Abstract
AIM: Transcatheter arterial embolization (TAE) of the hepatic artery has been accepted as an effective treatment for unresectable hepatocellular carcinoma (HCC). However, embolized vessel recanalization and collateral circulation formation are the main factors of HCC growth and recurrence and metastasis after TAE. Vascular endothelial growth factor (VEGF) plays an important role in tumor angiogenesis. This study was to explore the inhibitory effect of VEGF antisense oligodeoxynucleotides (ODNs) on VEGF expression in cultured Walker-256 cells and to observe the anti-tumor effect of intra-arterial infusion of antisense ODNs mixed with lipiodol on rat liver cancer.
METHODS: VEGF antisense ODNs and sense ODNs were added to the media of non-serum cultured Walker-256 cells. Forty-eight hours later, VEGF concentrations of supernatants were detected by ELISA. Endothelial cell line ECV-304 cells were cultured in the supernatants. Seventy-two hours later, growth of ECV-304 cells was analyzed by MTT method. Thirty Walker-256 cell implanted rat liver tumor models were divided into 3 groups. 0.2 mL lipiodol (LP group, n = 10), 3OD antisense ODNs mixed with 0.2 mL lipiodol (LP+ODNs group, n = 10) and 0.2 mL normal saline (control group, n = 10) were infused into the hepatic artery. Volumes of tumors were measured by MRI before and 7 d after the treatment. VEGF mRNA in cancerous and peri-cancerous tissues was detected by RT-PCR. Microvessel density (MVD) and VEGF expression were observed by immunohistochemistry.
RESULTS: Antisense ODNs inhibited Walker-256 cells’ VEGF expression. The tumor growth rate was significantly lower in LP+ODNs group than that in LP and control groups (140.1 ± 33. 8%, 177. 9 ± 64. 9% and 403.9 ± 69.4% respectively, F = 60.019, P < 0.01). VEGF mRNAs in cancerous and peri-cancerous tissues were expressed highest in LP group and lowest in LP+ODNs group. The VEGF positive rates showed no significant difference among LP, control and LP+ODNs groups (90%, 70% and 50%, H = 3.731, P>0.05). The MVD in LP+ODNs group (53.1 ± 18.4) was significantly less than that in control group (73.2 ± 20.4) and LP group (80.3 ± 18.5) (F = 5.44, P < 0.05)
CONCLUSION: VEGF antisense ODNs can inhibit VEGF expression of Walker-256 cells. It maybe an antiangiogenesis therapy agent for malignant tumors. VEGF antisense ODNs mixed with lipiodol embolizing liver cancer is better in inhibiting liver cancer growth, VEGF expression and microvessel density than lipiodol alone.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in human beings[1,2]. In China, HCC is responsible for 130 000 deaths every year and the second cause of cancer deaths[3]. Surgical resection is still the only potentially curative treatment for HCC, particularly for small HCC[3,4]. To date, the resection rate for HCC is unfortunately less than 30%[3,5,6]. Since HCC’s blood supply is derived almost exclusively from hepatic arteries, transcatheter arterial embolization (TAE) of the hepatic artery has been accepted as an effective treatment for unresectable HCC[5-10]. Although embolic materials and technique have been improved during the last decade, the outcome of TAE is not satisfactory. It hardly leads to tumor necrosis totally. The 3-year survival rate is about 14-35%[11-14]. Recanalization of the embolized vessel and collateral circulation formation, which are related with tumor angiogenesis, are the main factors of HCC growth, recurrence and metastasis after TAE[15,16]. In TAE, HCC cells undergo coagulative necrosis, a pathologic feature of anoxia. Anoxia and hypoxic liver injury are caused by the absolute and relative deficiency of oxygen, respectively. It is well known that hypoxia tension is a key factor of the gene expression of angiogenic factors such as VEGF, acidic and basic fibroblast growth factors (FGF), platelet derived growth factor (PDGF). These factors could promote tumor angiogenesis, growth, recurrence and metastasis[17,18]. It is possible that in addition to elimination of cancer cells, TAE may play a role in enhancing some cells’ malignant potency and ability to escape anoxia and ischemia after treatment.
VEGF is a key mediator of pathological angiogenesis. Many researchers found that it overexpressed in many solid tumor tissues including HCC, and was associated with tumor growth, recurrence, metastasis and patient’s prognosis[19-23]. It has been reported that preoperative TAE enhanced VEGF expression in both HCC cells and non-carcinoma liver cells. Antisense RNA could inhibit and block gene expression effectively[24-26]. In this study, we devised antisense ODNs specific to VEGF mRNA, and observed their inhibitory effects on VEGF expression in Walker-256 cell lines in vitro, and the anti-tumor effect of them mixed with lipiodol arterial embolization on Walker-256 cell transplanted rat liver cancer models.
