Abstract
AIM: To study the effects of daidzein on human pancreatic cancer cells in vitro.
METHODS: Human estrogen-receptor (ER)-positive pancreatic cancer cells MiaPaCa-2 and ER-negative pancreatic cancer cells PANC-1 were treated by 0.1 μmol/L, 1 μmol/L, 10 μmol/L, 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L of daidzein, respectively. Its antiproliferative effect was studied by MTT assay.
RESULTS: Daidzein inhibited the growth of MiaPaCa-2 and PANC-1 cells at the concentrations from 0.1 μmol/L to 100 μmol/L. A dose- and time-dependent manner was found. The IC50 of daidzein on MiaPaCa-2 and PANC-1 cells was 45 μmol/L and 75 μmol/L, respectively. After MiaPaCa-2 cells were treated by daidzein for 3 d and at the concentrations more than IC50, the inhibitory manner was identical and the inhibition appeared a saturation phenomenon, but the inhibitory manner of daidzein on PANC-1 cells was different from that of MiaPaCa-2 cells.
CONCLUSION: Daidzein has antiproliferative effects on human estrogen-receptor-positive and negative pancreatic cancer cells, but their mechanisms may be different.
INTRODUCTION
Phytoestrogens are natural compounds, which exhibit estrogen-like activities. They have been thought to have a preventive effect against a wide range of human conditions, including breast cancer, bowel cancer, prostate cancer, colon cancer and other cancers, cardiovascular diseases, brain function, alcohol abuse, osteoporosis and menopausal symptoms[1-4]. There are three main classes of phytoestrogens, namely isoflavones, coumestans, and lignans[5]. They are widely distributed in soybeans, oil seeds, and vegetables. Soy contains a number of isoflavones (such as genistein, genistin, daidzein, and biochanin A), which are found to have potential anticancer properties[6]. Interestingly, phytoestrogens can not only inhibit the growth of estrogen-receptor (ER)-dependent cancer cells, but also the growth of ER-independent cancer cells[7]. The mechanisms of their anticancer effects include induction of apoptosis, alterations of cell cycle distribution, etc.[8,9].
The morbidity of pancreatic carcinoma has taken an upward trend all over the world. In Occidental countries, the morbidity of pancreatic carcinoma has increased by 3 to 7 times in nearly thirty years, and pancreatic carcinoma has become one of the ten commonest malignant tumors[10]. Only 15% of patients with pancreatic cancer could undergo resection and the overall 5-year survival rate was about 10%[11]. In recent years, many advances in the diagnosis and treatment (including surgical and adjuvant treatment) of pancreatic cancer have been achieved[12-14].
Estrogen receptor has been found in many cases of pancreatic tumor tissues and antiestrogen therapy has benefit to some patients with pancreatic cancers[15,16]. There is evidence to support the hypothesis that the increased incidence of pancreatic cancer in Western communities might be related to the relatively low dietary content and protective role of naturally occurring plant hormones and related compounds[17]. Generally speaking, treatment of pancreatic cancer is still a serious challenge to us[10]. The key problem is to seek novel diagnostic methods, effective adjunctive therapy and the mechanism of pathogenesis and progression of pancreatic cancer. Pancreatic cancer is often detected in its late stages. As a result, it is very important to develop new potential chemopreventive and anticancer reagents for pancreatic cancer, especially those from nature. The effects of daidzein, one of the rich compounds in Eastern countries’ traditional diet, on human pancreatic cancer cells are unclear. In this study, we observed the effects of daidzein on the growth of human pancreatic cancer cells in vitro.
MATERIALS AND METHODS
Materials
Daidzein was purchased from Sigma Chemical Co. (St. Louis, MO) and dissolved in dimethylsulfoxide (DMSO). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was from Sigma. RPMI-1640 medium was from Life Technologies (Grand Island, NY). Human pancreatic cancer cell lines MiaPaCa-2 and PANC-1 were obtained from Department of General and Gastroenterological Surgery, Osaka Medical College, Takatsuki City, Japan.
