Abstract
The surgical management of patients with malignant biliary and duodenal obstruction is complex. Tumor excision is no longer possible in the majority of patients with malignant obstructive jaundice and duodenal obstruction. The aim of the present study was to evaluate the effectiveness of intraluminal dual stent placement in malignant biliary and duodenal obstruction. In total, 20 patients with malignant obstructive jaundice and duodenal obstruction, including 6 with pancreatic carcinoma, 11 with cholangiocarcinoma, 1 with duodenal carcinoma and 2 with abdominal lymph node metastasis, were treated with intraluminal stent placement. Bile duct obstruction with late occurrence of duodenal obstruction was observed in 16 cases, and duodenal obstruction followed by a late occurrence of bile duct obstruction was observed in 3 cases, while, in 1 case, bile duct obstruction and duodenal obstruction occurred simultaneously. After X-ray fluoroscopy revealed obstruction in the bile duct and duodenum, stents were placed into the respective lumens. Percutaneous transhepatic placement was employed for the biliary stent, while the duodenal stent was placed perioraly. The clinical outcomes, including complications associated with the procedures and patency of the stents, were evaluated. The biliary and duodenal stents were successfully implanted in 18 patients and the technical success rate was 90% (18/20). A total of 39 stents were implanted in 20 patients. In 2 cases, duodenal stent placement failed following biliary stent placement. Duodenal obstruction remitted in 15 patients, and 1 patient succumbed to aspiration pneumonia 5 days after the procedure. No severe complications were observed in any other patient. The survival time of the 18 patients was 5–21 months (median, 9.6 months), and 6 of those patients survived for >12 months. The present study suggests that X-ray fluoroscopy-guided intraluminal stent implantation is an effective procedure for the treatment of malignant biliary and duodenal obstruction.
Keywords: biliary obstruction, duodenal obstruction, stent, radiology, interventional
Introduction
Biliary and duodenal obstruction is a common complication in patients with gastroduodenal or pancreatobiliary malignancies. Stent implantation has been widely used in clinical practice, which is the preferred method for palliative management of malignant biliary and duodenal obstruction (1–6). Obstructive jaundice accompanied by duodenal obstruction is mainly caused by periampullary or pancreatic head carcinoma, malignant duodenal tumor and lymph node metastasis. Biliary and duodenal obstruction causes cholestasis and hepatic insufficiency. Patients with malignant obstructive jaundice and duodenal obstruction are in a poor condition or the tumor has already invaded the surrounding tissue or organs, thus tumor excision in no longer possible (7).
The most commonly used surgical approach for biliary and duodenal obstruction is palliative cholangioenteric anastomosis, gastroenterostomy or jejunostomy; however, these approaches are not considered safe for patients who are weak, have electrolyte imbalance or experience multiple organ system failure (8,9). In comparison to the surgical approaches, percutaneous transhepatic biliary stenting (PTBS) combined with peroral duodenal stent implantation has certain advantages: Ease and safety of the procedure, minimum invasiveness, rapid recovery with less complications and smaller effect on the gastrointestinal function. The results of studies that focused on the combination of these two stenting methods reported high technical and clinical success rates (8,9).
The present study investigated the short-term therapeutic effectiveness of combined metallic stenting under fluoroscopic guidance in 20 patients with malignant biliary and duodenal obstruction.
Materials and methods
Patients
The present study was approved by the Institutional Review Board of the Third Affiliated Hospital of Harbin Medical University (Harbin, China) and informed consent was provided by all participants. In total, 20 patients (male, 13; female, 7; age range, 35–72 years; mean age, 63.1±8.2 years) with malignant obstructive jaundice and duodenal obstruction were enrolled in the study between June 2004 and June 2013. Computed tomography (CT), magnetic resonance imaging, ultrasound and/or gastroenterography were performed in all patients prior to the interventional procedures. Among the patients, 6 cases were diagnosed with pancreatic carcinoma, 11 with cholangiocarcinoma, 1 with duodenal carcinoma and 2 with abdominal metastatic lymph nodes. In total, 16 patients initially presented with bile duct obstruction, which was manifested as skin and sclera yellowing, dark urine and pale stools, with 7 complaining of skin itching. The patients were treated by PTBS, and gastrointestinal obstruction symptoms, including nausea and vomiting, were observed 1–8 month(s) following the procedure. Gastroenterography showed duodenal obstruction. In addition, 3 patients initially presented with duodenal obstructive symptoms, which were followed by biliary obstruction. In 1 case, bile duct obstruction and duodenal obstruction occurred simultaneously.