MATERIALS AND METHODS
Cell culture
Walker 256 carcinosarcoma cells were purchased from China Center for Type Culture Collection. After recovery, the cells were inoculated in the abdominal cavity of male pathogen-free Wistar rats, weighing 100-120 g (supplied by Department of Experimental Animal, Tongji Medical College). Three days later, cancerous ascites was aspirated and cultured in RPMI-1640 containing 50 mL/L fetal calf serum (FCS) (Gibco, Grand Island, NY) and equilibrated with 950mL/L air and 50mL/L CO2. Cells were passaged every 2 d. The cells at passage 3 were used for experiments.
Oligodeoxynucleotides (ODNs)
Phosphorothioate ODNs were synthesized by Shanghai Sangon Biological Engineering Technology and Service Co., LTD. The sequences of ODNs were designed as follows: antisense ODNs: 5’-GCA GTA GCT GCG CTG ATA GCG C-3’, complementary to the linkage area of VEGF exon 2 and exon 3; sense ODNs: 5’-GCA CTA TCA GCG CAG CTA CTG C-3’, equivalent to the linkage area of VEGF exon 2 and exon 3.
Cell proliferation studies
Walker-256 carcinosarcoma cells were planted into a 24-well plate at 1×105/well (1 mL/well) and cultured in non-serum medium. Ten μL of 0.25, 0.5, 1.0, 2.0 μmol/L antisense ODNs and sense ODNs was added to the media every 24 h. Ten μL of non-serum medium was added to the blank control wells. Forty-eight hours later, the supernatant fluids were collected. The concentration of VEGF was measured by ELISA. Two hundred μL of supernatant fluids was added triplicate to a 96-well plate to culture ECV-304 cells (endothelial cell line, purchased from China Center for Type Culture Collection, 1×104/ well), equilibrated with 950 mL/L air and 50 mL/L CO2. Seventy-two hours later, ECV-304 cells were collected. MTT method was used to detect the growth of ECV-304 cells.
Tumor model and treatment schedule
Walker- 256 carcinosarcoma cells were inoculated subcutaneously in the right flank of rats with 107 tumor cells in approximately 0.1 mL of cell suspension. Tumors were palpable 7 d after transplantation. Fresh tumor tissues were isolated and cut into 1 mm3 size. Following midline laparotomy, the Walker 256 carcinosarcoma tissue pieces were implanted into the left hepatic lobe of rats. Seven days later, after anesthesia and laparotomy, the gastroduodenal artery was retrograde catheterized with a Portex PE10 tube (inner diameter 0.28 mm, external diameter 0.61 mm, Neolab, Germany) under a binocular operative microscope (Suzhou Medical Instruments Factory, Jiangsu, China) and infused embolic materials to perform TAE. The common hepatic artery and right hepatic artery were temporarily ligated during infusion. Thirty tumor-bearing rats were randomly divided into 3 groups, 10 rats each. LP group: the hepatic arteries were embolized with 0.2 mL lipiodol (Lipiodol Ultra-Fluid; Andre Guerbet, Aulnay-Sous-Bois, France), LP+ODNs group: the hepatic arteries were embolized with 3OD antisense ODNs mixed with 0.2 mL lipiodol, control group: 0.2 mL normal saline was infused into hepatic arteries. Then the gastroduodenal artery was ligated and the abdominal cavity was closed.
MR scans were performed on 1.5-Tesla system (Magnetom Vision, Siemens, Germany) supplemented by a cervical coil before and 7 d after TAE. T1-weighted (TR/TE, 450/12 ms) and T2-weighted (TR/TE, 2800/96 ms) transverse SE images (slice thickness 2 mm) were acquired using acquisition times of 7:25 and 6:16 min, respectively. Tumor volume was determined from MR measurements of the largest and smallest diameters and calculated according to the following formula: Tumor volume (mm3, V) = largest diameter (mm) × [smallest diameter (mm)]2/2. The tumor growth rate = V post/Vpre × 100%.
The rats were sacrificed 7 d after TAE. Liver cancerous and peri-cancerous tissues were dissected. Some of them were frozen at -70 °C for RNA isolation. The reminders were fixed in 40g/L formaldehyde, dehydrated and embedded in paraffin. Five-μm sections were stained with hematoxylin-eosin for light microscopy and measurement of the degree of tumor necrosis.