Cell culture and treatment
Cells were cultured in plastic flasks or multi-well plates at 37 °C in a humidified atmosphere of 50 ml/L CO2 and 950 ml/L air with RPMI-1640 medium containing 100 ml/L fetal calf serum, 50000 U/L penicillin and 50 mg/L streptomycin. The medium was changed every other day. Exponentially growing cells were used in experiments. For quantitative assays of proliferation, 1 × 104 MiaPaCa-2 or PANC-1 cells were seeded in 96-well plates in regular growth medium. Twenty-four hours later, cells were incubated in the medium with test compounds or 1 ml/L DMSO (as control). There were 7 test groups. Their concentrations of daidzein were 0.1 μmol/L, 1 μmol/L, 10 μmol/L, 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L, respectively. After cells were treated for 1 d, 2 d, 3 d, 4 d, 5 d and 6 d, the growth of cells was monitored by MTT assay.
MTT assay
Sets of 12 wells were used for each dose and control in this assay. When the growth time reached, 30 μL MTT solution (2 g/L in PBS) was added into each well of a 96-well plate. After cells were treated for 4 h at 37 °C, the medium was removed and then 150 μL DMSO was added into each well to solubilize formazan. After that, the microplate was shaken on a rotary platform for 10 min. Finally, absorbance was measured at 550 nm with a Wellscan (Labsystems, USA). The inhibitive rate (%) = ((Acontrol-Aexperiment)/Acontrol) × 100%.
Statistics
Statistical analysis was performed using SPSS software 10.0. Student’s t-test was used to make a statistical comparison between groups. The level of significance was set at P < 0.05.
RESULTS
Morphologic change of the cells
The effects of daidzein on the growth of pancreatic cancer MiaPaCa-2 and PANC-1 cells were tested over a range of concentrations from 0.1 μmol/L to 100 μmol/L. The changes of configuration and number were observed under a microscope (Figure 1, Figure 2). Both pancreatic cancer cells treated by daidzein grew slower than control (Figures 1, Figure 2). The higher the concentration of daidzein, the less the number of cells.
Figure 1.
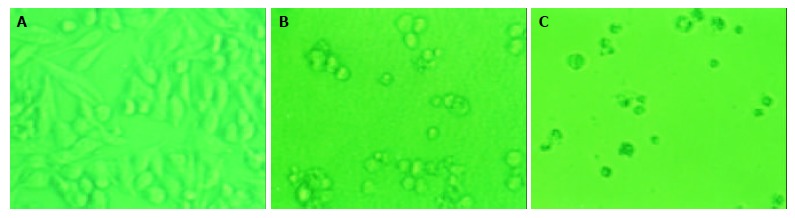
Morphologic changes of MiaPaCa-2 cells treated by daidzein. A: Control, B: Cells treated by 10 μmol/L of daidzein for 3 d, C: Cells treated by 75 μmol/L of daidzein for 3 d (×200).
Figure 2.
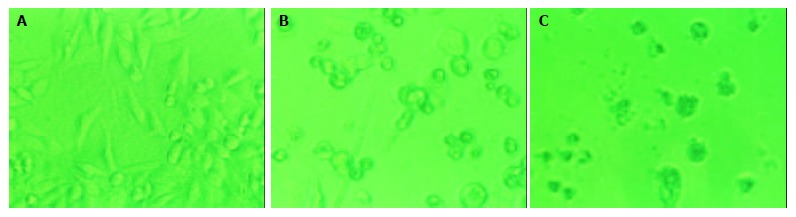
Morphologic changes of PANC-1 cells treated by daidzein. A: Control, B: Cells treated by 10 μmol/L of daidzein for 3 d, C: Cells treated by 75 μmol/L of daidzein for 3 d (×200).
Inhibitory effect of daidzein on estrogen-receptor-positive pancreatic cancer cells
Estrogen-receptor-positive MiaPaCa-2 cells were treated by various concentrations of daidzein for 1 d to 6 d. At the concentrations from 0.1 μmol/L to 100 μmol/L, especially at 50 μmol/L, 75 μmol/L, and 100 μmol/L, daidzein inhibited the growth of cells (P < 0.01) in a time-dependent manner (Figure 3). When cells were treated for 3 d, 50% inhibitory rate might be reached. The IC50 was 45 μmol/L. At the concentrations of more than IC50, the inhibitory patterns were identical (P > 0.05, Figure 3).
Figure 3.
