Abstract
AIM: Whether operative procedure is a risk factor influencing recurrence following resection of carcinoma in the head of pancreas or not remains controversies. In this text we compared the recurrence rate of two operative procedure: the Whipple procedure and extended radical operation, and inquired into the factors influencing recurrence after radical resection.
METHODS: From January 1995 to December 1998, 35 cases of carcinoma of pancreas underwent the Whipple operadure, 21 patients received the Extended radical operation. All patients were followed up for more than 3 years. Prognostic factors included operative procedure, size of tumor, lymph node, interstitial invasion.
RESULTS: Deaths duo to recurrence within 3 years after operation were studied. The death rate was 51.4% in the Whipple procedure and 42.9% in the Extended radical operative procedure. There was a significant difference between the two groups. Recurrence occurred in 75% patients with tumor large than 4 cm, in 87.5% patients with lymph node involvement, and in 50% patients with the presence of interstitial invasion.
CONCLUSION: Tumor exceeding 4 cm, lymph node involvement, and presence of interstitial invasion are high risk factors of recurrence after Whipple’s procedure and extended radical operation.
INTRODUCTION
Recurrence of pancreatic cancer is common after operation. Intraabdominal recurrence ranged 38% to 86%[1-3]. Factors influencing recurrence in some studies included lymph node metastasis[4,5], tumor size[5,6], and tumor in surgical resection[5-7]. In the present study we retrospectively analysis 56 patients with carcinoma located in pancreatic head after operation in our department of surgery, The aim was to find the factors influencing recurrence following surgical resection for patients with pancreatic cancer hoping to improve the therapeutic results of carcinoma in the head of pancreas.
MATERIALS AND METHODS
Materials
Fifty six curative surgical resections were performed for pancreatic cancer in our department of surgery between January 1995 and December 1998. The patients did not receive any anticancer therapy before or after surgery.
Methods
Our radical procedures employed for carcinoma of pancreas was the Whipple operation in 35 cases, male/female ratio was 2.2:1(24/11), patients with an average of age were (57.3 ± 4.6) years. According to the General Rules for Cancer of the Pancreas (4th edition, 1996), lymphatic clearance was limited to the regional lymph nodes immediately adjacent to the pancreatic head (D1-). In the pancreas, the line of resection was on the left border of the superior mesenteric vein. Extended radical operation (D2+) was performed in the other 21 cases, the male/female ratio was 2.5:1(15/6) with an average of age 58.9 ± 5.1 years (Figure 1A and B). On the basis of n1 and n2 group and neighboring connective tissue clearance, the n3 group lymph nodes and soft tissues were properly cleared, nerve-plexus dissection around the retroperitoneum in 13 cases. Resection and reconstruction of the portal -vein system were performed in 6 cases, the line of resection of the pancreas was 1-2 cm outside the left border of the aorta.
Figure 1.
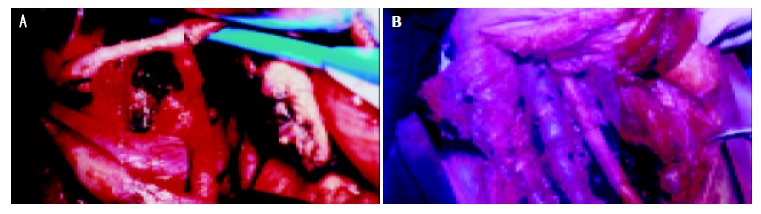
A: Ranges of lymphatic and neighboring connective tissure dissection n1, n2, and part of n3 group nodes were cleared with neighboring connective tissue, B: lymph node dissection around aorta, inferior vein,resection and reconstruction portal vein.