Procedure
Under monitoring with X-ray fluoroscopy, the puncture site was selected at an intercostal space under the right costophrenic angle. The puncture site was locally anesthetized and a 22 G MReye® Chiba needle (Cook Medical, Inc., Bloomington, IN, USA) was used to puncture the skin at the right midaxillary line at T11 level. The depth of the puncture was ~2 cm to the right border of the vertebra. The needle was slowly withdrawn with simultaneous infusion of the contrast agent [Omnipaque™; GE Healthcare (Shanghai) Co., Ltd., Shanghai, China]. When the bile duct became visible, a Radifocus® guidewire (Terumo, Tokyo, Japan) was inserted, the Chiba needle was removed and a sheath was positioned using the guidewire. A cholangiography was performed to detect the location of the stenosis and a stent (SMART® or Zilver®; Cordis Corp., Hialeah, USA or Cook Medical, Inc., respectively) was implanted at the site of the stenosis via the guidewire. A balloon catheter, 6mm in diameter, would be used for the dilation of the stenosis, if the rate of the remaining stenosis was 30% following PTBS.
With regard to the duodenal obstruction, a nasogastric tube (Freka; Frensenius Kabi AG, Miekinia, Poland) was inserted to decompress the stomach overnight, prior to the procedure. A duodenal stent (Hanarostent®; M.I. Tech Co., Ltd., Seoul, Korea) was implanted through the mouth, under the guidance of X-ray fluorescence. A local anesthetic spray was used to numb the pharynx, and a Cobra angiographic catheter (Radiofocus®; Terumo) was placed into the proximal segment of stenosis via a guidewire (Radifocus; Terumo, Tokyo, Japan) and an infused contrast agent was used to confirm the duodenal obstruction. The guidewire and the catheter were then advanced to the distal segment of the stenosis with further radiography to demonstrate the length of the stenosis. An Amplatz Super Stiff™ guidewire (Boston Scientific, MA, USA) was used to exchange the catheter and position an intestinal stent at the stenotic segment, and was then deployed. In cases where the rate of stenosis was >40% following stent implantation, further balloon dilation was performed (Fig. 1). Subsequent to biliary stenting, the guidewire should be carefully used, in order to avoid going through the biliary stent mesh.
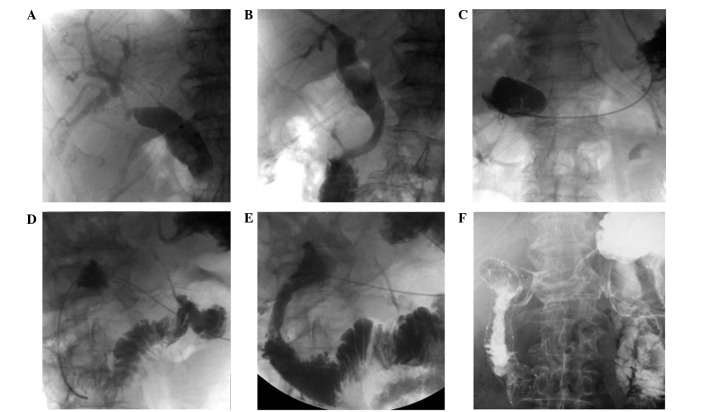
Figure 1.
Male patient aged 53 years with pancreatic cancer. (A) Cholangiography demonstrated obstruction of the lower bile duct. (B) The contrast agent is seen passing into the duodenum through the bile duct following PTBS. (C and D) At 2 months after PTBS, the patient presented with duodenal obstruction. Transcatheter gastroenterography revealed a descendant segment of duodenum obstruction. (E) The contrast agent is seen passing into the jejunum from the duodenum following duodenal stent implantation. (F) The duodenal stent was fully inflated and the contrast agent passed through, 2 weeks after duodenal stent implantation.
In cases that developed duodenal obstruction following PTBS, duodenal stenting was performed. Similarly, PTBS was attempted in the patients who developed biliary obstruction following duodenal stent placement (Fig. 2). In cases with simultaneous biliary and duodenal obstruction without any previous interventions, a duodenal stent (Hanarostent; M.I. Tech, Co., Ltd.) was inserted several days after the placement of a biliary drainage catheter (Ultrathane; Cook Medical, Inc.), which was followed by implantation of the biliary stent (SMART; Cordis Corp.).
