Abstract
Fibronectin (FN) is one of the most important extracellular matrix proteins and plays an important role in the pathogenesis of atherosclerosis (AS). The aim of the present study was to evaluate the effect of a potent, water-soluble antioxidant, protocatechuic aldehyde (PA), which is derived from the Chinese herb Salvia miltiorrhiza, on the expression of FN in human umbilical vein endothelial cells (HUVECs) stimulated with tumor necrosis factor-α (TNF-α). The pharmacological effects of PA on the production of FN were investigated using ELISA and western blot analysis. In addition, ELISA and western blot analysis were used to examine the activation and suppression of the mitogen-activated protein kinase (MAPK) pathways and nuclear factor (NF)-κB in TNF-α-stimulated HUVECs, in order to explore the underlying pharmacological mechanism of PA. The inhibitory effect of PA on the total generation of reactive oxygen species (ROS) in TNF-α-stimulated HUVECs was assessed using 2′,7′-dichlorofluorescein diacetate. Pretreatment of HUVECs with PA (0.15, 0.45 and 1.35 mM) for 18 h markedly attenuated the TNF-α-stimulated FN surface expression and secretion in a dose-dependent manner. Intracellular ROS generation and the expression of extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 MAPK (p38) were significantly induced by TNF-α (2 ng/ml) in HUVECs. TNF-α-induced ROS generation and JNK activation were inhibited by PA in a concentration-dependent manner. By contrast, ERK1/2 and p38 activation was not significantly affected by PA. Pretreatment of HUVECs with PA for 18 h markedly attenuated TNF-α-stimulated NF-κB activation. In conclusion, the present findings suggest that PA inhibits TNF-α-induced FN expression in HUVECs through a mechanism that involves ROS/JNK and NF-κB.
Keywords: tumor necrosis factor-α, protocatechuic aldehyde, mitogen-activated protein kinase, fibronectin, endothelial cell
Introduction
Atherosclerosis (AS) has come to be recognized as an active and inflammatory process, rather than simply a passive process of lipid infiltration or a reparative process following endothelial injury (1–3). One of the major inflammatory cytokines is tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine that is released in response to a pathological condition (4). TNF-α can injure the structure of endothelial cells and induce inflammatory responses by enhancing the expression and secretion of adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), endothelial cell selectin and fibronectin (FN) (5,6), which results in leukocyte recruitment to the endothelium and the initiation of AS. A number of chemicals derived from plants with anti-inflammatory properties have been reported to exert an anti-leukocyte recruitment effect (7,8). FN is a 250-kDa adhesive glycoprotein and one of the most abundant proteins in the extracellular matrix (ECM). FN generates a scaffold that allows the attachment of other ECM components, and large quantities of FN have been detected in atherosclerotic plaques, suggesting that it may play a role in the pathogenesis of AS (9,10). Physiologically, FN plays an important role in a number of processes, including cell adhesion, motility and tissue repair; however, its overproduction may decrease the motility and replication of various cell types, including endothelial cells (11).
Oxidative stress and the production of intracellular reactive oxygen species (ROS) have been implicated in the pathogenesis of AS (12). ROS and their by-products may not only be cytotoxic to cells but also play a role in signal transduction processes, such as cell growth and the post-translational modification of proteins, which contributes to the formation of AS (13). The common key point in the pathophysiology of AS is believed to be the intracellular redox signal-induced expression of specific inflammatory genes (14). Salvia miltiorrhiza (S.M.), a herb that is often used in Traditional Chinese Medicine, has been found to exert beneficial effects on the circulatory system (15). Aqueous extracts of S.M. that are rich in antioxidants have been described as being effective in reducing AS in experimental studies in vitro and in vivo (16,17). Our previous studies have demonstrated that the main compounds of S.M. inhibit endothelin-1 expression, stimulate nitric oxide production (18) and attenuate plasminogen activator inhibitor type 1 production in TNF-α-treated human umbilical vein endothelial cells (HUVECs) (19). Furthermore, we reported that protocatechuic aldehyde (PA, also known as 3,4-dihydroxybenzaldehyde), a compound isolated from the aqueous extract of S.M., selectively inhibits TNF-α-induced VCAM-1 and ICAM-1 expression and reduces monocyte adhesion to endothelial cells (20); however, no studies examining the effect of PA on the regulation of FN in endothelial cells exist, to the best of our knowledge.
