Abstract
Patients with biliary tract cancer (BTC) have a poor prognosis. Advanced BTC patients have been treated with cisplatin in combination with gemcitabine, however, the treatment has had little impact on survival rates, and more effective treatments are urgently required for this disease. Previous studies discovered that buthionine sulfoximine (BSO), a potent inhibitor of glutathione (GSH) synthesis, was able to enhance the cytotoxic effect of various drugs in cancer cells. Phase I studies demonstrated that continuous-infusion of BSO was relatively non-toxic and resulted in the depletion of tumor GSH. However, the synergistic effect of BSO and cisplatin in BTC cells remains unknown, and no reports are available regarding sensitization to gemcitabine by BSO. In the present study, the effect of BSO in combination with cisplatin or gemcitabine in the treatment of BTC cells was examined in vitro. Cytotoxic effects were measured using an MTT assay, Annexin V assay and fluorescence-activated cell sorting analysis. Antiapoptotic protein expression levels were examined using western blot analysis. The results revealed that a sub-toxic concentration of BSO was capable of significantly enhancing cisplatin-induced apoptosis in BTC cells. The mechanisms of BSO's effect on BTC cells may be attributable to the reduction of GSH levels and downregulation of the expression of antiapoptotic proteins (Bcl-2, Bcl-xL and Mcl-1). Furthermore, BSO enhanced the antiproliferative effect of gemcitabine. In conclusion, the present data are the first results to indicate that BSO may sensitize BTC cells to standard first-line chemotherapeutic agents (cisplatin and gemcitabine). Combining BSO with cisplatin and gemcitabine is a promising therapeutic strategy for the treatment of BTC.
Keywords: biliary tract cancer, buthionine sulfoximine, cisplatin, gemcitabine, chemoresistance
Introduction
Biliary tract cancer (BTC) is a collective term for a heterogenous group of tumors, including cancer arising from the gallbladder and bile ducts, as well as adenocarcinoma of the ampulla of Vater. Although considered relatively rare in the US [5,000 new cases diagnosed annually (1)] and European countries (1,200 cases per annum in the UK) (2,3), it has a much higher prevalence in Latin America (4) and East Asia. In Japan, the incidence is 10-fold of that in the West, with 17,311 mortalities from BTC in 2007, making it the sixth leading cause of cancer mortality in Japan (5). Furthermore, the incidence, particularly of intrahepatic cholangiocarcinoma, has been increasing in the US, Japan, UK and Australia since the 1970s (6–8). Surgical removal of the tumor is the only curative treatment. However, the majority of patients are diagnosed when the disease has reached an advanced-stage, making them ineligible for complete surgical resection. Furthermore, recurrence is common even following complete resection, and is usually only amenable to palliative chemotherapy (9). In 2010, a phase III trial found that cisplatin in combination with gemcitabine is an appropriate option for the treatment of patients with advanced BTC (10). However, patients with advanced BTC still have a poor prognosis, with a median survival of <1 year (10–12). Therefore, future research on BTC must aim to enhance the effectiveness of chemotherapeutic regimens.
It has been well established increased intracellular glutathione (GSH) is associated with resistance to chemotherapy and irradiation and, correspondingly, that the reduction of GSH levels is associated with sensitization to these two types of therapy (13). We previously demonstrated that emodin, a natural anthraquinone isolated from traditional Chinese herbal medicines, enhances cisplatin-induced apoptosis in gallbladder cancer cells, in vitro and in vivo, via depletion of GSH and downregulation of multidrug resistance-related protein 1 (14). Although emodin provides a therapeutically feasible approach to overcome chemoresistance in gallbladder cancer cells, little clinical trial data regarding emodin is available, and the development of an emodin-based drug remains in the experimental stages. Oral ingestion of large amounts of emodin may lead to stomach cramping, gas production, bloating and diarrhea (15–17). Therefore, it is considered that emodin tends to exacerbate the gastrointestinal side effects and contribute to patient intolerance of chemotherapy. For these reasons, emodin may not be an ideal chemosensitizer. Other compounds must be tested to develop effective and safe drugs capable of enhancing the chemosensitivity of BTC cells.
Buthionine sulfoximine (BSO) is a specific inhibitor of γ-glutamyl-cysteine synthetase and is thus able to block the rate-limiting step of GSH biosynthesis. Depletion of GSH by BSO restores the sensitivity of resistant tumors to drugs in vitro and in vivo (18). A number of research groups undertook phase I clinical studies to determine clinically whether BSO produced the desired biochemical end point of GSH depletion. In these preliminary studies, it was revealed that continuous infusion of BSO was relatively non-toxic and resulted in the depletion of tumor GSH in patients with advanced cancers (ovarian, lung, breast and colon cancer, and melanoma) (19–21). These results prompted the current study, which aimed to investigate the effect of BSO combined with cisplatin and gemcitabine in BTC cells.
