Abstract
AIM: To establish a stable and reliable model of Helicobacter pylori infection model in Mongolian gerbils and to observe pathological changes in gastric mucosa in infected animals.
METHODS: Mongolian gerbils were randomly divided into 18 groups; 6 groups were infected with H pylori clinical strain Y06 (n = 6, groups Y), 6 groups were infected with H pylori strain NCTC11637 (n = 6, groups N), and 6 uninfected groups as negative controls (n = 4, groups C). H pylori suspensions at the concentrations of 2 × 108 and 2 × 109 CFU/mL of strain NCTC11637 and strain Y06 were prepared. The animals in three groups N and in three groups Y were orally challenged once with 0.5 mL of the low concentration of the bacterial suspension. The animals in another three groups N and in another three groups Y were orally challenged with 0.5 mL of the high concentration of the bacterial suspension for 3 times at the intervals of 24 h, respectively. For the negative controls, the animals in six groups C were orally given with the same volume of Brucella broth at the corresponding inoculating time. The animals were killed after 2nd, 4th and 6th week after the last challenge and the gastric mucosal specimens were taken for urease test, bacterial isolation, pathological and immunohistochemical examinations.
RESULTS: Positive isolation rates of H pylori in the animals of groups Y at the 2nd, 4th and 6th week after one challenge were 0%, 16.7% and 66.7%, while in the animals of groups N were 0%, 0% and 16.7%, respectively. Positive isolation rates of H pylori in the animals of groups Y at the 2nd, 4th and 6th week after three challenges were 66.7%, 100% and 100%, while in the animals of groups N were 66.7%, 66.7% and 100%, respectively. In animals with positive isolation of H pylori, the bacterium was found to colonized on the surface of gastric mucosal cells and in the gastric pits, and the gastric mucosal lamina propria was infiltrated with inflammatory cells.
CONCLUSION: By using H pylori suspension at high concentration of 2 × 109 CFU/mL for multiple times, the orally challenged Mongolian gerbils can be used as a stable and reliable H pylori infection model. The 2 strains of H pylori can colonize in gastric mucosa of the infected animals and cause mild inflammation reaction.
INTRODUCTION
Helicobacter pylori, a microaerophilic, spiral and Gram-negative bacillus, is recognized as an important pathogen causing human gastritis and peptic ulcer and a high risk factor for gastric carcinoma[1,2]. In China, chronic gastritis and peptic ulcer are two of the most common digestive diseases, and gastric cancer is one of the malignant tumors with high morbidities[3-34]. An ideal public measure to prevent and control these H pylori infection-associated diseases may be a vaccine that could induce strong humoral and cellular immune responses. However, no commercial H pylori vaccine is available so far, and the development of H pylori vaccine by using genetic engineering techniques is being active[35-39]. A stable and reliable H pylori infection animal model would be necessary for evaluating vaccine efficacy and helpful for understanding the pathological mechanism of the organism. Therapeutic drugs for H pylori eradication differ from those for many other bacteria such as using metronidazole[40,41]. Therefore, H pylori infection animal models would contribute to screen new drugs against H pylori. In recent published data, Mongolian gerbils have been considered as ideal animals to establish infection model by using internationally collected H pylori strains[42-44]. In this study, we used a clinical H pylori isolate named Y06 to establish a stable and reliable infection model in Mongolian gerbils. The colonization sites of H pylori and pathological changes in gastric mucosa of the animals were also observed.
MATERIALS AND METHODS
H pylori strains
A clinical strain of H pylori named Y06 was isolated from a patient with gastric ulcer. This strain was identified based on their characteristic morphology by Gram staining under microscope, and positive for urease and oxidase activities, further confirmed by slide agglutination test using commercial rabbit antiserum against whole cell of H pylori (DAKO). A reference H pylori strain, NCTC11637, was used as an infection control. The two strains were subcultured in Columbia agar (bioMérieux) containing 80 mL/L sheep blood under microaerobic conditions containing 100 mL/L CO2, 50 mL/L O2 and 850 mL/L N2.