The aim of the present study was four-fold: i) To examine the effect of TNF-α on FN secretion and expression in cultured HUVECs; ii) to investigate the effect of PA on TNF-α-induced FN expression in HUVECs; iii) to explore the effect of PA on the TNF-α-induced activation of extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38) in HUVECs; and iv) to investigate the activity of the key AS-related transcription factor nuclear factor-κB (NF-κB).
Materials and methods
Reagents
PA was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). PA was dissolved in warm culture medium just before incubation with HUVECs. Direct exposure of the PA to light and air was avoided during the experiments. Recombinant human TNF-α, the CellTiter Aqueous One Solution Cell Proliferation Assay (MTS) and the Gel Shift Assay Core system were purchased from Promega Corp. (Madison, WI, USA). Antibodies to FN (rabbit polyclonal; 1:500; cat. no. sc-9068), phosphorylated (P-)ERK1/2 (rabbit polyclonal; 1:200; cat. no. sc-101761), ERK1/2 (rabbit polyclonal; 1:250; cat. no. sc-292838), p38 (rabbit polyclonal; 1:400; cat. no. sc-535) and JNK (rabbit polyclonal; 1:400; cat. no. sc-571) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and the antibodies to P-JNK (rabbit monoclonal; 1:500; cat. no. 4668) and P-p38 (rabbit polyclonal; 1:500; cat. no. 9211) and the anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody (1:1,200; cat. no. 7074P2) were obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA). The FN ELISA kit was obtained from Shanghai Sun Biotech Co., Ltd. (Shanghai, China).
Cell culture
HUVECs were obtained from Cascade Biologics, Inc. (Portland, OR, USA), in the form of cryopreserved primary cultures, and grown in culture flasks (Costar® Corning, New York, NY, USA) in M200 endothelial cell growth medium (Cascade Biologics, Inc.) supplemented with 2% low serum growth supplement (LSGS; Cascade Biologics, Inc.) according to the manufacturer's instructions. The growth medium was changed every other day until the cells reached confluence. Passage 3 and 4 cells were grown in monolayers at 37°C in a humidified atmosphere of 5% CO2 and 95% air and used for experiments at 80% confluency. At 24 h prior to the experiments, the control medium was removed and replaced with LSGS-free medium containing 0.4% fetal bovine serum. For the experiments, HUVECs were cultured in medium containing 0.4% fetal bovine serum with or without the PA for 18 h, prior to further culture for 6 h with TNF-α (2 ng/ml).
Assessment of cell viability and cellular ROS generation
In order to evaluate cytotoxicity, cells were seeded at a density of 5,000 cells/well into 96-well culture plates (Costar) and grown for 48 h. The cells were incubated with PCA (6.75, 13.5, 27, 54 or 108 nM) with or withour TNF-α (2 ng/ml) in M200 media (serum-free) containing 0.4% fetal bovine serum for 24 h, and 20 µl MTS was then added to each well for further incubation at 37°C for 2 h. The absorbance of the solubilized formazan was read at 490 nm using a Victor 1420 Multilabel Counter instrument (Wallac, Turku, Finland). Cells incubated in control media were taken to be 100% viable. The generation of ROS was assessed using the ROS-sensitive fluorescence indicator 2′,7′-dichlorofluorescein diacetate (DCFH-DA). A total of 10 µmol/l DCFH-DA was added to the cell culture wells in a potassium phosphate buffer for 30 min. The fluorescence of 2′,7′-dichlorofluorescein, the oxidation product of DCFH-DA, was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm on a fluorescence spectrophotometer (Victor 1420 Multilabel Counter; Wallac).