Previous studies have demonstrated that BSO is able to enhance the cytotoxic effect of certain drugs, including cisplatin, azathioprine and melphalan, in cancer cells (22–25). However, the synergistic effect of BSO and cisplatin in BTC cells remains unknown, and there are no available reports regarding sensitization to gemcitabine by BSO. Therefore, the purpose of the present study was to demonstrate whether BSO was capable of potentiating the anticancer effects of cisplatin or gemcitabine in BTC cells, and to investigate the possible mechanism.
Materials and methods
Cell culture and reagents
Human gallbladder cancer (GBC-SD) and human cholangiocarcinoma (RBE) cell lines were obtained from the Cell Bank of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). GBC-SD and RBE cells were maintained in RPMI-1640 (GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells were cultured in a humidified atmosphere of 5% CO2 at 37°C.
BSO was purchased from Sigma-Aldrich (St. Louis, MO, USA). Gemcitabine was purchased from Jiangsu Hansoh Pharmaceutical Co., Ltd. (Lianyungang, China), and cisplatin was obtained from Qilu Pharmaceutical Co., Ltd. (Jinan, China).
Human GBC-SD and RBE cells were pretreated with 50 µM BSO for 24 h before exposure to 4 or 8 µg/ml cisplatin or 0.5 mg/ml gemcitabine for 24 h. The cells were then collected and the cytotoxic effects examined.
Cell viability and apoptosis analysis
Cell viability was assayed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich), as previously described (26). Briefly, the cells were seeded in a 96-well plate at a density of 10,000 cells/well. Following overnight incubation in a humidified atmosphere of 5% CO2 at 37°C, each well was refreshed with 0.2 ml serum-free medium (SFM) containing 50 µM BSO for a further day. The cells were then pretreated with 0.2 ml SFM containing 50 µM BSO for 24 h. Gemcitabine (500 µg/ml) or cisplatin (4 or 8 µg/ml) were subsequently added to the medium for an additional 24 h. Cells were not washed between treatments. Finally, cell viability was assessed with an MTT reagent and by measuring the absorbance at a wavelength of 570 nm using a VersaMax™ ELISA Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA). Relative viability was obtained from the absorbance of the drug-treated cells divided by that of the untreated cells. The same experiment was repeated three times.
Cell apoptosis was assessed using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit (BD Pharmingen, San Diego, CA, USA) and analyzed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) (27). Briefly, the cells were seeded into 6-well plates and treated with BSO, gemcitabine, cisplatin, BSO/gemcitabine or BSO/cisplatin. The cells were collected 24 h later and washed twice using cold phosphate-buffered saline (Gibco; Thermo Fisher Scientific, Inc.). The cells were then stained using an Annexin V/PI double staining solution at room temperature. After 15 min, the Annexin V/PI-stained cells were analyzed by flow cytometry, and the percentage of apoptotic and necrotic cells was calculated. Cells that were positively stained by Annexin V-FITC only (early apoptosis), or positive for Annexin V-FITC and PI (late apoptosis/necrosis) were quantitated, and these two sub-populations were considered as the overall population of apoptotic cells.
GSH/oxidized GSH (GSSG) ratio assay
GSH is a tripeptide with a free thiol group that functions as a major antioxidant in cells. Usually, cellular GSH exists predominantly in its reduced form, whereas GSSG is present in small amounts (28). The GSH/GSSG ratio is often used as an indicator of cellular redox status (28). Total GSH and GSSG levels were determined by colorimetric microplate assay kit (Beyotime Institute of Biotechnology, Haimen, China) as previously described (29,30). Following treatment with 50 µM BSO, cells were collected by centrifugation at 10,000 × g for 10 min at 4°C and re-suspended in 20 µl cell culture medium. Cells (10 µl) were mixed with 30 µl 5% metaphosphoric acid (Beyotime Institute of Biotechnology), then frozen and thawed twice in liquid nitrogen and 37°C water respectively. The samples were centrifuged again (using the same conditions) and the supernatant was used for GSH and GSSG assays. The total GSH level was measured by performing a DTNB-GSSG recycling assay (29). The GSSG level was quantified by the same method as for total GSH after the supernatant was treated with 1 mol/l 2-vinylpyridine solution to remove the reduced GSH. The quantity of reduced GSH was obtained by subtracting the quantity of GSSG from that of total GSH. The GSH/GSSG ratio was calculated using the following formula: Ratio = (total GSH − 2GSSG) / GSSG.