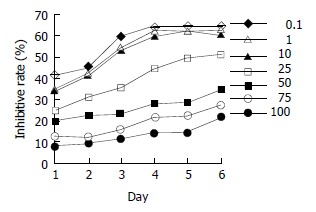
Inhibitory effects of daidzein on growth of estrogen-receptor-positive pancreatic cancer MiaPaCa-2 cells. 0.1 μmol/L, 1 μmol/L, 10 μmol/L, 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L of daidzein were used, respectively.
Inhibitory effect of daidzein on estrogen-receptor-negative pancreatic cancer cells
ER-negative pancreatic cancer PANC-1 cells were treated by daidzein. A dose-dependent effect was observed (Figure 4; 10 μmol/L, 25 μmol/L, 50 μmol/L and 75 μmol/L, P < 0.05; 100 μmol/L, P < 0.01). When cells were treated for 2 d, 50% inhibitory rate might be observed. The IC50 was 80 μmol/L on day 2 and 75 μmol/L on day 3.
Figure 4.
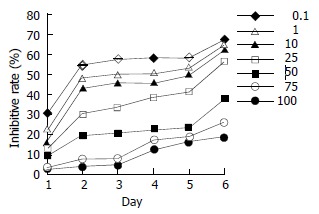
Inhibitory effects of daidzein on growth of estrogen-receptor-negative pancreatic cancer PANC-1 cells. 0.1 μmol/L, 1 μmol/L, 10 μmol/L, 25 μmol/L, 50 μmol/L, 75 μmol/L and 100 μmol/L of daidzein were used, respectively.
DISCUSSION
There has been an increase in the incidence of pancreatic cancer recent years[18-21]. The pattern of its increase had some similarities to the high incidence of breast cancer in women and prostate cancer in men in Western communities[17]. Typical Western diets have a low content of natural plant hormones, phytoestrogens, which are beneficial to our health[1-6]. They exerted their anticancer effects by their specific bi-effects, estrogenic and antiestrogenic properties[22-24]. On the one hand, they acted as antiestroges by competing with endogenous estrogens for receptor binding sites, and decrease the promoting effects of high levels of endogenous estrogens[25]. On the other hand, they could inhibit the activities of tyrosine protein kinase, DNA topisomerase, angiogenesis and antioxidant[26-29].
Hormone products were related to the pathological behaviors of several carcinomas (including colorectal carcinoma, gastric carcinoma, pancreatic carcinoma, etc.) and might help to predict the prognosis and effectiveness of endocrine therapy for some kinds of carcinomas[30-32]. As to pancreatic carcinoma, there were indications of possible effects of sex hormones[33]. The pancreatic cell lines, MiaPaCa-2 and PANC-1, used in our study were ER positive and negative cells, respectively[34,35]. We found that daidzein inhibited the growth of both ER positive and negative pancreatic cancer cells. However, their inhibitory patterns were different. For ER-positive cells, MiaPaCa-2, when the concentrations of daidzein were higher than the level of IC50, the inhibitory effect would not significantly increase. It indicated that its antiprolifertive effect was exerted by binding to ER and appeared a saturation phenomenon. For PANC-1, an ER-negative cell, the saturation phenomenon was not found and the inhibition was in a dose-dependent manner. Its IC50 was higher than that of MiaPaCa-2, indicating that for the chemoprevention of ER-negative pancreatic cancer, high concentrations of daidzein should be used.
The antiestrogen reagent, tamoxifen was a choice of hormonal therapy for pancreatic cancer[16,33]. The favorable response to this therapy and survival of patients were dependent on whether the patients’ tumor cells were estrogen receptor positive or not[16]. The presence or absence of estrogen receptors was also important in determining the effects of phytoestrogens on pancreatic cancer cells. A report used four kinds of phytoestrogens to study human pancreatic cancer cells showed that equol and coumestrol had anticarcinogenic effects on human ER positive pancreatic cancer Su86.86 cells. However, they stimulated the growth of human ER negative pancreatic cancer HPAF-11 cells, genistein stimulated the growth of HPAF-11 cells but had little effect on Su86.86 cells. Another phytoestrogen, biochanin A, inhibited the growth of both Su86.86 cells and HPAF-11 cells[36]. Our results showed that daidzein, another main phytoestrogen, had inhibitory effects on both ER positive and negative pancreatic cancer cells. As phytoestrogens are a large group of compounds, the possibility of gender differences in response to these reagents may be important in determining their ability to act as chemoprotective reagents for pancreatic cancer.