Cell surface immunoassay and FN secretion
To detect the expression of adhesion molecules, ELISA was performed as previously described (19). In brief, the HUVECs were plated onto 96-well plates overnight and growth-arrested for 24 h with 0.4% serum-containing medium. The cells were then stimulated with TNF-α with or without PA, washed with phosphate-buffered saline (PBS) and fixed. Anti-FN antibody (Shanghai Sun Biotech Co., Ltd.) was added to the wells for 1 h at 37°C. Cells were washed and the expression of FN was quantified by the addition of o-phenylenediamine dihydrochloride in phosphate-citrate buffer. Following incubation for 20 min at 37°C, the reaction was terminated through the addition of 5 N H2SO4, and the absorbance of each well was measured at 490 nm using a Multilabel reader. In order to measure the FN secretion by the HUVECs, an ELISA kit was used to determine the levels of soluble FN antigens in the culture supernatant of the HUVECs, according to the manufacturer's instructions (Shanghai Sun Biotech Co., Ltd.).
Preparation of nuclear extracts
Upon reaching 80% confluency, the HUVECs were administered the indicated treatment and nuclear protein extracts were prepared, as described previously (19). All nuclear extraction procedures were performed on ice with ice-cold reagents. The cells were harvested, washed with PBS, resuspended in Buffer A, which contained 10 mM HEPES (pH 7.6), 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol (DTT) and 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and incubated for 10 min at 4°C. Following centrifugation at 300 × g for 10 min, the pellets containing the nuclei were suspended in Buffer B, which contained 20 mM HEPES (pH 7.6), 10 mM KCl, 1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 25% glycerol and 0.4 M NaCl, for 30 min. Nuclear proteins were isolated by centrifugation at 12,000 × g for 20 min. The protein concentrations were determined using a Bicinchoninic Acid Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA, and the proteins were stored at −80°C until use in the NF-κB binding activity assay.
NF-κB binding activity assay
The NF-κB activity in the nuclear protein (20 µg) of treated or control HUVECs was measured using a DNA-binding ELISA kit (TransAM™ NF-κB p65 assay; Active Motif, Carlsbad, CA, USA) according to the manufacturer's instructions and analyzed using a microplate absorbance reader.
Western blotting and activity assay of mitogen-activated protein kinases (MAPKs) (ERK1/2, JNK and p38)
Following treatment with the reagents, the cells were washed with PBS and harvested in 200 µl lysis buffer containing 20 mM HEPES (pH 7.9), 10 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.1% Nonidet P40 (V/V) and protease inhibitor (0.1 µg/ml leupeptin, 5 µg/ml aprotinin and 0.5 mM PMSF). Cell lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and proteins were transferred to a polyvinylidene difluoride membrane (Schleicher and Schuell Biosciences, Inc., Keene, NH, USA). The membrane was blocked for 1 h at room temperature with Tris buffered saline-Tween 20 (0.5%)/5% non-fat skim milk. The blots were then incubated overnight with antibodies against FN, or the phosphorylated or unphosphorylated forms of ERK1/2, JNK or p38 at 4°C, followed by incubation for 1 h with HRP-conjugated secondary antibody. Immunoreactive bands were visualized using Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Inc.), and the blots were exposed to XBT-1 film (Kodak, Xiamen, China).
Statistical analysis
All values are expressed as the mean ± standard error of the mean of independent determinations. Statistical analysis was performed with analysis of variance and the Tukey test using GraphPad Prism® software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of PA on FN secretion and cell surface expression
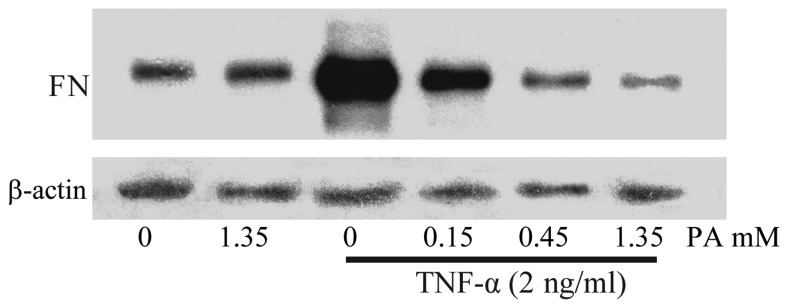
To examine the effect of TNF-α on FN secretion and surface expression in HUVECs, the HUVECs were exposed to 2 ng/ml TNF-α for 6, 12 and 18 h. The expression of FN was determined in accordance with the aforementioned method. As shown in Fig. 1, the surface expression of FN increased in a time-dependent manner (maximum at 18 h). To investigate whether PA affected the TNF-α-induced FN secretion and expression, the effect of various concentrations of PA on TNF-α-induced FN secretion and expression was examined using ELISA and cell surface ELISA. PA at doses of 0.15, 0.45 and 1.35 mM induced a significant dose-dependent inhibition of FN protein surface expression. FN protein surface expression was reduced to 73.4, 55.8 and 55.5% of the control, respectively, and FN secretion was significantly reduced to 48.2, 17.4 and 18.2%, respectively (Fig. 1). The FN expression in the cytoplasm was also examined using western blot analysis. As shown in Fig. 2, the FN expression in the TNF-α-stimulated HUVECs increased notably, whereas the pretreatment of HUVECs with PA for 18 h markedly attenuated the TNF-α-stimulated FN expression in a dose-dependent manner.