Assays of antiapoptotic protein expression
Myeloid cell leukemia 1 (Mcl-1), B-cell lymphoma-extra large (Bcl-xL) and B-cell lymphoma 2 (Bcl-2) protein expression was determined by western blot analysis as previously described (31). Cells were lysed in sample solution. Proteins were separated with 10% SDS-PAGE gels (Sangon Biotech, Shanghai, China) run at 80 V for 30 min, then 120 V for 60 min at room temperature, and transferred to nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). After blocking with 5% milk in Tris-buffered saline in Tween 20 buffer (TBST; Gibco; Thermo Fisher Scientific, Inc.), the membrane was incubated overnight at 4°C with polyclonal rabbit anti-human Bcl-2 (1:200 dilution; cat no. sc-492) and Bcl-xl (1:200 dilution; cat no. sc-7195; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies, and monoclonal rabbit anti-human Mcl-1 antibody (1:800 dilution; cat no. ab32087; Abcam, Cambridge, UK). The membranes were also incubated with monoclonal mouse anti-human β-actin antibody (1:1,000 dilution; cat no. sc-47778; Santa Cruz Biotechnology, Inc.) as the loading control. Following incubation, the membranes were washed three times with TBST. The membranes were subsequently incubated with goat anti-rabbit or anti-mouse peroxidase-conjugated secondary antibodies (cat nos. 111-035-003 and 115-035-003; Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) for 2 h at 37°C, prior to detection using ECL system (cat no. WBKLS0500; EMD Millipore).
Statistical analysis
Data are presented as the mean value ± standard deviation. SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Analysis of variance was applied for comparison of the means of two or multiple groups. A value of P<0.05 was considered to indicate statistically significant differences.
Results
BSO enhances cisplatin-induced inhibition of cell viability in BTC cells by increasing apoptosis
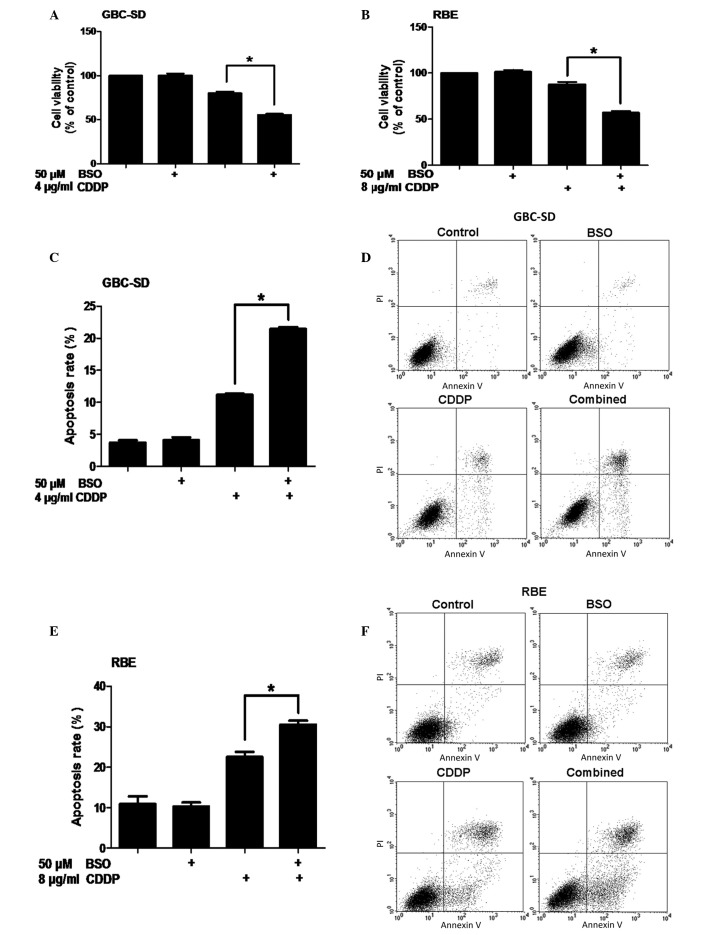
To examine the synergistic effect of BSO on the inhibition of cell viability by cisplatin, GBC-SD human gallbladder cancer cells and RBE cholangiocarcinoma cells were pretreated with 50 µM BSO for 24 h before exposure to cisplatin for 24 h. Cell metabolic activity was measured using an MTT assay. No significant difference in cell viability was detected between untreated cells and cells treated only with BSO (P>0.05). However, compared with the control, GBC-SD and RBE cells that were not pretreated with BSO experienced 20 and 12% decreases in viability, respectively, following 24-h exposure to cisplatin (P<0.0001 and P=0.0029, respectively). In addition, pretreatment of BTC cells with BSO followed by treatment with cisplatin resulted in reductions in cell viability of 44% and 43% in the GBC-SD and RBE cell lines, respectively (Fig. 1A and B).