The resected specimens were fixed in 40g/L formaldehyde solution, and sliced into 5 μm sections. Histologic sections were stained with hematoxylin and eoxin. We measure the maximum size of the tumor, metastasis in lymph nodes, and determined whether tumors extended directly beyond the posterior confines of the pancreas. The maxinum tumor sizes were classified into four grades: 0 < t1 ≤ 2 cm, 2.0 < t2 ≤ 4.0 cm (t2), 4.0 < t3 ≤ 6.0 cm, and t4 > 6.0 cm. The lymph node involvement were gradeded into n0, n1, n2, and n3 accoding to the General Rules for Pancreatic Cancer Study (4th edition, 1996) proposed by the Japanese Pancreatic Society. The primary group included N06: infrapyloric, N08: anterosuperior nodes along the common hepatic artery, N012inferior: inferior nodes along the proper hepatic artery, along the bile duct, and along the posterior to the portal vein, N013: posterior surface of the head of pancreas, N01: origins of the superior mesenteric artery, the inferior pancreaticoduodenal artery, and the middle colic artery along the first jejunal branch, and the the superior mesenteric vein, N017: on the anterior surface of the head of pancreas. The second group included (N2): N09: around the celiac artery, N011: along the splenic artery, N012superior: superior nodes along the proper hepatic artery, the bile duct, superrior to the portal vein, around the cystic duct, N016: paraabdominal aorta. The third group (N3) included N03: lessur curvature, N04: greater curvature, N05: suprapyloric, N07: left gastric artery. Retroperitoneal invasion was classified into two grades Rp (+) and Rp(-) on the basis of whether the tumors extended directly beyond the posterior confines of the pancreas.
After surgery, all patients were followed up by serial determinations of plasma carcinoembryonic antigen (CEA), CA19-9, ultrasonograms and computed tomograms (CT) to determine whether and where cancer recurrence developed. The mode of clinical recurrence was classified into four types: hepatic metastasis (H), retroperitoneal recurrence (R), peritoneal dissemination (P), and distant metastasis (M). Retroperitoneal recurrence was divided into two subtypes: (1) local retroperitoneal recurrence was defined as infiltration of nerves, lymphatic vessels, and connective soft tissue, and (2) lymph node metastasis (LN).
The cumulative recurrence rate was analysed by using a χ2 test. P value less than 0.05 was considered statistically significant.
RESULTS
No operative death occurred within 1 mo after excision. The follow-up period was more than 3 years for all patients of the two groups. In D1- group, 6 cases were lost to be followed, 7 cases died of other disesses unrelated to cancer within three years, the remaining 22 patients died of recurrence, of which 18 patients was dead within 3 years. In D2+ group, 2 patients were lost to be followed, 3 patients died of other diseases within 3 years, the remaining 9 patients died of recurrence within 3 years. The 3 years cumulative rate of death duo to recurrence was 51.4% in D1- group and 42.9% in D2+ group, there was a significant difference between the 2 groups (P < 0.05). The histopathological backgrounds in patients who died of recurrence are showed in Table 1.
Table 1.
Histopathological findings in patients died of Recurrence
| Operative procedure (No.of patients) |
Maximum size |
Nodal involvement |
Interstitial invasion |
|||||||||
| t1 | t2 | t3 | t4 | n0 | n1 | n2 | n3 | Ii (-) | Ii (+) | |||
| D1- | 1/5 | 7/16 | 10/14 | - | 3/9 | 15/26 | - | - | 6/13 | 12/22 | ||
| D2+ | 0/4 | 3/9 | 3/5 | 3/3 | 0/6 | 2/7 | 4/5 | 3/3 | 1/5 | 8/16 | ||
Recurrent styles
In D1- group at least more than 2 recurrent sites could be found. Eighteen patients had retroperitoneal recurrence, among them 7 patients were complicated with peritoneal dissemination, 2 patients were complicated with liver metastasis, and 1 patient was complicated with extroabdomen metastasis. In D2+ group, the major recurrent styles of were as fellows: hepatic metastasis alone or in combination with retroperitoneal recurrence (n = 5), peritoneal dissemination alone or combined with abdomen lymph node enlargement (n = 4), or combined with other organ out of abdomen cavity metastasis (n = 1).
Histopathological diagnosis
The distribution of cases was histopathologically (Figure 2) based on 3 factors: maxinum tumor size, lymph node involvement, and interstitial invasion (Table 2).
Figure 2.
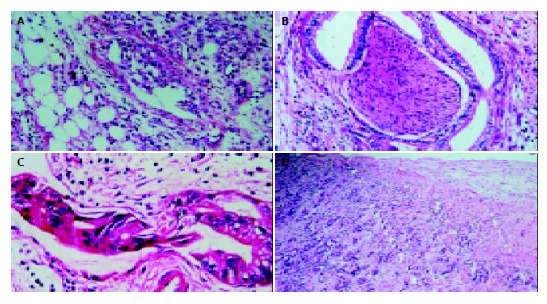
A: peritoneal dissemination, B: nerve invasion, C: cancer thrombus in lymphatic vessel, D: portal venious wall invasion. (HE original magnification × 200).