Figure 1.
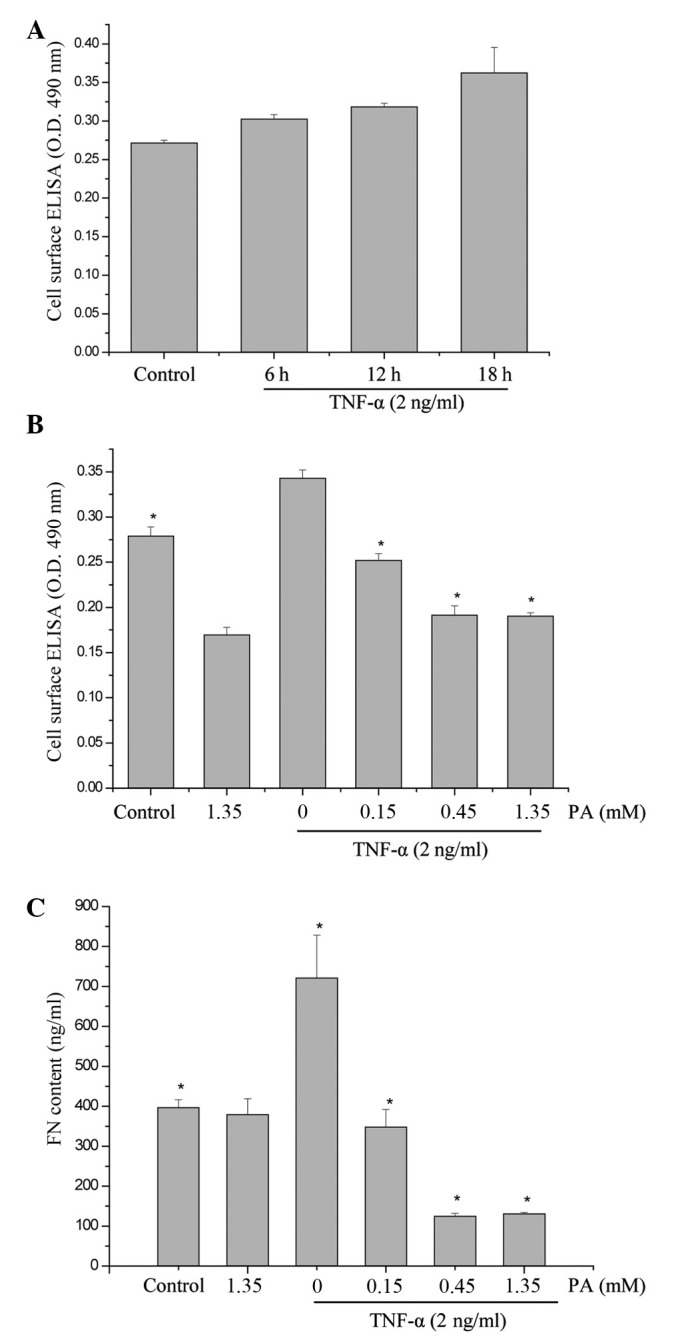
Effects of PA on the cell surface expression and secretion of FN in human umbilical vein endothelial cells stimulated with TNF-α. (A) HUVECs were exposed to 2 ng/ml TNF-α for 6, 12 and 18 h. (B and C) Cells were pretreated with PA (0.15, 0.45 and 1.35 mM) for 18 h and then activated with TNF-α (2 ng/ml) for 18 h. Following incubation, (B) the expression of FN was assessed by cell ELISA and (C) the concentration of FN in the culture medium was examined. The data are presented as the mean ± standard error of the mean (n=4). Asterisks indicate a statistically significant difference when compared with results from the TNF-α-stimulated cells in the absence of PA (*P<0.05). PA, protocatechuic aldehyde; FN, fibronectin; TNF-α, tumor necrosis factor-α; OD, optical density.