Animals
Eight-week-old specific pathogen-free male Mongolian gerbils with body mass of 75±5 g were provided by Experimental Animal Center, Zhejiang Academy of Medical Sciences. These gerbils were randomly divided into eighteen groups: six groups infected with the clinical strain Y06 (groups Y, n = 6 in each group), six groups infected with the reference H pylori strain NCTC11637 (groups N, n = 6 in each group) and six groups as negative controls (groups C, n = 4 in each group).
Dosages and pathway of inoculation
Bacterial cells grown on the 2 strains on Columbia agar for 3-5 d were collected and diluted to the final concentrations of 2 × 108 CFU/mL and 2 × 109 CFU/mL, respectively, by using Brucella broth (bioMérieux). Each Mongolian gerbils in 3 groups Y and 3 groups N were orally challenged with 0.5 mL of 2 × 108 CFU/mL H pylori suspension, while the animals in another 3 groups Y and 3 groups N were attacked with 0.5 mL of 2 × 109 CFU/mL H pylori suspension through the same pathway. For the negative controls, the animals in 6 groups C were orally given with 0.5 mL of Brucella broth. Each animal in the groups was given with the different concentrations of H pylori suspensions or Brucella broth, respectively, as the described above for 3 times at an interval of 24 h. The animals were deprived of food but offered with water for 12 h before the challenge, and supplied with food and water after 4 h of H pylori inoculation.
Isolation and identification of H pylori
Six animals in group Y, 6 in group N and 4 animals in group C were killed, respectively, at 2, 4 and 6 wk after the last challenge. Two gastric mucosal specimens at the adjacent position were taken from antrum and corpus, respectively. One of the specimens was used for H pylori isolation, and the others were fixed with 40 g/L formaldehyde solution. The colonies on Columbia plates were identified by microscopy after Gram-staining, assays for urease and oxidase activities and slide agglutination test using the commercial H pylori-specific antiserum. The bacterium was defined to be H pylori if it was Gram-negative with arc shape or “seagull-like”, positive for the two enzymes and immune agglutination.
Pathological and immunohistochemical examinations
The gastric mucosal specimens fixed with formaldehyde were pathologically examined after embedding, section and haematoxylin-esosin (HE) staining. H pylori in gastric mucosal specimens were detected by the immunohistochemical method using a commercial rabbit anti-H pylori antibody and goat anti-rabbit HRP-labeled IgG antibody (Jackson Immunoresearch).
RESULTS
Infection of Mongolian gerbils with H pylori strains
The results of H pylori isolation from gastric mucosal specimens of Mongolian gerbils are shown in Table 1.
Table 1.
The detection results of H pylori isolated from gastric mucosa of experimental infected Mongolian gerbils
| Group | Detection time (wk) |
Infection rate (%) (positive/total cases) |
|
| 1 × 108 CFU (1 challenge) | 1 × 109 CFU (3 challenges) | ||
| Y | 2 | 0(0/6) | 66.7(4/6) |
| N | 2 | 0(0/6) | 66.7(4/6) |
| C | 2 | 0(0/4) | 0(0/4) |
| Y | 4 | 16.7(1/6) | 100(6/6) |
| N | 4 | 0(0/6) | 66.7(4/6) |
| C | 4 | 0(0/4) | 0(0/4) |
| Y | 6 | 66.7(4/6) | 100(6/6) |
| N | 6 | 16.7(1/6) | 100(6/6) |
| C | 6 | 0(0/4) | 0(0/4) |
Y, groups infected with strain Y06; N, groups infected with strain NCTC11637; C, negative controls.
Pathological and immunohistochemical findings
In animals with positive H pylori isolation, the organisms were found to colonize the surface of gastric mucosa and the gastric pits (Figure 1). In the presence of H pylori infection, infiltration of chronic inflammatory cells in the lamina propria and erosions on the surface of gastric mucosa were observed (Figure 2).
Figure 1.