Figure 1.
Effect of BSO on cell viability and apoptosis of biliary tract cancer cells in response to cisplatin. (A) GBC-SD cells were pretreated with 50 µM BSO for 24 h before exposure to 4 or 8 µg/ml CDDP for 24 h. (B) Cell viability of RBE cells (MTT assay). (C) Apoptosis of GBC-SD cells (Annexin V/PI flow cytometry, bar charts). (D) Apoptosis of GBC-SD cells (density plots). (E) Apoptosis of RBE cells (Annexin V/PI flow cytometry, bar charts). (F) Apoptosis of RBE cells (density plots). Data shown is the average of three independent experiments. *P<0.05. BSO, buthionine sulfoximine; CDDP, cisplatin; PI, propidium iodide.
To determine whether the reduction in cell viability could be attributed to an increase in apoptosis, Annexin V-FITC/PI double-labeling flow cytometry was conducted. Compared with cisplatin alone, pretreatment with BSO followed by cisplatin treatment resulted in an increase in apoptosis in GBC-SD cells (P=0.0013; Fig. 1C and D). Similarly, the BSO/cisplatin co-treatment also led to an increase in apoptosis in RBE cells (P=0.0374; Fig. 1E and F). Together, these data demonstrate that BSO is capable of enhancing cisplatin-induced apoptosis in BTC cells.
BSO depletes GSH and decreases the GSH/GSSG ratio in BTC cells
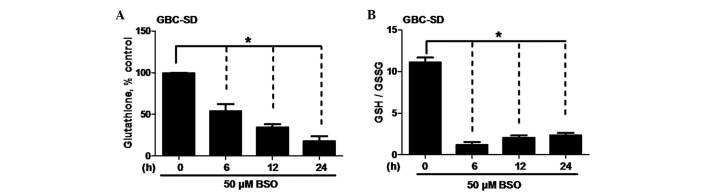
BSO has been demonstrated to deplete intracellular GSH and induce oxidative stress (19–27,29–32), and this may sensitize various types of cancer cells to certain drugs (33–36). To study the oxidative impact of BSO on the cellular redox state of BTC cells, intracellular GSH levels and the GSH/GSSG ratio were measured in GBC-SD cells treated with 50 µM BSO. Notably, BSO reduced the intracellular GSH levels in a time-dependent manner (P=0.0321; Fig. 2A). Furthermore, BSO decreased the GSH/GSSG ratio, a reflection of the cellular redox state (P=0.0016; Fig. 2B). These data provide evidence that the enhancement of BSO on cisplatin-induced apoptosis in BTC cells is associated with the depletion of GSH.
Figure 2.
Effect of BSO on GSH levels and GSH/GSSG ratio in biliary tract cancer cells. (A) GSH levels and (B) GSH/GSSG ratio in GBC-SD cells. Cells were exposed to 50 µM BSO for the indicated times. Data shown is the average of three independent experiments. *P<0.05. BSO, buthionine sulfoximine; GSH, glutathione
BSO downregulates antiapoptotic protein expression in BTC cells
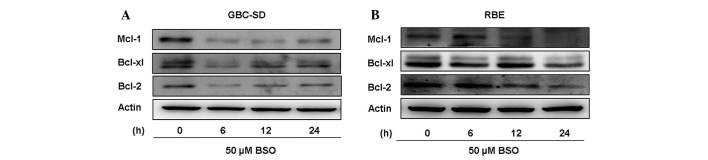
Antiapoptotic proteins, including Bcl-2, Bcl-xL and Mcl-1, are overexpressed in numerous types of cancer cells and contribute to tumor drug resistance (37). Therefore, inhibition of their expression may lead to an increased sensitivity to anticancer drugs. To better understand the mechanism responsible for the ability of BSO to overcome cisplatin resistance, the expression of these antiapoptotic proteins was analyzed in lysates from BTC cells treated with 50 µM BSO for 24 h. Results from western blot analyses revealed that BSO effectively downregulated Mcl-1, Bcl-2 and Bcl-xL expression (Fig. 3). The observation that BSO increases the cytotoxicity of cisplatin may be explained, at least partially, by downregulation of antiapoptotic proteins in BTC cells.