Figure 2.
Western blotting shows the dose-dependent effect of PA on FN expression in TNF-α-induced human umbilical vein endothelial cells. The cells were pretreated with PA (0.15, 0.45 and 1.35 mM) for 18 h and then activated with TNF-α (2 ng/ml) for 6 h. PA, protocatechuic aldehyde; FN, fibronectin; TNF-α, tumor necrosis factor-α.
PA attenuates TNF-α-induced ROS production in HUVECs
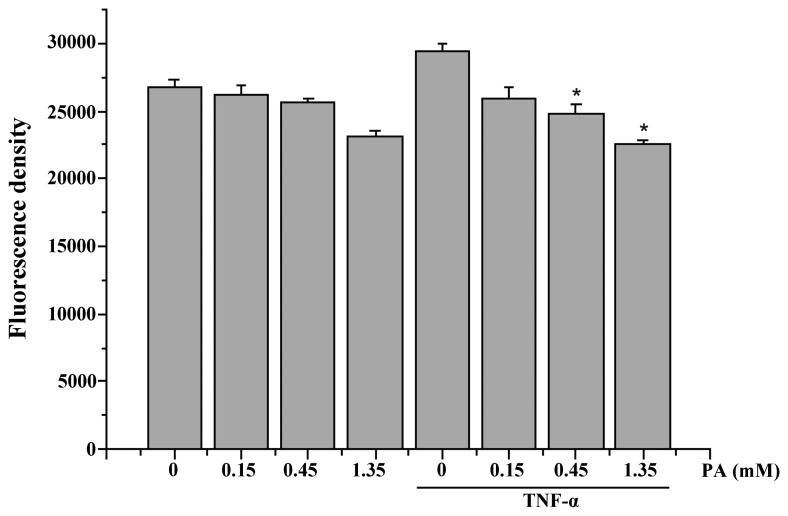
As shown in Fig. 3, incubating the HUVECs for 15 min with 2 ng/ml TNF-α significantly increased the intracellular ROS generation. PA was found to inhibit the TNF-α-induced increase in ROS generation in a dose-dependent manner (Fig. 3).
Figure 3.
Effect of PA on TNF-α-induced intracellular ROS generation in HUVECs. HUVECs were treated with PA (0.15, 0.45 and 1.35 mM) for 30 min before treatment with TNF-α (2 ng/ml) for 15 min; ROS were quantitatively analyzed. The data are presented as the mean ± standard error of the mean. *P<0.05, vs. the TNF-α-stimulated cells in the absence of PA. PA, protocatechuic aldehyde; FN, fibronectin; TNF-α, tumor necrosis factor-α; ROS, reactive oxygen species; HUVECs, human umbilical vein endothelial cells.
Effect of PA on TNF-α-induced ERK1/2, JNK and p38 activation in HUVECs
To examine whether PA affects TNF-α-induced MAPK activation, the effect of various concentrations of PA on TNF-α-induced ERK1/2, JNK and p38 activation in HUVECs was assessed. The cells were pretreated with PA for 60 min prior to the addition of TNF-α (2 ng/ml) for 15 min for ERK1/2, JNK and p38 activation. TNF-α-induced JNK activation was inhibited by PA in a concentration-dependent manner (0.15, 0.45, and 1.35 mM). By contrast, ERK1/2 and p38 activation were not affected by PA (Fig. 4). Western blot analysis with anti-ERK1/2, -JNK and -p38 antibodies revealed no differences in the total quantities of ERK1/2, JNK and p38. These findings suggest that TNF-α-induced JNK activation, but not ERK1/2 and p38 activation, is specifically sensitive to PA in HUVECs.
Figure 4.