Table 2.
Comparision of tumor Size, nodal involvement and interstitial invasion between two groups
| Operative procedure (No.of patients) |
Maximum size |
Nodal involvement |
Interstitial invasion |
|||||||||
| t1 | t2 | t3 | t4 | n0 | n1 | n2 | n3 | Ii (-) | Ii (+) | |||
| D1- | 5 | 16 | 14 | - | 9 | 26 | - | - | 13 | 22 | ||
| D2+ | 4 | 9 | 5 | 3 | 6 | 7 | 5 | 3** | 2 | 19 | ||
*5 is positive also in n1, **3 is also positive in n1,and n2.
In D2+ group, tumors were less than 2 cm in diameter (4 cases), one case had lymph-node metastasis, and 2 lymph node vessels and perineural invasion respectively. In t2 group, 77.8%(7/9) of cases was associated with lymph-vessel invasion. Perineural invasion was present in 88.9%(8/9) of the tumor, and loose connective tissue invasion occurred in 55.6%(5/9). Tumors larger than 4.1 cm were all associated with lymph-vessel, perineural, and loose connective tissue invasion. Metastatic rate of lymph node was 69.2% (n = 15). Lymph node metastatic rate was 69.2% (n = 15). Rates of histologically proved metastasis to individual lymph nodes observed in our series were as follows: N1: N06: 23.8%(n = 5), N08: 14.4%(n = 3), N012inferior: 33.3%(n = 7), N013:33.3%(n = 7), N014:28.6%(n = 6), N017:33.3%(n = 7); N2: N09:14.4%(n = 3), N011:19.1%(n = 4), N012superior23.8%(n = 5), N016:23.8%(n = 5); N3: N03:0%, N04:0%, N05:14.4%(n = 3), N07: 13.3%(n = 2). In tumors with negative lymph nodes, 5/6 had lymph-vessel invasion, and 4/6 had perineural invasion. The tumors with nodal involvement were all associated with lymph-vessel, perineural, and loose connective tissue invasion.
DISCUSSION
Argument existed about whether operative procedure on the risk factors influencing recurrence or not[8-11]. Factors that influence the recurrent rate after resection were the absence of lymph node involvement[12,13], and retroperitoneal invasion[14], and microscopic curative resection[12,14]. Such a procedure is also called Ro surgery. In our current study we confirmed that D2+ procedure could decrease recurrence in compassion with D1-. In D2+ group we found there exists wide extension of nodal involvement,and ‘interstitial invasion’ required careful dissection. D1- procedure only provided simple lymphadenectomy limited to the region of the head of pancreas without resection of surrounding connective tissues, and dissection of the second and tertiary group lymph node was inadequate for the purpose of lymphatic clearance. Theoretically D2+ procedure could achieve a microscopic curative resection[15,16]. Macroscopic curative resection has been proven to be microscopic noncurative resection by precise serial section analysis. Even the patients with microscopic curative resection had a surgical margin of only a few millimeters away from tumor[17], that could not assure avodance of future metastasis. Only in those with small (t1/t2), noninvasive lesions or slight retroperitoneal invasion, could D2+ actually decrease recurrence. In those with t3/t4 tumors, even after extended lymphatic and soft tissue dissection that goes beyond the regional lymph-node stations, D2+ procedure still has a higher recurrence.
The rate of recurrence in patients with t1 and t2 tumors generally was lower than that in those with t3 and t4 tumors after D2+ procedure. The collective recurrence rate in t3 and t4 tumors was 75%(6 of 8). Tumors larger than 4.1 cm were all associated with lymph-vessel and perineural invasions. Therefore, our conclusion is that the larger the tumor the more extensive infiltration within interstitial invasion and nodal involvement, or the higher the recurrent risk, this is in accord with that reported in the literature[18-21].
In comparision with D1-, D2+ procedure decreased recurrence in no and n1 group. There was a close relation between lymph node involvement and ‘interstitial invasion’ . Positive lymph node was often accompanied by lymph vessels invasion. Even if in pNo stage, lymph vessels invasion was present in 64% of the cases[19]. Lymph vessel invasion might imply lymphatic metastasis before cancer cells flowed into lymph nodes. If nodal involvement was found in n1 region, microinvasion had already occurred in the n2 region[22]. If n2 and n3 groups were invaded , the chance of distant recurrence was much increased.