Figure 3.
Effect of BSO on antiapoptotic protein expression in BTC cells immunoblot analysis of Mcl-1, Bcl-xL and Bcl-2 expression in (A) GBC-SD and (B) RBE cells. Cells were treated with 50 µM BSO and harvested at the indicated times. β-actin was used as a loading control. BSO, buthionine sulfoximine.
BSO enhances the antiproliferative effect of gemcitabine on BTC cells
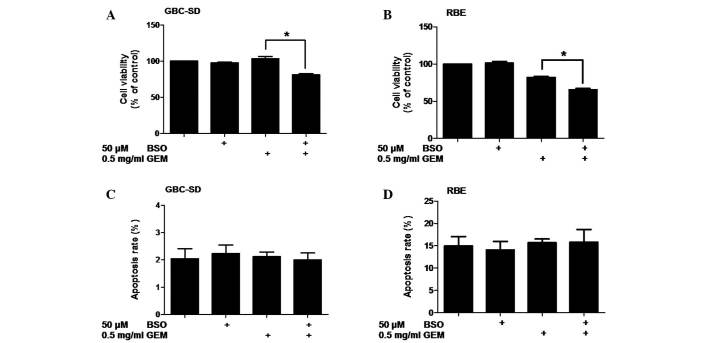
Patients with advanced BTC are currently being treated with a combination of cisplatin and gemcitabine (10). Therefore, the potential synergistic effect of BSO with gemcitabine was also examined. Cell viability was assessed following the treatment of BTC cells with BSO and/or gemcitabine. The results revealed that a dose of 500 µg/ml gemcitabine was significantly more effective at reducing cell viability when it was combined with 50 µM BSO in GBC-SD (P=0.0020) and RBE (P=0.0005) cells (Fig. 4A and B). These results suggest that the effects of BSO are not specific to cisplatin and that it may also enhance the antiproliferative effect of gemcitabine in BTC cells.
Figure 4.
Effect of BSO on sensitivity of biliary tract cancer cells to gemcitabine. (A) GBC-SD cells were pretreated with 50 µM BSO for 24 h before exposure to gemcitabine for 24 h. (B) Cell viability of RBE cells (MTT). (C) Apoptosis of GBC-SD cells (Annexin V/PI flow cytometry, bar charts). (D) Apoptosis of RBE cells (Annexin V/PI flow cytometry, bar charts). Data shown is the average of three independent experiments. *P<0.05. GEM: gemcitabine. BSO, buthionine sulfoximine; PI, propidium iodide.
To determine whether BSO enhances the induction of apoptosis by gemcitabine, the induction of apoptosis in BTC cells treated with BSO and/or gemcitabine was investigated by Annexin V-FITC/PI flow cytometry. No increase in apoptosis was detected in the two cell lines following treatment with gemcitabine alone or treatment with BSO and gemcitabine (P>0.05; Fig. 4C and D).
Discussion
Chemoresistance remains a major obstacle in improving responses to BTC treatment, and new drug combinations offer promising innovations to BTC patients. The present data suggest that BSO may sensitize BTC cells to the standard first-line chemotherapeutic agents cisplatin and gemcitabine. This conclusion is supported by multiple lines of evidence: (i) Cisplatin-induced apoptosis in BTC cells was significantly enhanced by a sub-toxic concentration of BSO; (ii) BSO sensitized BTC cells to cisplatin through GSH depletion and downregulation of antiapoptotic proteins (Mcl-1, Bcl-2 and Bcl-xL); iii) BSO also enhanced the antiproliferative effect of gemcitabine in BTC cells.
The therapeutic effect of cisplatin is considered to be due to the formation of covalent adducts with DNA, which prompt DNA damage signals to induce apoptosis in a number of types of solid tumor (14). Cancer cells may become resistant to platinum-based drugs through multiple mechanisms, including an increased ability to repair platinum-induced DNA damage, neutralization of platinum toxicity, and an increase in drug export (38). GSH, the most abundant cellular antioxidant, is important in the promotion of cell survival. GSH is able to confer resistance to platinum drugs, owing to its ability to form conjugates with platinum compounds and thus neutralize drug toxicity and promote the export of the drug (39). In the present study, a marked reduction of intracellular GSH was detected following treatment with BSO. Consistently, an increase of cisplatin-induced cytotoxicity following combined treatment with BSO and cisplatin was observed, which may be attributed to a reduction in GSH availability to form platinum conjugates and thereby the reduction of cellular efflux of the drug. This hypothesized mechanism may be further tested by examining the total intracellular platinum and DNA-bound platinum in BTC cells following treatment with BSO in combination with cisplatin.