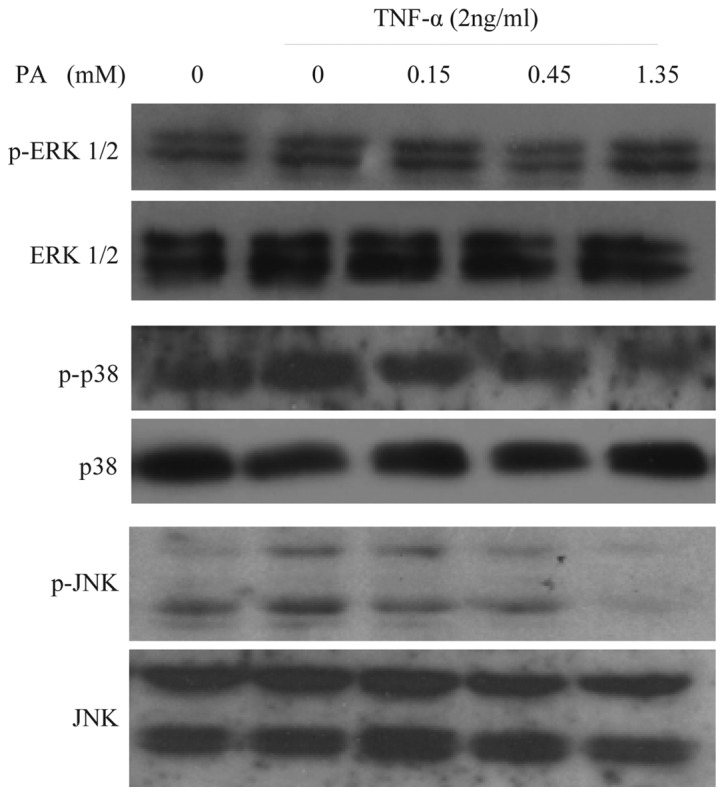
PA inhibits the TNF-α-induced JNK activation in a concentration-dependent manner in human umbilical vein endothelial cells, but not ERK1/2 and p38 activation. Cells were pretreated with PA at the indicated concentrations for 60 min. The cells were then stimulated with 2 ng/ml TNF-α for 15 min for ERK1/2, JNK and p38 activation. Cells were harvested, lysed and used for subsequent analysis. No significant differences in the levels of ERK1/2, JNK and p38 were observed using immunoblot analysis with anti-ERK1/2, -JNK and -p38 antibodies. PA, protocatechuic aldehyde; FN, fibronectin; TNF-α, tumor necrosis factor-α; p-ERK1/2, phosphorylated extracellular signal-regulated kinase 1 and 2; JNK, c-Jun N-terminal kinase; p38, p38 mitogen-activated protein kinase.
PA inhibits the TNF-α-induced transcriptional activation of NF-κB in HUVECs
To examine the effect of PA on constitutive NF-κB activation, an NF-κB p65 activity assay was performed using a DNA-binding ELISA kit. As shown in Fig. 5, increased DNA binding activity for NF-κB was observed in the TNF-α-stimulated HUVECs, whereas the pretreatment of the HUVECs with PA for 18 h markedly attenuated the TNF-α-stimulated NF-κB activation in a dose-dependent manner.
Figure 5.
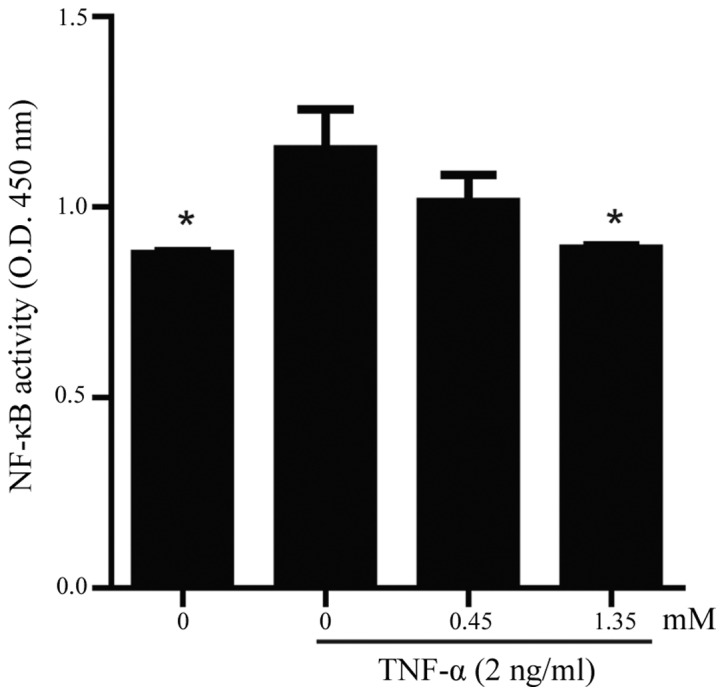
NF-κB DNA binding activity was measured using a NF-κB p65 assay. Human umbilical vein endothelial cells were pretreated with 0, 0.45 and 1.35 mM PA for 18 h and then treated with or without TNF-α (2 ng/ml) for 6 h. The data are presented as the mean ± standard error of the mean (n=4). Asterisks indicate a statistically significant difference when compared with results from the TNF-α-stimulated cells in the absence of PA (*P<0.05). PA, protocatechuic aldehyde; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α; OD, optical density.