Footnotes
Supported by Scientific Research Fund of Zhejiang Provincial Education Department, No.20010217
Edited by Zhang JZ and Wang XL Proofread by Xu FM
References
- 1.Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A. Phyto-oestrogens: where are we now. Br J Nutr. 1998;79:393–406. doi: 10.1079/bjn19980068. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906–913. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- 3.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 4.Morrissey C, Watson RW. Phytoestrogens and prostate cancer. Curr Drug Targets. 2003;4:231–241. doi: 10.2174/1389450033491154. [DOI] [PubMed] [Google Scholar]
- 5.Peeters PH, Keinan-Boker L, van der Schouw YT, Grobbee DE. Phytoestrogens and breast cancer risk. Review of the epidemiological evidence. Breast Cancer Res Treat. 2003;77:171–183. doi: 10.1023/a:1021381101632. [DOI] [PubMed] [Google Scholar]
- 6.Zhou JR, Mukherjee P, Gugger ET, Tanaka T, Blackburn GL, Clinton SK. Inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res. 1998;58:5231–5238. [PubMed] [Google Scholar]
- 7.Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, Andò S. Estrogen receptor alpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol Pharmacol. 2001;60:595–602. [PubMed] [Google Scholar]
- 8.Yanagihara K, Ito A, Toge T, Numoto M. Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 1993;53:5815–5821. [PubMed] [Google Scholar]
- 9.Su SJ, Yeh TM, Lei HY, Chow NH. The potential of soybean foods as a chemoprevention approach for human urinary tract cancer. Clin Cancer Res. 2000;6:230–236. [PubMed] [Google Scholar]
- 10.Tan ZJ, Hu XG, Cao GS, Tang Y. Analysis of gene expression profile of pancreatic carcinoma using cDNA microarray. World J Gastroenterol. 2003;9:818–823. doi: 10.3748/wjg.v9.i4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YJ, Ji XR. Relationship between expression of E-cadherin-catenin complex and clinicopathologic characteristics of pancreatic cancer. World J Gastroenterol. 2003;9:368–372. doi: 10.3748/wjg.v9.i2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng M, Liu LX, Zhu AL, Qi SY, Jiang HC, Xiao ZY. K-ras gene mutation in the diagnosis of ultrasound guided fine-needle biopsy of pancreatic masses. World J Gastroenterol. 2003;9:188–191. doi: 10.3748/wjg.v9.i1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar A, Russell RC. Recent advances in the surgical treatment of pancreatic cancer. World J Gastroenterol. 2001;7:622–626. doi: 10.3748/wjg.v7.i5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaneh P, Slavin J, Sutton R, Hartley M, Neoptolemos JP. Adjuvant therapy in pancreatic cancer. World J Gastroenterol. 2001;7:482–489. doi: 10.3748/wjg.v7.i4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Baker PR, Poulsom R, Wright NA, Sheppard MC, Langman MJ, Neoptolemos JP. Expression of oestrogen receptor and oestrogen-inducible genes in pancreatic cancer. Br J Surg. 1997;84:1085–1089. [PubMed] [Google Scholar]
- 16.Horimi T, Takasaki M, Toki A, Nishimura W, Morita S. The beneficial effect of tamoxifen therapy in patients with resected adenocarcinoma of the pancreas. Hepatogastroenterology. 1996;43:1225–1229. [PubMed] [Google Scholar]
- 17.Stephens FO. The increased incidence of cancer of the pancreas: is there a missing dietary factor Can it be reversed. Aust N Z J Surg. 1999;69:331–335. doi: 10.1046/j.1440-1622.1999.01567.x. [DOI] [PubMed] [Google Scholar]