Besides reduced drug accumulation, cisplatin resistance develops through an increased ability to avoid drug-induced cell damage, cell shrinkage and, therefore, initiation of apoptosis (40). Apoptosis is regulated in part by the Bcl-2 family of proteins, which consist of both proapoptotic [Bcl-2-associated X and Bcl-2-antagonist/killer] and antiapoptotic (Bcl-2, Bcl-xL and Mcl-l) proteins (41). Thus, downregulation of antiapoptotic protein expression may negate cisplatin resistance in BTC cells. The current study provided evidence that Mcl-1, Bcl-2 and Bcl-xL expression in BTC cells was significantly downregulated by BSO treatment, indicating that BSO may exert synergistic anticancer actions through antiapoptotic protein downregulation. As shown in Fig. 3, BSO induced more significant downregulation of antiapoptotic proteins in GBC-SD cells than in RBE cells, which may explain the fact that BSO in combination with cisplatin increased cell apoptosis to a greater extent in GBC-SD cells than in RBE cells. However, it remains unclear how BSO actually suppresses the expression of antiapoptotic proteins. Previous work has revealed that mild oxidative stress may induce S-glutathionylation of signal transducer and activator of transcription 3 (STAT3), leading to the suppression of the STAT3 pathway, downregulation of STAT3-dependent gene expression and chemosensitization of tumor cells to chemotherapy (42). It has also been established that Mcl-1 and Bcl-2 are regulated by STAT3 (43,44). The present results indicated that BSO treatment was capable of inducing oxidative stress in BTC cells (Fig. 2B), suggesting that BSO may also affect the STAT3 pathway. Thus, further studies are required to test whether BSO is able to downregulate the expression of antiapoptotic proteins through S-glutathionylation of STAT3.
In addition, the present findings revealed that BSO was able to enhance gemcitabine-induced inhibition of cell viability, but did not affect apoptosis in gemcitabine-treated cells. This result indicates that the reduction in BTC cell viability could not be attributed to an increase in apoptosis. BSO in combination with gemcitabine may decrease cell viability through induction of cell cycle arrest or other mechanisms. The exact mechanism of growth inhibition under these conditions, however, requires further investigation.
In conclusion, the current results indicate that BSO may significantly enhance the anticancer effects of cisplatin and gemcitabine in BTC cells in vitro. In phase I trials, the concentration of BSO in blood has been reported to reach 0.5–1 mM (19,21). Thus the concentration of BSO used in the present investigation is clinically achievable. In addition, it is notable that phase I studies of BSO administered with the anticancer drug melphalan demonstrated that continuous infusion of BSO was relatively non-toxic and resulted in depletion of tumor GSH (16,17). This study adds to a growing body of evidence that BSO may be a highly effective sensitizer for chemotherapeutic treatment of BTC patients. Further research is necessary to evaluate the feasibility of using this therapeutic strategy in vivo and in clinical trials.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81072011 awarded to Dr Jian Wang).