RNA isolation and RT-PCR analysis
Total RNA was extracted from liver cancerous and peri-cancerous tissues using the TRIzol reagent (Gibco). Reverse transcription of 5 μg total RNA was performed in a volume of 20 μL for 60 min at 37 °C, containing AMV 5U, Oligo dT 0.050 μg, RNasin 20 U, 10 mmol/L dNTP 2 μL. The samples were heated to 95 °C for 5 min to terminate the reverse transcription reaction. By using a Perkin-Elmer DNA thermocycler 2 400 (Perkin-Elmer, Norwalk, CT), 2 μL cDNA mixture obtained from the reverse transcription reaction was then amplified for VEGF and G3PDH. G3PDH was used as a housekeeping gene and amplified with VEGF as control. The amplification reaction mixture consisted of 10×buffer 2.5 μL, 5 mmol/L dNTP 0.5 μL, 25 mmol/L MgCl2 1.5 μL,10 pmol/L each of sense and antisense primers, Taqase 5U. The reaction mixture was first heated at 95 °C for 5 min and amplification was carried out for 35 cycles at 94 °C for 30 s, at 60 °C for 30 s, and at 72 °C for 30 s, followed by incubation for 10 min at 72 °C. The PCR primers used were: VEGF, sense 5’-GAA GTG GTG AAG TTC ATG GAT GTC-3’ and antisense 5’-CGA TCG TTC TGT ATC AGT CTT TCC-3’; G3PDH, sense 5’-TCC CTC AAG ATT GTC AGC AA-3’ and antisense 5’-AGA TCC ACA ACG GAT ACA TT-3’. The length of PCR products for VEGF and G3PDH was 394 bp and 309 bp. PCR products were checked with 15 g/L agarose gel electrophoresis stained with ethidum bromide.
Immunohistochemical analysis
Five micron paraffin-embedded tissue sections were deparaffinized and rehydrated. Rabbit anti-mouse vWF, VEGF monoclonal antibody and SABC kit were provided by Beijing Zhongshan Biological Technology Co., Ltd. Immunohistochemical studies were performed by SABC methods according to the manufacturer’s instructions. VEGF staining was evaluated semiquantitatively on the basis of the percentage of positive cells, and classified as follows: diffusely positive (+++) when positive cells accounted for more than 50% of the total cells, moderately positive (++) when positive cells were 16-50%, weakly positive (+) when positive cells accounted for 5-15%, and negative (-) when positive cells accounted for less than 5%. For MVD determination, 5 areas were randomly selected and counted at a magnification of 200. Briefly, the stained sections were screened at a magnification of 40 under a light microscope to identify 3 regions of the section with the highest microvessel density. Microvessels were counted in these areas at a magnification of 200, and the average number of microvessels was recorded.
Statistical analysis
Experimental results were analyzed with analysis of variance (ANOVA) and Kruskal-Wallis rank test. The difference between 2 groups was analyzed by SNK-q and Dunn test respectively. Statistical significance was determined at P < 0.05.
RESULTS
VEGF concentration in supernatant of walker-256 cells
After incubated with walker-256 cells for 48 h, antisense ODNs decreased the concentration of VEGF in the supernatant of walker-256 cells in a dose-dependent manner, whereas no significant changes were seen in sense ODNs (Table 1).
Table 1.
Concentration of VEGF in supernatant of Walker-256 cells (pg/ml)(mean ± SD)
| Group | 0.25 μmol/L | 0.5 μmol/L | 1.0 μmol/L | 2.0 μmol/L |
| Antisense ODNs | 84.2 ± 2.2 | 79.2 ± 2.6 | 74.4 ± 2.1 | 65.2 ± 2.0 |
| Sense ODNs | 89.2 ± 1.9 | 90.4 ± 2.6 | 88.2 ± 1.9 | 88.6 ± 2.5 |
| Control | 90.2 ± 1.9 | 90.8 ± 1.8 | 89.4 ± 2.1 | 90.4 ± 1.7 |
| F values | 7.877 | 23.132 | 49.906 | 133.636 |
| P values | 0.0211 | 0.0021 | 0.0001 | 0.0001 |
SNK-q test: The difference between sense ODNs and control groups was not statistically significant.
Cell proliferation study
The supernatants of Walker-256 cells that were incubated with antisense ODNs could inhibit the growth of ECV-304 cells in a dose-dependent manner. No significant effects were seen in those incubated with sense ODNs (Table 2).
Table 2.
Inhibitory effect of supernatants of Walker-256 cells cultured with ODNs on ECV-304 proliferation (OD) (mean ± SD)
| Group | 0.25 μmol/L | 0.5 μmol/L | 1.0 μmol/L | 2.0 μmol/L |
| Antisense ODNs | 0.339 ± 0.012 | 0.332 ± 0.006 | 0.327 ± 0.008 | 0.321 ± 0.019 |
| Sense ODNs | 0.345 ± 0.021 | 0.348 ± 0.014 | 0.336 ± 0.022 | 0.359 ± 0.014 |
| Control | 0.357 ± 0.010 | 0.359 ± 0.008 | 0.356 ± 0.005 | 0.360 ± 0.009 |
| F values | 2.477 | 5.573 | 12.931 | 7.296 |
| P values | 0.164 | 0.0431 | 0.0071 | 0.0251 |
SNK-q test: The difference between sense ODNs and control groups was not statistically significant.