- 18.Xu HB, Zhang YJ, Wei WJ, Li WM, Tu XQ. Pancreatic tumor: DSA diagnosis and treatment. World J Gastroenterol. 1998;4:80–81. doi: 10.3748/wjg.v4.i1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao XY, Yu SY, Da SP, Bai L, Guo XZ, Dai XJ, Wang YM. A clinical evaluation of serological diagnosis for pancreatic cancer. World J Gastroenterol. 1998;4:147–149. doi: 10.3748/wjg.v4.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo XZ, Friess H, Shao XD, Liu MP, Xia YT, Xu JH, Buchler MW. KAI1 gene is differently expressed in papillary and pancreatic cancer: influence on metastasis. World J Gastroenterol. 2000;6:866–871. doi: 10.3748/wjg.v6.i6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue H, Na YL, Feng XL, Ma SR, Song FL, Yang B. Expression of p57kip2, Rb protein and PCNA and their relationships with clinicopathology in human pancreatic cancer. World J Gastroenterol. 2003;9:377–380. doi: 10.3748/wjg.v9.i2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So FV, Guthrie N, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997;112:127–133. doi: 10.1016/s0304-3835(96)04557-0. [DOI] [PubMed] [Google Scholar]
- 23.Shao ZM, Alpaugh ML, Fontana JA, Barsky SH. Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by P21WAF1/CIP1 induction, G2/M arrest, and apoptosis. J Cell Biochem. 1998;69:44–54. [PubMed] [Google Scholar]
- 24.Hopert AC, Beyer A, Frank K, Strunck E, Wünsche W, Vollmer G. Characterization of estrogenicity of phytoestrogens in an endometrial-derived experimental model. Environ Health Perspect. 1998;106:581–586. doi: 10.1289/ehp.98106581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scambia G, Ranelletti FO, Panici PB, Piantelli M, De Vincenzo R, Ferrandina G, Bonanno G, Capelli A, Mancuso S. Quercetin induces type-II estrogen-binding sites in estrogen-receptor-negative (MDA-MB231) and estrogen-receptor-positive (MCF-7) human breast-cancer cell lines. Int J Cancer. 1993;54:462–466. doi: 10.1002/ijc.2910540318. [DOI] [PubMed] [Google Scholar]
- 26.Robinson MJ, Corbett AH, Osheroff N. Effects of topoisomerase II-targeted drugs on enzyme-mediated DNA cleavage and ATP hydrolysis: evidence for distinct drug interaction domains on topoisomerase II. Biochemistry. 1993;32:3638–3643. doi: 10.1021/bi00065a016. [DOI] [PubMed] [Google Scholar]
- 27.Spinozzi F, Pagliacci MC, Migliorati G, Moraca R, Grignani F, Riccardi C, Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk Res. 1994;18:431–439. doi: 10.1016/0145-2126(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 28.Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993;20:1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- 30.Xin Y, Li XL, Wang YP, Zhang SM, Zheng HC, Wu DY, Zhang YC. Relationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomas. World J Gastroenterol. 2001;7:53–59. doi: 10.3748/wjg.v7.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao XH, Gu SZ, Liu SX, Pan BR. Expression of estrogen receptor and estrogen receptor messenger RNA in gastric carcinoma tissues. World J Gastroenterol. 2003;9:665–669. doi: 10.3748/wjg.v9.i4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003;9:858–861. doi: 10.3748/wjg.v9.i4.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theve NO, Pousette A, Carlström K. Adenocarcinoma of the pancreas--a hormone sensitive tumor A preliminary report on Nolvadex treatment. Clin Oncol. 1983;9:193–197. [PubMed] [Google Scholar]
- 34.Kuramoto M, Yamashita J, Ogawa M. Tissue-type plasminogen activator predicts endocrine responsiveness of human pancreatic carcinoma cells. Cancer. 1995;75:1263–1272. doi: 10.1002/1097-0142(19950315)75:6<1263::aid-cncr2820750608>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.Abe M, Yamashita J, Ogawa M. Medroxyprogesterone acetate inhibits human pancreatic carcinoma cell growth by inducing apoptosis in association with Bcl-2 phosphorylation. Cancer. 2000;88:2000–2009. [PubMed] [Google Scholar]
- 36.Lyn-Cook BD, Stottman HL, Yan Y, Blann E, Kadlubar FF, Hammons GJ. The effects of phytoestrogens on human pancreatic tumor cells in vitro. Cancer Lett. 1999;142:111–119. doi: 10.1016/s0304-3835(99)00157-3. [DOI] [PubMed] [Google Scholar]