Our study confirmed that pancreatic cancer tended to be accompanied by ‘interstitial invasion’ and positive of ‘interstitial invasion’ was a factor influencing recurrence. The so-called ‘interstitial invasion’includes lymph vessel, nerves, and loose connective tissue invasions. The recurrence rate in patients with or without ‘interstitial invasion’ was 50% and 20%, respectively. The significance of nerve invasion has been annotated by other researchers[23-25]. Peritoneal dissemination after excision could not be treated by surgery alone, bcause cancer cells either as single cells or cell clumps were randomely allocated on the large area of loose connective tissue of the peritonum[26]. About 40% of patients had small distant metastases. Such metastases were typical 1-2 mm nodules located on the surface of the peritoneum[27]. So far as peritoneal dissemination concerned, there is no effective treatment. Even extensive lymph node dissection and resection of surrounding connective tissues and major vessels combined with radiotherapy and chemotherapy could not assure avoidance of recurrence up to now[28-30].
In summary, the long term survival following resection depends on decrease of recurrence. Therefore rationally standardized operative procedure with due to attention to factors of recurrence may help improve the long term survival of pancreatic cancer patients.
Footnotes
Edited by Wang XL Proofread by Xu FM
References
- 1.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving. Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, Evans RG. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Westerdahl J, Andrén-Sandberg A, Ihse I. Recurrence of exocrine pancreatic cancer--local or hepatic. Hepatogastroenterology. 1993;40:384–387. [PubMed] [Google Scholar]
- 4.Meyer W, Jurowich C, Reichel M, Steinhäuser B, Wünsch PH, Gebhardt C. Pathomorphological and histological prognostic factors in curatively resected ductal adenocarcinoma of the pancreas. Surg Today. 2000;30:582–587. doi: 10.1007/s005950070096. [DOI] [PubMed] [Google Scholar]
- 5.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Mosella G. Survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Chir Ital. 2000;52:263–270. [PubMed] [Google Scholar]
- 6.Yamaguchi K, Mizumoto K, Noshiro H, Sugitani A, Shimizu S, Chijiiwa K, Tanaka M. Pancreatic carcinoma: <or = 2 cm versus> 2 cm in size. Int Surg. 1999;84:213–219. [PubMed] [Google Scholar]
- 7.van Geenen RC, van Gulik TM, Offerhaus GJ, de Wit LT, Busch OR, Obertop H, Gouma DJ. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: an update. Eur J Surg Oncol. 2001;27:549–557. doi: 10.1053/ejso.2001.1162. [DOI] [PubMed] [Google Scholar]
- 8.Iacono C, Facci E, Bortolasi L, Zamboni G, Scarpa A, Talamini G, Prati G, Nifosí F, Serio G. Intermediate results of extended pancreaticoduodenectomy. Verona experience. J Hepatobiliary Pancreat Surg. 1999;6:74–78. doi: 10.1007/s005340050086. [DOI] [PubMed] [Google Scholar]
- 9.Tsiotos GG, Farnell MB, Sarr MG. Are the results of pancreatectomy for pancreatic cancer improving. World J Surg. 1999;23:913–919. doi: 10.1007/s002689900599. [DOI] [PubMed] [Google Scholar]
- 10.Pedrazzoli S, Pasquali C, Sperti C. General aspects of surgical treatment of pancreatic cancer. Dig Surg. 1999;16:265–275. doi: 10.1159/000018735. [DOI] [PubMed] [Google Scholar]
- 11.Benassai G, Mastrorilli M, Mosella F, Mosella G. Significance of lymph node metastases in the surgical management of pancreatic head carcinoma. J Exp Clin Cancer Res. 1999;18:23–28. [PubMed] [Google Scholar]
- 12.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–57; discussion 257-60. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagakawa T, Nagamori M, Futakami F, Tsukioka Y, Kayahara M, Ohta T, Ueno K, Miyazaki I. Results of extensive surgery for pancreatic carcinoma. Cancer. 1996;77:640–645. [PubMed] [Google Scholar]