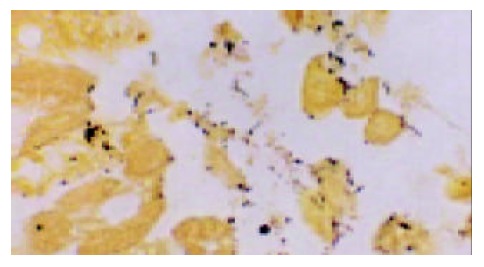
The H pylori bodies located on the surface of gastric mucosal cells and in gastric pits ( × 1000).
Figure 2.
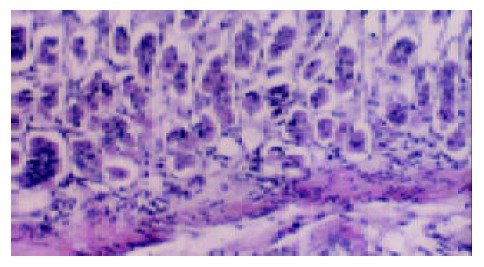
The infiltrated chronic inflammatory cells in the gastric mucosal lamina propria of the specimens with positive H pylori isolation ( × 400).
DISCUSSION
In previous published data, animals for establishment of H pylori infection models included guinea pigs, rats, nude mice, chimpanzee etc.[45-48]. These animal models have many disadvantages such as low infection rates, instability, immunodeficiency and high costs. In 1997, Lee et al[48] successfully established an animal model infected with a H pylori strain named as SS1 in C57BL/6 and BaLb/c mice. This animal model showed a high frequency and stability for H pylori infection. However, strain SS1 is a mutant of H pylori and its virulence is considerably low. Recently, Mongolian gerbils, which has distinct advantages such as high frequency and stability of infection, large colonization of H pylori, longer living suitable for study with long period of time and pathological changes similar to those observed in human with chronic gastritis, have become the predominant animals for preparing H pylori infection model[42-44]. In 1999, Chi et al[49] established a stable model of H pylori infection in Mongolian gerbils, which was orally pretreated with alcohol. Therefore, Mongolian gerbils are regarded as an ideal animal for H pylori infection models.
Previous studies have shown that infection model in Mongolian gerbils by oral challenge once with H pylori suspension at the concentration of 1 × 108 CFU of H pylori is stable[42-44]. In the present study, H pylori was undetectable in the gastric mucosa from Mongolian gerbils at 2 wk after challenge once with 1 × 108 CFU of the bacterium. Furthermore, the infection rates at 6 wk after one challenge by H pylori strain Y06 and strain NCTC11637 were only 66.7% and 16.7%, respectively. On contrast, by using three oral challenges at the dosage of 1 × 109 CFU, H pylori colonization rates in the gastric mucosa at 2 and 6 wk after challenge by H pylori strain Y06 or strain NCTC11637 were both 66.7% and 100%, respectively, which indicates that multiple challenges with a high concentration of H pylori contribute to the increased infection rates.
The infection rates of H pylori strain Y06 and strain NCTC11637 in Mongolian gerbils were 100% and 66.7% at 4 wk for 3 challenges using the low concentration of bacterial suspension, and 16.7% and 0% at 4 wk and 66.7% and 16.7% at 6 wk after one challenge using the high concentration. H pylori strain Y06, a fresh clinical isolate, seems to be more virulent than strain NCTC11637 and more beneficial for establishing H pylori infection model in Mongolian gerbils with a higher infection rate in a shorter period of time.
H pylori was found on the surface of gastric mucosa and in the gastric pits of Mongolian gerbils when infection was established. The observations that there were erosions of mucosal surface and infiltration of chronic inflammatory cells in the lamina propria of gastric mucosa of H pylori infected Mongolian gerbils indicates that the two tested H pylori strains are able to colonize gastric mucosa of Mongolian gerbils and cause chronic inflammation and gastric erosions.