Glossary
Abbreviations
- BTC
biliary tract cancer
- BSO
buthionine sulfoximine
- GSH
glutathione
- FITC
fluorescein isothiocyanate
- PI
propidium iodide
- GSSG
oxidized glutathione
- Mcl-1
myeloid cell leukemia 1
- Bcl-xL
B-cell lymphoma-extra large
- Bcl-2
B-cell lymphoma 2
- STAT3
signal transducer and activator of transcription 3
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
References
- 1.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Keane MG, Horsfall L, Rait G, Pereira SP. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open. 2014;4:e005720. doi: 10.1136/bmjopen-2014-005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, La Vecchia C. Epidemiology of biliary tract cancers: An update. Ann Oncol. 2009;20:146–159. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda T, Marugame T. International comparisons of cumulative risk of gallbladder cancer and other biliary tract cancer, from Cancer Incidence in Five Continents Vol. VIII. Jpn J Clin Oncol. 2007;37:74–75. doi: 10.1093/jjco/hyl158. [DOI] [PubMed] [Google Scholar]
- 6.Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/S0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 9.Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, Fartoux L, Faivre S, Blanc JF, Viret F, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819–828. doi: 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 11.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P, Chaudhary SP. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: A randomized controlled study. J Clin Oncol. 2010;28:4581–4586. doi: 10.1200/JCO.2010.29.3605. [DOI] [PubMed] [Google Scholar]
- 13.Awasthi YC, Chaudhary P, Vatsyayan R, Sharma A, Awasthi S, Sharma R. Physiological and pharmacological significance of glutathione-conjugate transport. J Toxicol Environ Health B Crit Rev. 2009;12:540–551. doi: 10.1080/10937400903358975. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Sun YP, Huang XZ, He M, Chen YY, Shi GY, Li H, Yi J, Wang J. Emodin enhances sensitivity of gallbladder cancer cells to platinum drugs via glutathion depletion and MRP1 downregulation. Biochem Pharmacol. 2010;79:1134–1140. doi: 10.1016/j.bcp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Omar JM, Yang H, Li S, Marquardt RR, Jones PJ. Development of an improved reverse-phase high-performance liquid chromatography method for the simultaneous analyses of trans-/cis-resveratrol, quercetin and emodin in commercial resveratrol supplements. J Agric Food Chem. 2014;62:5812–5817. doi: 10.1021/jf5001277. [DOI] [PubMed] [Google Scholar]
- 16.Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 17.Xu JD, Wang W, Li LS, Chen X, Zhu JX. Involvement of endogenous prostaglandin in emodin-evoked rat colonic anion secretion. Biol Pharm Bull. 2007;30:2058–2062. doi: 10.1248/bpb.30.2058. [DOI] [PubMed] [Google Scholar]
- 18.Anderson CP, Tsai JM, Meek WE, Liu RM, Tang Y, Forman HJ, Reynolds CP. Depletion of glutathione by buthionine sulfoxine is cytotoxic for human neuroblastoma cell lines via apoptosis. Exp Cell Res. 1999;246:183–192. doi: 10.1006/excr.1998.4303. [DOI] [PubMed] [Google Scholar]
- 19.Bailey HH, Ripple G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Mahvi D, Schink J, Pomplun M, Mulcahy RT, Wilding G. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst. 1997;89:1789–1796. doi: 10.1093/jnci/89.23.1789. [DOI] [PubMed] [Google Scholar]
- 20.Bailey HH, Mulcahy RT, Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M, Spriggs DR. Phase I clinical trial of intravenous L-buthionine sulfoximine and melphalan: An attempt at modulation of glutathione. J Clin Oncol. 1994;12:194–205. doi: 10.1200/JCO.1994.12.1.194. [DOI] [PubMed] [Google Scholar]
- 21.O'Dwyer PJ, Hamilton TC, LaCreta FP, Gallo JM, Kilpatrick D, Halbherr T, Brennan J, Bookman MA, Hoffman J, Young RC, et al. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J Clin Oncol. 1996;14:249–256. doi: 10.1200/JCO.1996.14.1.249. [DOI] [PubMed] [Google Scholar]
- 22.Jia Y, Zhang W, Liu H, Peng L, Yang Z, Lou J. Inhibition of glutathione synthesis reverses Krüppel-like factor 4-mediated cisplatin resistance. Cancer Chemother Pharmacol. 2012;69:377–385. doi: 10.1007/s00280-011-1708-7. [DOI] [PubMed] [Google Scholar]