- 14.Nakao A, Harada A, Nonami T, Kaneko T, Takagi H. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357–361. doi: 10.1097/00006676-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Nakao A, Kaneko T, Takeda S, Inoue S, Harada A, Nomoto S, Ekmel T, Yamashita K, Hatsuno T. The role of extended radical operation for pancreatic cancer. Hepatogastroenterology. 2001;48:949–952. [PubMed] [Google Scholar]
- 16.Imamura M, Hosotani R, Kogire M. Rationale of the so-called extended resection for pancreatic invasive ductal carcinoma. Digestion. 1999;60 Suppl 1:126–129. doi: 10.1159/000051468. [DOI] [PubMed] [Google Scholar]
- 17.Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–2123. doi: 10.1002/1097-0142(19931001)72:7<2118::aid-cncr2820720710>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Nagai H, Kuroda A, Morioka Y. Lymphatic and local spread of T1 and T2 pancreatic cancer. A study of autopsy material. Ann Surg. 1986;204:65–71. doi: 10.1097/00000658-198607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebhardt C, Meyer W, Reichel M, Wünsch PH. Prognostic factors in the operative treatment of ductal pancreatic carcinoma. Langenbecks Arch Surg. 2000;385:14–20. doi: 10.1007/s004230050004. [DOI] [PubMed] [Google Scholar]
- 20.Takao S, Shinchi H, Sha K, Natsugoe S, Maenohara S, Suenaga T, Nishimata Y, Aikou T. Clinical and biological features of t1 ductal adenocarcinoma of the pancreas. Hepatogastroenterology. 1998;46:498–503. [PubMed] [Google Scholar]
- 21.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, Mazzeo F. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73:212–218. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa O, Ohhigashi H, Sasaki Y, Kabuto T, Fukuda I, Furukawa H, Imaoka S, Iwanaga T. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg. 1988;208:215–220. doi: 10.1097/00000658-198808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16–22. doi: 10.1007/BF02482964. [DOI] [PubMed] [Google Scholar]
- 24.Dang C, Qin Z, Ji Z, Li Y, Zhao J, Takashi E, Naito Z, Yokoyama M, Asano G. Morphological characteristics and clinical significance of nerve distribution in pancreatic cancers. Nihon Ika Daigaku Zasshi. 1997;64:526–531. doi: 10.1272/jnms1923.64.526. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi S, Hasebe T, Oda T, Sasaki S, Kinoshita T, Konishi M, Ueda T, Ochiai T, Ochiai A. Extra-tumor perineural invasion predicts postoperative development of peritoneal dissemination in pancreatic ductal adenocarcinoma. Anticancer Res. 2001;21:1407–1412. [PubMed] [Google Scholar]
- 26.Hiraoka T, Uchino R, Kanemitsu K, Toyonaga M, Saitoh N, Nakamura I, Tashiro S, Miyauchi Y. Combination of intraoperative radiation with resection of cancer of the pancreas. Int J Pancreatol. 1990;7:201–207. doi: 10.1007/BF02924238. [DOI] [PubMed] [Google Scholar]
- 27.Warshaw AL, Tepper JE, Shipley WU. Laparoscopy in the staging and planning of therapy for pancreatic cancer. Am J Surg. 1986;151:76–80. doi: 10.1016/0002-9610(86)90015-2. [DOI] [PubMed] [Google Scholar]
- 28.Cellini N, Trodella L, Valentini V, Doglietto GB, Morganti AG, Ziccarelli P, Alfieri S, Bossola M, Brizi MG, Crucitti F. Radiotherapy, local control and survival in carcinomas of the exocrine pancreas. Rays. 1998;23:528–534. [PubMed] [Google Scholar]
- 29.Alfieri S, Morganti AG, Di Giorgio A, Valentini V, Bossola M, Trodella L, Cellini N, Doglietto GB. Improved survival and local control after intraoperative radiation therapy and postoperative radiotherapy: a multivariate analysis of 46 patients undergoing surgery for pancreatic head cancer. Arch Surg. 2001;136:343–347. doi: 10.1001/archsurg.136.3.343. [DOI] [PubMed] [Google Scholar]
- 30.Foo ML, Gunderson LL, Nagorney DM, McLlrath DC, van Heerden JA, Robinow JS, Kvols LK, Garton GR, Martenson JA, Cha SS. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/- 5 fluorouracil. Int J Radiat Oncol Biol Phys. 1993;26:483–489. doi: 10.1016/0360-3016(93)90967-z. [DOI] [PubMed] [Google Scholar]