Tumor growth and histopathological findings
Seven days after implantation, the tumor was located in the left lobe of liver as a solitary mass. There was no statistical difference between the volumes of tumors in control group, LP group and LP+ODNs group (212.3 ± 117.5 mm3, 174.6 ± 106.5 mm3, 173.9 ± 91.8 mm3 respectively) before TAE treatment. Seven days after the treatment, all tumors grew. The volumes and growth rates in LP group (286.0 ± 186.4 mm3, 177.9 ± 64.9%, respectively) and LP+ODNs group (337.6 ± 98.1 mm3, 140.1 ± 33.8%, respectively) were significantly less than those in control group (823.3 ± 426.1 mm3, 403.9 ± 69.4%, respectively, P < 0.01) (Table 3).
Table 3.
Volumes and growth rates of transplanted liver tu-mors (mean ± SD)
| Group | Volume of pre-TAE(mm3) | Volume of post-TAE(mm3) | Growth rate (%) |
| Control | 212.3 ± 117.5 | 823.3 ± 426.1 | 403.9 ± 69.4 |
| LP | 174.6 ± 106.5 | 286.0 ± 186.4 | 177.9 ± 64.9 |
| LP+ODNs | 173.9 ± 91.8 | 337.6 ± 98.1 | 140.1 ± 33.8 |
| F values | 0.432 | 14.319 | 60.019 |
| P values | 0.653 | 0.0001 | 0.000 |
SNK-q test: The difference between antisense LP and LP+ODNs groups was not statistically significant.
Hematoxylin-eosin (H & E) stained sections of the liver specimens showed a poorly differentiated carcinoma, which was spherical or ovoid in shape. Tumor cells arranged in irregular nests and signs of malignancy including hyperchromatosis, polymorphism and numerous mitoses were detected. The mass had a sharp demarcation from the surrounding normal hepatic parenchyma, its capsules were thin and composed of collagen fibers, which were caused by the compression of the tumor. The tumor showed inhomogeneous signs of hypervascularization consisting mainly of small arteries and capillaries. Seven days after therapy, spotty and scattered necrosis were seen in all cases of control group (Figure 1A). Satellite nodules could be seen in some tumors. In LP group, the necrotic area was increased. Many patched necrotic zones were seen in tumor tissues (Figure 1B). In LP+ODNs group, the tumor necrotic area was much wider. It showed a big area of central necrosis. The residual tumors could be seen only in the margin of tumor (Figure 1C).
Figure 1.
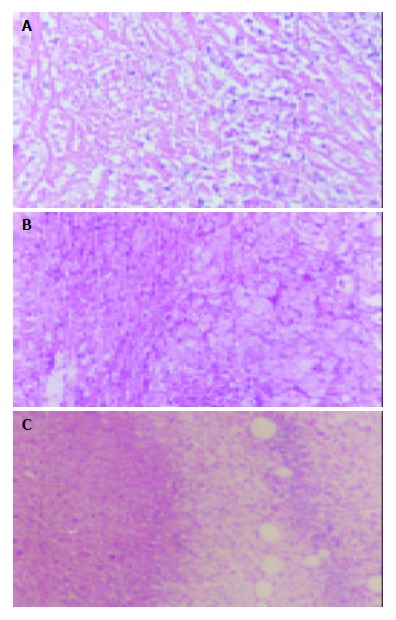
Pathological changes of tumor tissues 7 d after TAE. A: Control group showing spotty and scattered necrosis. B: LP group showing many patched necrotic zones. C: LP+ODNs group showing big areas of central necrosis. Hematoxylin-eosin ×40.
RT-PCR analysis of VEGF mRNA expression
VEGF mRNA expression was detected in cancerous and peri-cancerous tissues. The expression level in cancerous tissue was higher than that in peri-cancerous tissues. The VEGF mRNA levels in cancerous and peri-cancerous tissues in LP group were higher than those in control group, and those in LP+ODNs group were lower than those in control and LP groups (Figure 2).
Figure 2.
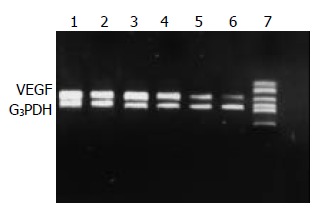
RT-PCR analysis of VEGF mRNA level in cancerous and peri-cancerous tissues using G3PDH as internal control. Control group: 1 tumor, 2 peri-tumor. LP group: 3 tumor, 4 peri-tumor. LP+ODNs group: 5 tumor, 6 peri-tumor, 7 marker.