Discussion
S.M. has been demonstrated to be clinically effective for the prevention and treatment of AS (21,22). Previous studies have demonstrated that S.M. is an antioxidative, antithrombogenic and anti-inflammatory plant (23). AS is characterized by endothelial cell injury and dysfunction. One of the earliest events in atherogenesis is the adhesion of monocytes to the endothelium, followed by their infiltration and differentiation into macrophages (2). This key step is mediated by the interaction of monocytes with molecules expressed on the surface of endothelial cells (24). FN is a large, multifunctional glycoprotein that is important in ECM organization, tissue remodeling and wound healing (25). The overproduction of FN can decrease the motility and replication of numerous types of cells, including endothelial cells. Inflammation or injury can trigger the deposition of transitional ECM proteins, such as FN and fibrinogen (FG), into the subendothelial matrix (26). In vivo, FN and FG are deposited at AS-prone sites prior to other signs of AS (27). Cellular FN (cFN) normally makes up <2% of the total FN present in the plasma and is synthesized locally by endothelial cells, smooth muscle cells or fibroblasts in response to cytokine stimulation or vascular injury (28). cFN and soluble VCAM-1 have been found to be reliable markers of endothelial injury (29). In the present study, the effect of PA on the expression and secretion of endothelial FN was investigated. It was found that the surface expression, secretion and content of FN in the cytoplasm increased significantly following the treatment of HUVECs with TNF-α. PA treatment attenuated basal and TNF-α-induced FN surface expression and secretion in a dose-dependent manner.
In previous study, we reported that the aqueous compound of S.M., PA, selectively inhibited cytokine-induced VCAM-1 and ICAM-1 expression and reduced monocyte adhesion to endothelial cells through an antioxidative mechanism (20). In the present study, PA was found to dose-dependently attenuate the ROS production in HUVECs with or without TNF-α stimulation. ROS overproduction leads to oxidative modifications of DNA, lipid oxidation, protein modification and the activation of redox-sensitive genes (13,30,31), including the upregulation of FN expression (32), as well as that of VCAM-1 and ICAM-1. The earliest stages of AS are associated with the increased attraction and adhesion of monocytes to the endothelium, which is mediated by the adhesive molecules expressed by the activated endothelium. FN plays an important role in the initiation and progression of AS.
In response to TNF-α treatment, the transcription factor NF-κB and MAPKs, including ERK, p38 and JNK, are activated in most types of cells (33). Activation of NF-κB and MAPKs plays an important role in the induction of numerous cytokines and immune-regulatory proteins and is pivotal for several inflammatory responses (34). In the present study, the mechanisms underlying the action of PA were investigated by examining the effects of PA on the MAPK and NF-κB pathways. It was found that PA specifically inhibited TNF-α-induced JNK activation, but not ERK1/2 and p38 activation, in HUVECs. The effect of PA on JNK activation by TNF-α would be expected to have important consequences for the expression of adhesion molecules by endothelial cells (35). The most likely mechanism for the PA-induced inhibition of TNF-α would be crosstalk between the MAPK and NF-κB signaling pathways. At another level, each of the three major MAPK pathways (p38, ERK and JNK) has been shown to phosphorylate p65 or p50 in various cell types, affecting transcriptional activity (36). To directly assess whether NF-κB activity was attenuated in endothelial cells treated with PA, the DNA binding activity of NF-κB was analyzed using a commercial DNA-binding ELISA assay, and it was found that the TNF-α-induced NF-κB activity was significantly inhibited by PA.
