mönig SP, Zirbes TK, Schröder W, Baldus SE, Lindemann DG, Dienes HP, Hölscher AH. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol. 1999;173:365–367. doi: 10.2214/ajr.173.2.10430138. [DOI] [PubMed] [Google Scholar]
- 61.Bollschweiler E, Boettcher K, Hoelscher AH, Sasako M, Kinoshita T, Maruyama K, Siewert JR. Preoperative assessment of lymph node metastases in patients with gastric cancer: evaluation of the Maruyama computer program. Br J Surg. 1992;79:156–160. doi: 10.1002/bjs.1800790221. [DOI] [PubMed] [Google Scholar]
- 62.Guadagni S, de Manzoni G, Catarci M, Valenti M, Amicucci G, De Bernardinis G, Cordiano C, Carboni M, Maruyama K. Evaluation of the Maruyama computer program accuracy for preoperative estimation of lymph node metastases from gastric cancer. World J Surg. 2000;24:1550–1558. doi: 10.1007/s002680010276. [DOI] [PubMed] [Google Scholar]
- 63.Hasegawa S, Furukawa Y, Li M, Satoh S, Kato T, Watanabe T, Katagiri T, Tsunoda T, Yamaoka Y, Nakamura Y. Genome-wide analysis of gene expression in intestinal-type gastric cancers using a complementary DNA microarray representing 23,040 genes. Cancer Res. 2002;62:7012–7017. [PubMed] [Google Scholar]