Immunohistochemical analysis of VEGF protein expression and MVD
VEGF immunoreactivity was observed mainly in the cytoplasm of tumor cells, and also frequently in hepatic cells in peri-cancerous tissues. The distribution of strong VEGF-staining zones and microvessels was mainly in the survival nidui and margins of the tumor. The positive rate of VEGF in LP group, control group and LP+ODNs group was 90%, 70% and 50% respectively, and the difference was not statistically significant (P = 0.065) (Table 4, Figure 3). The MVD in LP+ODNs group (53.1 ± 18.4) was significantly less than that in control group (73.2 ± 20.4) and LP group (80.3 ± 18.5). Although the MVD in control group and LP group showed no significant difference, but abundant tumor vessels were seen in residual nidui in LP group (Table 4, Figure 4).
Table 4.
Expression of VEGF and MVD by immunohistochemi-cal staining (n=10)
| Group | MVD (mean ± SD) |
VEGF |
||||
| +++ | ++ | + | - | Positive rate (%) | ||
| Control | 73.2 ± 20.4 | 3 | 2 | 2 | 3 | 70 |
| LP | 80.3 ± 18.5 | 3 | 4 | 2 | 1 | 90 |
| LP+ODNs | 53.1 ± 18.4 | 1 | 2 | 2 | 5 | 50 |
| Test values | F = 5.440 | H = 3.731 | ||||
| P values | 0.0101 | 0.1545 | ||||
SNK-q test: The difference between LP and control groups was not statistically significant.
Figure 3.
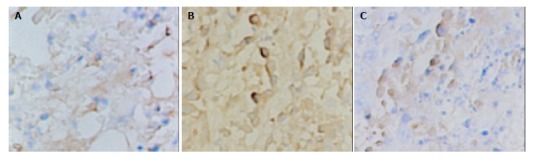
Immunohistochemical staining of VEGF in tumor tissues 7 d after TAE, showing strong expression in LP group and low expression in LP+ODNs group. A: Control group, B: LP group, C: LP+ODNs group. SABC ×200.
Figure 4.
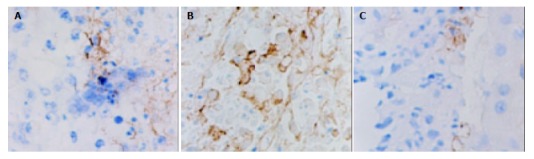
Immunohistochemical staining of vWF in tumor tissues 7 d after TAE, showing plenty of microvessels in LP group and a few microvessels in LP+ODNs group. A: Control group, B: LP group, C: LP+ODNs group. SABC×200.
DISCUSSION
Many authors have reported the effects of TAE on VEGF expression of HCC. An et al[27] found that preoperative TAE enhanced VEGF expression in both HCC cells and non-carcinoma liver cells. Kobayashi et al[28] reported that the frequency of Bcl-2 positive cells was higher in HCCs undergone TAE than that in HCCs not undergone TAE, and the immunohistochemical staining intensity for VEGF was higher in Bcl-2 positive than in Bcl-2 negative area. Suzuki et al[29] reported that the serum levels of VEGF in HCC patients increased significantly 7 d after TAE. Guo et al[30] reported that blockage of hepatic arterial blood supply resulted in decreased blood perfusion and increased expression of metastasis-associated genes VEGF and MMP-1 of transplanted liver cancer in rats. The results in this study showed that VEGF mRNA and protein had an increasing tendency after TAE. These findings suggested that TAE could enhance the expression of VEGF in HCC cells. The rationale is based on the following points: TAE was hard to lead to total tumor necrosis and to make the tumor tissue anoxia further. Hypoxia induced transcription of VEGF mRNA was mediated by the binding of hypoxia-inducible factor 1 (HIF-1) to an HIF-1 binding site located in the VEGF promoter[31-33]. VEGF plays an important role in each stage of tumor angiogenesis. The overexpression of VEGF in cancerous and peri-cancerous tissues of HCC could certainly promote tumor angiogenesis and collateral vessels formation, and enhance the possibility of recurrence and metastasis[34-37]. In this study, although we found that the MVD in LP group was higher than that in control group, but the difference was not significant and abundant tumor vessels were seen in residual nidui in LP group. It must be due to tumor angiogenesis enhancement caused by VEGF overexpression after TAE. In clinical setting, we also found that the collateral vessels were increased with the time of increased TAE. These vessels are very small and hard to catheterize, resulting in the embolized HCC tissue receiving blood and escaping from anoxia stress. Some HCC vessels not embolized completely would grow acceleratedly and were prone to recurrence and metastasis. So it is very important to reduce VEGF expression and collateral vessel formation after TAE.