- 23.Tagde A, Singh H, Kang MH, Reynolds CP. The glutathione synthesis inhibitor buthionine sulfoximine synergistically enhanced melphalan activity against preclinical models of multiple myeloma. Blood Cancer J. 2014;4:e229. doi: 10.1038/bcj.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Breijo B, Monserrat J, Ramirez-Rubio S, Cuevas EP, Vara D, Díaz-Laviada I, Fernández-Moreno MD, Román ID, Gisbert JP, Guijarro LG. Preclinical evaluation of azathioprine plus buthionine sulfoximine in the treatment of human hepatocarcinoma and colon carcinoma. World J Gastroenterol. 2011;17:3899–3911. doi: 10.3748/wjg.v17.i34.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury AA, Chaudhuri J, Biswas N, Manna A, Chatterjee S, Mahato SK, Chaudhuri U, Jaisankar P, Bandyopadhyay S. Synergistic apoptosis of CML cells by buthionine sulfoximine and hydroxychavicol correlates with activation of AIF and GSH-ROS-JNK-ERK-iNOS pathway. PloS one. 2013;8:e73672. doi: 10.1371/journal.pone.0073672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.CAN-2820-2. [DOI] [PubMed] [Google Scholar]
- 27.Jing Y, Yang J, Wang Y, Li H, Chen Y, Hu Q, Shi G, Tang X, Yi J. Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor kappaB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent. Free Radic Biol Med. 2006;40:2183–2197. doi: 10.1016/j.freeradbiomed.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4:1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 30.Fan J, Cai H, Yang S, Yan L, Tan W. Comparison between the effects of normoxia and hypoxia on antioxidant enzymes and glutathione redox state in ex vivo culture of CD34(+) cells. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:153–158. doi: 10.1016/j.cbpb.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Han Y, Huang C, Sun X, Xiang B, Wang M, Yeh ET, Chen Y, Li H, Shi G, Cang H, et al. SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress. J Biol Chem. 2010;285:12906–12915. doi: 10.1074/jbc.M109.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liaudat AC, Bohl LP, de Talamoni Tolosa NG, Maletto B, Pistoresi-Palencia MC, Picotto G. Oxidative stress, cell cycle arrest and differentiation contribute toward the antiproliferative action of BSO and calcitriol on Caco-2 cells. Anticancer Drugs. 2014;25:810–818. doi: 10.1097/CAD.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 33.Hadzic T, Aykin-Burns N, Zhu Y, Coleman MC, Leick K, Jacobson GM, Spitz DR. Paclitaxel combined with inhibitors of glucose and hydroperoxide metabolism enhances breast cancer cell killing via H2O2-mediated oxidative stress. Free Radic Biol Med. 2010;48:1024–1033. doi: 10.1016/j.freeradbiomed.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer. 2000;89:1440–1447. doi: 10.1002/1097-0142(20001001)89:7<1440::AID-CNCR5>3.3.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312–318. [PubMed] [Google Scholar]
- 36.Meurette O, Lefeuvre-Orfila L, Rebillard A, Lagadic-Gossmann D, Dimanche-Boitrel MT. Role of intracellular glutathione in cell sensitivity to the apoptosis induced by tumor necrosis factor {alpha}-related apoptosis-inducing ligand/anticancer drug combinations. Clin Cancer Res. 2005;11:3075–3083. doi: 10.1158/1078-0432.CCR-04-1764. [DOI] [PubMed] [Google Scholar]
- 37.Buolamwini JK. Novel anticancer drug discovery. Curr Opin Chem Biol. 1999;3:500–509. doi: 10.1016/S1367-5931(99)80073-8. [DOI] [PubMed] [Google Scholar]
- 38.Wu WJ, Zhang Y, Zeng ZL, Li XB, Hu KS, Luo HY, Yang J, Huang P, Xu RH. β-phenylethyl isothiocyanate reverses platinum resistance by a GSH-dependent mechanism in cancer cells with epithelial-mesenchymal transition phenotype. Biochem Pharmacol. 2013;85:486–496. doi: 10.1016/j.bcp.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum (II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- 40.Sorensen BH, Thorsteinsdottir UA, Lambert IH. Acquired cisplatin resistance in humane ovarian cancer A2780 cells correlates with shift in taurine homeostasis and ability to volume regulate. Am J Physiol Cell Physiol. 2014;307:C1071–C1080. doi: 10.1152/ajpcell.00274.2014. [DOI] [PubMed] [Google Scholar]
- 41.Heiser D, Labi V, Erlacher M, Villunger A. The Bcl-2 protein family and its role in the development of neoplastic disease. Exp Gerontol. 2004;39:1125–1135. doi: 10.1016/j.exger.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Butturini E, de Prati Carcereri A, Chiavegato G, Rigo A, Cavalieri E, Darra E, Mariotto S. Mild oxidative stress induces S-glutathionylation of STAT3 and enhances chemosensitivity of tumoural cells to chemotherapeutic drugs. Free Radic Biol Med. 2013;65:1322–1330. doi: 10.1016/j.freeradbiomed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Lu Z, Wang J, Zheng T, Liang Y, Yin D, Song R, Pei T, Pan S, Jiang H, Liu L. FTY720 inhibits proliferation and epithelial-mesenchymal transition in cholangiocarcinoma by inactivating STAT3 signaling. BMC Cancer. 2014;14:783. doi: 10.1186/1471-2407-14-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng H, Zhou Z, Tu W, Xia Y, Huang H, Tian D. Knockdown of astrocyte elevated gene-1 inhibits growth through suppression of IL-6 secretion in HepG2 human hepatoma cells. Oncol Lett. 2014;7:101–106. doi: 10.3892/ol.2013.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]