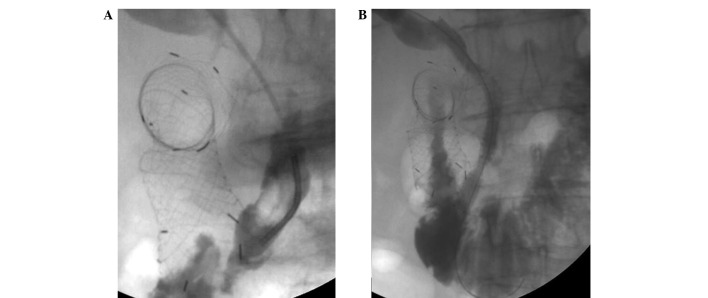
Figure 2.
Male patient aged 57 years who underwent duodenal stent implantation. (A) At 1 month after duodenal stent implantation, the patient presented with biliary obstruction. Cholangiography demonstrated obstruction of the bile duct and (B) satisfactory expansion of the biliary stents (rate of stenosis following PTBS, <30%).
The clinical outcomes, including complications associated with the procedures and patency of the stents, were evaluated. Cholangitis was diagnosed in the case of abdominal pain and fever without any other infection outside the hepatobiliary system that required antibiotic treatment within 24 h of the procedure. Pancreatitis was diagnosed following a 3-fold increase relative to the normal levels of serum amylase, which was accompanied by persistent abdominal pain for >24 h postoperatively. Duodenal stenting was suggested when recurrent vomiting was observed in the absence of features suggestive of peritoneal carcinomatosis, and was confirmed using imaging methods. Biliary stent occlusion was diagnosed upon recurrence of the typical symptoms of biliary tract obstruction, such as yellowing of skin and sclera, dark urine and pale stool. Follows-ups that included clinical examinations and assessments of stent patency continued until the death of the patients.
Statistical analysis
All analyses were conducted using SPSS software, version 20.0 (SPSS, Inc., Chicago, IL, USA). The paired Student's t-test was used to compare the mean values of serum bilirubin pre- and post-stent implantation. P<0.05 was considered to indicate a statistically significant difference.
Results
Stenting
A total of 39 stents were implanted in 20 patients with a technical success rate of 90% (18/20). Of these stents, 21 were biliary, including 11 SMART (Cordis Corp.) and 10 Zilver (Cook Medical Inc.) stents. In addition, 1 patient underwent an implantation of two biliary stents due to stent sliding, while 5 patients received subsequent balloon inflation, following which adequate stent expansion was achieved. The mean serum bilirubin level prior to PTBS was 326.5±105.7 mol/l and dropped to 35.9±12.2 mol/l at 7 days after stenting (P<0.05). The skin and sclera jaundice gradually vanished and the skin itching was relieved in all 7 patients 10–25 days after PTBS (median, 13.3 days). No aggravating jaundice was identified in the patients who had undergone duodenal stent implantation, which suggested that the biliary stent was not blocked by duodenal stent implantation.
Furthermore, 18 duodenal stents (Hanarostent; M.I. Tech Co., Ltd.) were implanted, all of which were successfully deployed and inflated, according to gastroenterography. However, 50–60% of stenosis remained following implantation of 2 stents, as observed by the slow passing of the contrast agent. Following the secondary balloon inflation, the remaining in-stent stenosis decreased by ~20%. At 1 week after the duodenal stent implantation, radiography confirmed the normal flow of the contrast agent through the stent. All patients were able to have a liquid or semi-liquid diet immediately following stent implantation and were allowed a solid diet 1 week later. The symptom of bloating after the meals persisted in 3 patients, but vomiting was not observed. Gastroenterography confirmed that the stent remained open, but gastrointestinal movement was decreased.
Duodenal obstruction occurred in 2 patients, 4 and 8 months after PTBS. Duodenal stent implantation was then planned. Transcatheter radiography revealed that the length of the free part of the biliary stent was relatively long and contained in the duodenal carcinoma.
Complications
The duodenal stent would have to pass through the mesh of the biliary stent, which could destroy its structure; therefore, the duodenal stent placement was not performed.
A total of 5 patients exhibited a temporary increase in serum amylase with no abdominal pain. The patients were treated with fasting, fluid infusion and heteropathy and serum amylase returned to its normal range after 24–48 h. In addition, 9 patients suffered from mild abdominal pain following stent implantation, which was relieved following heteropathy. Furthermore, 4 patients were found to be positive or strong positive for occult blood in the stool, which disappeared in 3–5 days.
Survival time
Additionally, 1 patient exhibited cough and fever, which, according to a CT scan, were considered to be symptoms of aspiration pneumonia. The patient succumbed to the disease 5 days after the procedure, with no cholangitis or pancreatitis detected.
The survival time of the 18 patients was 5–21 months (median, 9.6 months). In total, 6 of those patients survived for >12 months.