Footnotes
Supported by the Excellent Young Teacher Fund of Chinese Education Ministry and the General Research Plan of the Science and Technology Department of Zhejiang Province, No. 001110438
Edited by Xia HHX and Xu FM
References
- 1.Frenck RW, Clemens J. Helicobacter in the developing world. Microbes Infect. 2003;5:705–713. doi: 10.1016/s1286-4579(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Vakil N. Review article: Helicobacter pylori and reflux disease. Aliment Pharmacol Ther. 2003;17:297–305. doi: 10.1046/j.1365-2036.2003.01428.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779–782. doi: 10.3748/wjg.v7.i6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu XL, Qian KD, Tang XQ, Zhu YL, Du Q. Detection of H.pylori DNA in gastric epithelial cells by in situ hybridization. World J Gastroenterol. 2002;8:305–307. doi: 10.3748/wjg.v8.i2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao YL, Xu B, Song YG, Zhang WD. Overexpression of cyclin E in Mongolian gerbil with Helicobacter pylori-induced gastric precancerosis. World J Gastroenterol. 2002;8:60–63. doi: 10.3748/wjg.v8.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo DL, Dong M, Wang L, Sun LP, Yuan Y. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H. pylori -associated gastric diseases. World J Gastroenterol. 2002;8:1009–1013. doi: 10.3748/wjg.v8.i6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng ZS, Liang ZC, Liu MC, Ouyang NT. Studies on gastric epithelial cell proliferation and apoptosis in Hp associated gastric ulcer. Shijie Huaren Xiahua Zazhi. 1999;7:218–219. [Google Scholar]
- 8.Hiyama T, Haruma K, Kitadai Y, Miyamoto M, Tanaka S, Yoshihara M, Sumii K, Shimamoto F, Kajiyama G. B-cell monoclonality in Helicobacter pylori-associated chronic atrophic gastritis. Virchows Arch. 2001;438:232–237. doi: 10.1007/s004280000331. [DOI] [PubMed] [Google Scholar]
- 9.Xia HHX. Association between Helicobacter pylori and gastric cancer: current knowledge and future research. World J Gastroenterol. 1998;4:93–96. doi: 10.3748/wjg.v4.i2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan J, Fan XG. Progress in experimental research of Helicobacter pylori infection and gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:1068–1069. [Google Scholar]
- 11.Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its precancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649–651. [Google Scholar]
- 12.Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669–673. [Google Scholar]
- 13.Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532–539. doi: 10.3748/wjg.v6.i4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374–376. doi: 10.3748/wjg.v6.i3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao XX, Yin L, Zhang JY, Bai WY, Li YM, Sun ZC. Htert expression and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508–512. [Google Scholar]
- 16.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR, et al. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54–59. doi: 10.3748/wjg.v8.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901) World J Gastroenterol. 2002;8:224–229. doi: 10.3748/wjg.v8.i2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai L, Yu SZ. A molecular epidemiologic study on gastric cancer in Changle, Fujian Province. Shijie Huaren Xiaohua Zazhi. 1999;7:652–655. [Google Scholar]
- 20.Gao GL, Yang Y, Yang SF, Ren CW. Relationship between proliferation of vascular andothelial cells and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:282–284. [Google Scholar]
- 21.Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359–361. [Google Scholar]
- 22.Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281–284. doi: 10.3748/wjg.v7.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XY, Wei PK. Diagnosis of stomach cancer by serum tumor markers. Shijie Huaren Xiaohua Zazhi. 2001;9:568–570. [Google Scholar]
- 24.Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522–526. doi: 10.3748/wjg.v7.i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248–253. doi: 10.3748/wjg.v7.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613–618. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu ZM, Shou NH, Jiang XH. Expression of lung resistance protein in patients with gastric carcinoma and its clinical significance. World J Gastroenterol. 2000;6:433–434. doi: 10.3748/wjg.v6.i3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo CQ, Wang YP, Liu GY, Ma SW, Ding GY, Li JC. Study on Helicobacter pylori infection and p53, c-erbB-2 gene expression in carcinogenesis of gastric mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:313–315. [Google Scholar]