Antisense ODNs have shown great efficacy in selective inhibition of gene expression. They are designed and synthesized artificially, and can enter cells directly to hybridize with complementary mRNA and decrease protein expression. They have been used as an important tool to inhibit the expression of oncogenes and/or growth factors and some of them have been used as drugs in tumor gene therapies[38-41]. In this study, antisense ODNs were designed to complement the region between exon 2 and exon 3 of VEGF gene. They could inhibit 4 kinds of VEGF molecules’ expression[42,43]. To enforce their stability, ODNs were modified by phosphorothioate. Our previous study showed fluorescence labeled ODNs could transfect Walker-256 cells and keep in them for about 48 h (in press). The in vitro experimental results in this study showed that VEGF antisense ODNs could decrease VEGF expression of cultured Walker-256 cells in a dose-dependent manner. Similar effect has been reported on other tumor cell lines[41,44-46]. In in vivo study, we also found that VEGF antisense ODNs could inhibit VEGF expression of liver tumor tissues after TAE, reduce tumor MVD and growth rate in walker-256 cell transplanted rat liver cancer models. These imply that VEGF antisense ODNs could be used as an antiangiogenesis agent to inhibit HCC’s overexpression and collateral vessel formation after TAE.
Intravenous injection is the routine means for phosphothioate ODNs in clinical administration. However, intravenous infusion is not an ideal route for VEGF antisense ODNs in HCC treatment. First, it has a very short lifetime after injection into animal bodies. To enhance the target cell transfection rate and therapeutic effect, it is needed to increase the dose of antisense ODNs. A higher dose of ODNs may result in more side effects such as dose-dependent hypotension, complement activation, and transient prolongation of thromboplastin time[47-50]. Second, systemic administration of VEGF antisense ODNs might inhibit physiological angiogenesis, such as wound healing, menstruation. In this study, we mixed antisense ODNs with lipiodol and used them as an embolic agent in liver tumor TAE therapy. To infuse the agents into the left hepatic artery (the tumor was planted in the left lobe of the rat liver) and to make each tumor receive the same amount of agents, we catheterized the gastroduodenal artery retrograde and ligated the common hepatic artery and right hepatic artery temporarily during infusion. The results showed that antisense ODNs mixed with lipiodol was better in inhibiting tumor growth rate, VEGF expression and MVD than lipiodol alone. These indicated that antisense ODNs used in combination with lipiodol and transcatheter artery embolization were the ideal route in HCC treatment. The rationale is based on the following points. The blood supply of HCC is mainly from hepatic artery. ODNs hepatic artery infusion could increase the concentration in tumor tissue and reduce the dosage and systemic side-effects. When injected to the hepatic artery, lipiodol could stay in tumor tissue for a long time (several months), and could even be absorbed by tumor cells[51,52]. When mixed with lipiodol, the latter could act as a carrier, ODNs would give off slowly from it. It would prolong the contact time of ODNs and tumor cells, which is very important to increase the transfect rate. Our previous in vivo experimental study showed that fluorescence labeled ODNs could stay in tumor tissue for about 6 d when used in combination with lipiodol hepatic artery embolization on Walker-256 cell transplanted rat liver cancer models (in press). This is why the long-term inhibitory effect on VEGF expression and MVD was achieved in this study.