Discussion
With regard to patients with biliary and duodenal obstruction, particularly those who present obstruction in the descending segment of the duodenum, the present results suggest that the management begins with PTBS and peroral enteral nutrition using a jejunal feeding tube. The duodenal stent can be implanted when jaundice has decreased and the patient's general condition has improved. Another option for these patients is receiving PTBS following duodenal stent implantation, during which a guidewire is placed into the duodenum through the mesh of the duodenal stent using a catheter, under fluoroscopic guidance. A balloon catheter, 8–10 mm in diameter, is placed to dilate the mesh of the duodenal stent and allow biliary stent expansion. In the case that the biliary stent cannot fully expand, the balloon catheter is used to dilate the biliary stent. No interference has been identified between the biliary and duodenal stents. The duodenal stent is braided, so the balloon dilation, which is meant to expand the mesh, will not affect the entire structure of the stent (8).
Profili et al (10) reported 4 cases with pancreatic cancer treated with combined biliary and duodenal stenting for the palliative treatment of malignant biliary and duodenal obstruction under fluoroscopic guidance. The authors suggested that the biliary stent implantation should be performed prior to the duodenal stent implantation, since the existence of a stent in the duodenum may make the placement and expansion of a biliary stent difficult or even unsuccessful (10). In addition, the biliary stent can assist the duodenal stent positioning at the same time. Out of the 20 patients who participated in the present study, 4 received duodenal stenting first, and then PTBS was successfully performed. It is therefore suggested that duodenal stent implantation should be performed prior to PTBS, since biliary stenting through the duodenal stent mesh is not challenging. If malignant duodenal obstruction is detected in patients with a biliary stent, the guidewire should not pass through the mesh of the biliary stent, since the duodenal stent may not be sufficiently inflated and the chyme may block the biliary stent. The biliary stent is small and integrative, thus if the mesh cannot be inflated, it will lead to severe deformation (11). In the present study, duodenal stent implantation following PTBS failed in 2 patients, since the guidewire passed through the mesh of the biliary stent, but was unable to pass between the stent and the duodenum.
Combined duodenal and biliary stenting can also be performed either simultaneously or separately under endoscopic guidance (7,12,13). Kaw et al (7) reported the clinical outcome of combined duodenal and biliary stenting under endoscopic guidance in 18 patients. All patients had documented evidence of symptomatic duodenal and biliary obstruction, and received endoscopic duodenal stenting using enteral Wallstent and endoscopic biliary stenting with biliary Ultraflex or Wallstent (7). Combined metal stenting for malignant biliary and duodenal obstruction was successful in 17 patients, while one procedure failed due to a tortuous duodenal stricture. From the 17 patients who underwent a successful duodenal stenting, 16 had a good clinical outcome, with relief of the obstruction symptoms (7). No immediate stent-associated complications were noted; therefore, based on the results of the study by Kaw et al (7), the endoscopic approach can be considered if the peroral approach fails.
In the present study, duodenal stenting failed in 2 cases due to the fact that the free part of the biliary stent in the duodenal lumen was relatively long. The existence of a biliary stent in the duodenum may disturb the insertion of a duodenal stent, which may then destroy the structure of the biliary stent. It is possible, however, for the guidewire to pass through the space between the biliary stent and the duodenal wall under endoscopic guidance.
PTBS combined with para-oral duodenal stent is minimally invasive; however, the technique has been associated with complications, such as bleeding, choleperitonitis, cholangitis, duodenum perforation and aspiration pneumonia. In the present study, 1 patient was diagnosed with pancreatic cancer and obstructive jaundice, and postoperatively suffered from abdominal distention, nausea and vomiting, which were caused by duodenal obstruction. Gastroenterography demonstrated obstruction of the descending segment of the duodenum. An exchange guidewire was placed into the jejunum, through which a stent (18×120 mm) was implanted. Following stent implantation, 40% of the stenosis remained. Considering the local stenosis, it was decided to perform balloon dilation. A balloon catheter (18×60 mm) was introduced, but was not able to enter the stenosis segment. Several attempts were unsuccessful, probably due to the deformation of the stent. The patient exhibited nausea and vomiting during the 2-h interventional procedure, as well as cough and fever (maximum temperature, 39°C). A thoracic CT scan showed scattered patchy-like shadows in the lungs and aspiration pneumonia was diagnosed. The patient suffered from continuous hyperpyrexia and succumbed 5 days later.