- 29.Cai L, Yu SZ, Ye WM, Yi YN. Fish sauce and gastric cancer: an ecological study in Fujian Province,China. World J Gastroenterol. 2000;6:671–675. doi: 10.3748/wjg.v6.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801–804. doi: 10.3748/wjg.v7.i6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW, et al. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103–1107. doi: 10.3748/wjg.v8.i6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua JS. Effect of Hp: cell proliferation and apoptosis on stomach cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:647–648. [Google Scholar]
- 33.Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, et al. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500–505. doi: 10.3748/wjg.v7.i4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230–232. doi: 10.3748/wjg.v8.i2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao YF, Yan J, Li LW, Li SP. Construction of hpaA gene from a clinical isolate of Helicobacter pylori and identification of fusion protein. World J Gastroenterol. 2003;9:1529–1536. doi: 10.3748/wjg.v9.i7.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggiero P, Peppoloni S, Rappuoli R, Del Giudice G. The quest for a vaccine against Helicobacter pylori: how to move from mouse to man. Microbes Infect. 2003;5:749–756. doi: 10.1016/s1286-4579(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 37.Garhart CA, Nedrud JG, Heinzel FP, Sigmund NE, Czinn SJ. Vaccine-induced protection against Helicobacter pylori in mice lacking both antibodies and interleukin-4. Infect Immun. 2003;71:3628–3633. doi: 10.1128/IAI.71.6.3628-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garhart CA, Heinzel FP, Czinn SJ, Nedrud JG. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect Immun. 2003;71:910–921. doi: 10.1128/IAI.71.2.910-921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panthel K, Faller G, Haas R. Colonization of C57BL/6J and BALB/c wild-type and knockout mice with Helicobacter pylori: effect of vaccination and implications for innate and acquired immunity. Infect Immun. 2003;71:794–800. doi: 10.1128/IAI.71.2.794-800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheu BS, Huang JJ, Yang HB, Huang AH, Wu JJ. The selection of triple therapy for Helicobacter pylori eradication in chronic renal insufficiency. Aliment Pharmacol Ther. 2003;17:1283–1290. doi: 10.1046/j.1365-2036.2003.01527.x. [DOI] [PubMed] [Google Scholar]
- 41.Marais A, Bilardi C, Cantet F, Mendz GL, Mégraud F. Characterization of the genes rdxA and frxA involved in metronidazole resistance in Helicobacter pylori. Res Microbiol. 2003;154:137–144. doi: 10.1016/S0923-2508(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 42.Ohkusa T, Okayasu I, Miwa H, Ohtaka K, Endo S, Sato N. Helicobacter pylori infection induces duodenitis and superficial duodenal ulcer in Mongolian gerbils. Gut. 2003;52:797–803. doi: 10.1136/gut.52.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiyama T, Hige S, Asaka M. Development of an H. pylori-infected animal model and gastric cancer: recent progress and issues. J Gastroenterol. 2002;37 Suppl 13:6–9. doi: 10.1007/BF02990092. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Court M, Jeremy AH, Aboshkiwa MA, Robinson PA, Crabtree JE. Infection of Mongolian gerbils with Chinese Helicobacter pylori strains. FEMS Immunol Med Microbiol. 2003;36:207–213. doi: 10.1016/S0928-8244(02)00464-9. [DOI] [PubMed] [Google Scholar]
- 45.Fujioka T, Murakami K, Kodama M, Kagawa J, Okimoto T, Sato R. Helicobacter pylori and gastric carcinoma--from the view point of animal model. Keio J Med. 2002;51 Suppl 2:69–73. doi: 10.2302/kjm.51.supplement2_69. [DOI] [PubMed] [Google Scholar]
- 46.Eaton KA, Kersulyte D, Mefford M, Danon SJ, Krakowka S, Berg DE. Role of Helicobacter pylori cag region genes in colonization and gastritis in two animal models. Infect Immun. 2001;69:2902–2908. doi: 10.1128/IAI.69.5.2902-2908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keenan JI, Rijpkema SG, Durrani Z, Roake JA. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol Med Microbiol. 2003;36:199–205. doi: 10.1016/S0928-8244(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 48.Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 49.Chi J, Fu BY, Nakajima , Hatorri , Kushima Establishment of Mon-golian gerbil animal model infected with Hp infection and change of inflammation and proliferation before and after Hp eradication. Shijie Huaren Xiaohua Zazhi. 1999;7:557–560. [Google Scholar]