In conclusion, TAE can increase the formation of microvessels in residual tumor tissues. VEGF plays an important role in liver cancer angiogenesis and collateral vascular formation after TAE treatment. VEGF antisense ODNs are able to inhibit tumor cells’ VEGF expression. VEGF antisense ODNs can inhibit the residual tumor angiogenesis and growth and reduce the possibility of metastasis and recurrence. The combination of TAE and VEGF antisense ODNs will be a hopeful strategy for HCC treatment.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39770839
Edited by Zhang JZ and Wang XL Proofread by Xu FM
References
- 1.1 Pisani P, Parkin DM, Bray F, Ferlay J. Erratum: Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer, 83, 18-29 (1999) Int J Cancer. 1999;83:870–873. doi: 10.1002/(sici)1097-0215(19991210)83:6<870::aid-ijc35>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Akriviadis EA, Llovet JM, Efremidis SC, Shouval D, Canelo R, Ringe B, Meyers WC. Hepatocellular carcinoma. Br J Surg. 1998;85:1319–1331. doi: 10.1046/j.1365-2168.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 3.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 5.Acunaş B, Rozanes I. Hepatocellular carcinoma: treatment with transcatheter arterial chemoembolization. Eur J Radiol. 1999;32:86–89. doi: 10.1016/s0720-048x(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 6.Rose DM, Chapman WC, Brockenbrough AT, Wright JK, Rose AT, Meranze S, Mazer M, Blair T, Blanke CD, Debelak JP, et al. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405–410. doi: 10.1016/s0002-9610(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 7.Zangos S, Gille T, Eichler K, Engelmann K, Woitaschek D, Balzer JO, Mack MG, Thalhammer A, Vogl TJ. [Transarterial chemoembolization in hepatocellular carcinomas: technique, indications, results] Radiologe. 2001;41:906–914. doi: 10.1007/s001170170062. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T. Treatment of hepatocellular carcinoma by interventional methods. Eur Radiol. 2001;11:2207–2219. doi: 10.1007/s003300100889. [DOI] [PubMed] [Google Scholar]
- 9.Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 11.Loewe C, Cejna M, Schoder M, Thurnher MM, Lammer J, Thurnher SA. Arterial embolization of unresectable hepatocellular carcinoma with use of cyanoacrylate and lipiodol. J Vasc Interv Radiol. 2002;13:61–69. doi: 10.1016/s1051-0443(07)60010-4. [DOI] [PubMed] [Google Scholar]
- 12.Zheng C, Feng G, Liang H. Bletilla striata as a vascular embolizing agent in interventional treatment of primary hepatic carcinoma. Chin Med J. 1998;111:1060–1063. [PubMed] [Google Scholar]
- 13.Li X, Hu G, Liu P. Segmental embolization by ethanol iodized oil emulsion for hepatocellular carcinoma. J Tongji Med Univ. 1999;19:135–140. doi: 10.1007/BF02886895. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511–512. doi: 10.3748/wjg.v4.i6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian JM, Wang F, Ye H, Wang ZT, Sun F, Liu Q, Yang JJ, Cheng D. Classification study of arterial blood supply of hepatic cancer: regular, variant and parasitic blood supply. Linchuang Fangshexue Zazhi. 1997;16:40–43. [Google Scholar]
- 16.Li HP, Cao J, Wang XY, Luo JQ. Effect of hepatic artery chemoembolization in patients with primary liver carcinoma and analysis of factors affecting the prognosis. Linchuang Fangshexue Zazhi. 2001;20:66–69. [Google Scholar]
- 17.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 18.Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med (Berl) 1995;73:333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 21.Zheng S, Han MY, Xiao ZX, Peng JP, Dong Q. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J Gastroenterol. 2003;9:1227–1230. doi: 10.3748/wjg.v9.i6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao HQ, Qin LF, Lin YZ, Wang RN. Expression of vascular en-dothelial growth factor and its prognostic significance in gastric carcinoma. China Natl J New Gastroenterol. 1996;2:128–130. [Google Scholar]
- 23.Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61–65. doi: 10.3748/wjg.v6.i1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayanan R, Akhtar S. Antisense therapy. Curr Opin Oncol. 1996;8:509–515. doi: 10.1097/00001622-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Gu ZP, Wang YJ, Li JG, Zhou YA. VEGF165 antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol. 2002;8:44–48. doi: 10.3748/wjg.v8.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang YC, Li Y, Qian GX. Reduction of tumorigenicity of SMMC-7721 hepatoma cells by vascular endothelial growth factor antisense gene therapy. World J Gastroenterol. 2001;7:22–27. doi: 10.3748/wjg.v7.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An FQ, Matsuda M, Fujii H, Matsumoto Y. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2000;126:153–160. doi: 10.1007/s004320050025. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi N, Ishii M, Ueno Y, Kisara N, Chida N, Iwasaki T, Toyota T. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver. 1999;19:25–31. doi: 10.1111/j.1478-3231.1999.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Mori M, Kawaguchi C, Adachi M, Miura S, Ishii H. Serum vascular endothelial growth factor in the course of transcatheter arterial embolization of hepatocellular carcinoma. Int J Oncol. 1999;14:1087–1090. doi: 10.3892/ijo.14.6.1087. [DOI] [PubMed] [Google Scholar]