Based on the results and observations of the present study, the following conclusion were made: i) Sufficient gastrointestinal decompression is necessary to drain the gastric contents; ii) a long sheath should be placed into the pylorus in order to reduce the pharyngeal stimulation from the surgery; and iii) the duration of the surgery should be reduced.
In conclusion, dual stent implantation into the bile duct and duodenum can successfully treat biliary and duodenal obstruction. This technique as effective in treating these obstructions as palliative bypass surgery, while it improves the quality of life of the patients by promptly alleviating the symptoms.
References
- 1.de Baere T, Harry G, Ducreux M, Elias D, Briquet R, Kuoch V, Roche A. Self-expanding metallic stents as palliative treatment of malignant gastroduodenal stenosis. AJR Am J Roentgenol. 1997;169:1079–1083. doi: 10.2214/ajr.169.4.9308468. [DOI] [PubMed] [Google Scholar]
- 2.Pinto IT. Malignant gastric and duodenal stenosis: Palliation by peroral implantation of a self-expanding metallic stent. Cardiovasc Intervent Radiol. 1997;20:431–434. doi: 10.1007/s002709900188. [DOI] [PubMed] [Google Scholar]
- 3.Lee BH, Choe DH, Lee JH, Kim KH, Chin SY. Metallic stents in malignant biliary obstruction: Prospective long-term clinical results. AJR Am J Roentgenol. 1997;168:741–745. doi: 10.2214/ajr.168.3.9057527. [DOI] [PubMed] [Google Scholar]
- 4.Yasumoto T, Yokoyama S, Nagaike K. Percutaneous transcholecystic metallic stent placement for malignant obstruction of the common bile duct: Preliminary clinical evaluation. J Vasc Interv Radiol. 2010;21:252–258. doi: 10.1016/j.jvir.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Jung GS, Song HY, Kang SG, Huh JD, Park SJ, Koo JY, Cho YD. Malignant gastroduodenal obstructions: Treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000;216:758–763. doi: 10.1148/radiology.216.3.r00au05758. [DOI] [PubMed] [Google Scholar]
- 6.Oikarinen H, Leinonen S, Karttunen A, Tikkakoski T, Hetemaa T, Mäkelä J, Päivänsalo M. Patency and complications of percutaneously inserted metallic stents in malignant biliary obstruction. J Vasc Interv Radiol. 1999;10:1387–1393. doi: 10.1016/S1051-0443(99)70249-6. [DOI] [PubMed] [Google Scholar]
- 7.Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003;17:457–461. doi: 10.1007/s00464-002-8541-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim KO, Kim TN, Lee HC. Effectiveness of combined biliary and duodenal stenting in patients with malignant biliary and duodenal obstruction. Scand J Gastroenterol. 2012;47:962–967. doi: 10.3109/00365521.2012.677956. [DOI] [PubMed] [Google Scholar]
- 9.Maire F, Hammel P, Ponsot P, Aubert A, O'Toole D, Hentic O, Levy P, Ruszniewski P. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735–742. doi: 10.1111/j.1572-0241.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 10.Profili S, Feo CF, Meloni GB, Strusi G, Cossu ML, Canalis GC. Combined biliary and duodenal stenting for palliation of pancreatic cancer. Scand J Gastroenterol. 2003;38:1099–1102. doi: 10.1080/00365520310005532. [DOI] [PubMed] [Google Scholar]
- 11.Akinci D, Akhan O, Ozkan F, Ciftci T, Ozkan OS, Karcaaltincaba M, Ozmen MN. Palliation of malignant biliary and duodenal obstruction with combined metallic stenting. Cardiovasc Intervent Radiol. 2007;30:1173–1177. doi: 10.1007/s00270-007-9045-2. [DOI] [PubMed] [Google Scholar]
- 12.Tonozuka R, Itoi T, Sofuni A, Itokawa F, Moriyasu F. Endoscopic double stenting for the treatment of malignant biliary and duodenal obstruction due to pancreatic cancer. Dig Endosc. 2013;25(Suppl 2):100–108. doi: 10.1111/den.12063. [DOI] [PubMed] [Google Scholar]
- 13.Katsinelos P, Kountouras J, Germanidis G, Paroutoglou G, Paikos D, Lazaraki G, Pilpilidis I, Chatzimavroudis G, Fasoulas K, Zavos C. Sequential or simultaneous placement of self-expandable metallic stents for palliation of malignant biliary and duodenal obstruction due to unresectable pancreatic head carcinoma. Surg Laparosc Endosc Percutan Tech. 2010;20:410–415. doi: 10.1097/SLE.0b013e3182001f26. [DOI] [PubMed] [Google Scholar]