- 30.Guo WJ, Li J, Ling WL, Bai YR, Zhang WZ, Cheng YF, Gu WH, Zhuang JY. Influence of hepatic arterial blockage on blood perfusion and VEGF, MMP-1 expression of implanted liver cancer in rats. World J Gastroenterol. 2002;8:476–479. doi: 10.3748/wjg.v8.i3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura M, Yamagishi S, Harada S, Hayashi Y, Yamashima T, Yamashita J, Yamamoto H. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270:28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- 32.Sivridis E, Giatromanolaki A, Gatter KC, Harris AL, Koukourakis MI. Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer. 2002;95:1055–1063. doi: 10.1002/cncr.10774. [DOI] [PubMed] [Google Scholar]
- 33.Büchler P, Reber HA, Büchler M, Shrinkante S, Büchler MW, Friess H, Semenza GL, Hines OJ. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56–64. doi: 10.1097/00006676-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Carmeliet P, Collen D. Vascular development and disorders: molecular analysis and pathogenic insights. Kidney Int. 1998;53:1519–1549. doi: 10.1046/j.1523-1755.1998.00936.x. [DOI] [PubMed] [Google Scholar]
- 35.Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, Fan ST, Wong J. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227–235. doi: 10.1097/00000658-200102000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng C, Chen X. [Association of VEGF, uPA, ICAM-1 and PCNA expression with metastasis and recurrence in hepato cellular carcinoma] Zhonghua Wai Ke Za Zhi. 2002;40:673–675. [PubMed] [Google Scholar]
- 38.Oza AM, Elit L, Swenerton K, Faught W, Ghatage P, Carey M, McIntosh L, Dorr A, Holmlund JT, Eisenhauer E. Phase II study of CGP 69846A (ISIS 5132) in recurrent epithelial ovarian cancer: an NCIC clinical trials group study (NCIC IND.116) Gynecol Oncol. 2003;89:129–133. doi: 10.1016/s0090-8258(02)00144-0. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AH, al-Azzawi F, Pringle JH, Bell SC. Inhibition of endometrial carcinoma cell growth using antisense estrogen receptor oligodeoxyribonucleotides. Anticancer Res. 2002;22:3993–4003. [PubMed] [Google Scholar]
- 40.Yacyshyn BR, Barish C, Goff J, Dalke D, Gaspari M, Yu R, Tami J, Dorr FA, Sewell KL. Dose ranging pharmacokinetic trial of high-dose alicaforsen (intercellular adhesion molecule-1 antisense oligodeoxynucleotide) (ISIS 2302) in active Crohn's disease. Aliment Pharmacol Ther. 2002;16:1761–1770. doi: 10.1046/j.1365-2036.2002.01341.x. [DOI] [PubMed] [Google Scholar]
- 41.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 43.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong F, Jin YX. Inhibition of angiogenesis with antisense ODN of VEGF. Chin J Oncol. 1997;19:264–266. [PubMed] [Google Scholar]
- 45.Chen YF, Sun HW, Zou Q, Zou SQ. Inhibition of growth of hu-man cholangiocarcinoma in nude mice by vascular endthelial frowth factor antisense phosphorothioate oligodeoxynucleotides. Zhonghua Shiyan Waike Zazhi. 2000;17:22–23. [Google Scholar]
- 46.Shi W, Siemann DW. Inhibition of renal cell carcinoma angiogenesis and growth by antisense oligonucleotides targeting vascular endothelial growth factor. Br J Cancer. 2002;87:119–126. doi: 10.1038/sj.bjc.6600416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iversen PL, Copple BL, Tewary HK. Pharmacology and toxicology of phosphorothioate oligonucleotides in the mouse, rat, monkey and man. Toxicol Lett. 1995;82-83:425–430. doi: 10.1016/0378-4274(95)03572-9. [DOI] [PubMed] [Google Scholar]
- 48.Leeds JM, Henry SP, Bistner S, Scherrill S, Williams K, Levin AA. Pharmacokinetics of an antisense oligonucleotide injected intravitreally in monkeys. Drug Metab Dispos. 1998;26:670–675. [PubMed] [Google Scholar]
- 49.Tolcher AW, Reyno L, Venner PM, Ernst SD, Moore M, Geary RS, Chi K, Hall S, Walsh W, Dorr A, et al. A randomized phase II and pharmacokinetic study of the antisense oligonucleotides ISIS 3521 and ISIS 5132 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2002;8:2530–2535. [PubMed] [Google Scholar]
- 50.Webb MS, Tortora N, Cremese M, Kozlowska H, Blaquiere M, Devine DV, Kornbrust DJ. Toxicity and toxicokinetics of a phosphorothioate oligonucleotide against the c-myc oncogene in cynomolgus monkeys. Antisense Nucleic Acid Drug Dev. 2001;11:155–163. doi: 10.1089/108729001300338681. [DOI] [PubMed] [Google Scholar]
- 51.Han GH, Guo QL, Huang GS, Guo YL. Distribution of lipiodol in hepatocellular carcinoma after hepatic arterial injection and its significance. Zhonghua Fangshexue Zazhi. 1993;27:828–831. [Google Scholar]
- 52.Kan Z, Sato M, Ivancev K, Uchida B, Hedgpeth P, Lunderquist A, Rosch J, Yamada R. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861–866. doi: 10.1148/radiology.186.3.8381552. [DOI] [PubMed] [Google Scholar]