In conclusion, the present study has demonstrated that PA, the aqueous ingredient of S.M., significantly inhibits TNF-α-induced JNK activation, but not ERK1/2 and p38 activation, in HUVECs. Experimental data also showed that PA inhibits NF-κB transcriptional activation and the resultant expression and secretion of the adhesion molecule FN. These results may provide useful insight to enhance the understanding of the pharmacological action of S.M.
Acknowledgements
This study was supported by a grant from the China Postdoctoral Science Foundation (no. 2004036218).
References
- 1.Langheinrich AC, Bohle RM. Atherosclerosis: Humoral and cellular factors of inflammation. Virchows Arch. 2005;446:101–111. doi: 10.1007/s00428-004-1180-4. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Montecucco F, Mach F. Atherosclerosis is an inflammatory disease. Semin Immunopathol. 2009;31:1–3. doi: 10.1007/s00281-009-0146-7. [DOI] [PubMed] [Google Scholar]
- 4.Ding M, Ye TX, Zhao GR, Yuan YJ, Guo ZX. Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-alpha. Int Immunopharmacol. 2005;5:1641–1651. doi: 10.1016/j.intimp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Pellegatta F, Radaelli A, Ferrero E, Toninelli E, Vidal MJ, Chierchia SL, Zocchi MR. Inducible nitric oxide synthase modulates fibronectin production in the EA.hy926 cell line and cultured human umbilical vein endothelial cells. J Cardiovasc Pharmacol. 1994;24:1014–1019. doi: 10.1097/00005344-199424060-00023. [DOI] [PubMed] [Google Scholar]
- 6.Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271:13094–13102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JJ, Lee YJ, Park OJ, Lee SM, Lee YP, Cho NG, Kang DG, Lee HS. Doinseunggitang ameliorates endothelial dysfunction in diabetic atherosclerosis. Evid Based Complement Alternat Med. 2013;2013:783576. doi: 10.1155/2013/783576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Y, Wan J, Li P, Jia Y, Sun R, Pan G, Wan G. Protective effect of Xin Mai Jia ultrafiltration extract on human umbilical vein endothelial cell injury induced by hydrogen peroxide and the effect on the NO-cGMP signaling pathway. Exp Ther Med. 2014;8:38–48. doi: 10.3892/etm.2014.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohwedder I, Montanez E, Beckman K, Bengtsson E, Dunér P, Nilsson J, Soehnlein O, Fässler R. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Mol Med. 2012;4:564–576. doi: 10.1002/emmm.201200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson HP. Strethching fibronectin. J Muscle Res Cell Motil. 2002;23:575–580. doi: 10.1023/A:1023427026818. [DOI] [PubMed] [Google Scholar]
- 11.Madri JA, Pratt BM, Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988;132:18–27. [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander RW. Theodore cooper memorial lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: A new perspective. Hypertension. 1995;25:155–161. doi: 10.1161/01.HYP.25.2.155. [DOI] [PubMed] [Google Scholar]
- 13.Patel RP, Moellering D, Murphy-Ullrich J, Jo H, Beckman JS, Darley-Usmar VM. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radic Biol Med. 2000;28:1780–1794. doi: 10.1016/S0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.RES.86.5.494. [DOI] [PubMed] [Google Scholar]
- 15.Zheng CS, Xu XJ, Ye HZ, Wu GW, Xu HF, Li XH, Huang SP, Liu XX. Computational pharmacological comparison of Salvia miltiorrhiza and Panax notoginseng used in the therapy of cardiovascular diseases. Exp Ther Med. 2013;6:1163–1168. doi: 10.3892/etm.2013.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, Chen YL. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem. 2001;82:512–521. doi: 10.1002/jcb.1176. [DOI] [PubMed] [Google Scholar]
- 17.Chen YL, Yang SP, Shiao MS, Chen JW, Lin SJ. Salvia miltiorrhiza inhibits intimal hyperplasia and monocyte chemotactic protein-1 expression after balloon injury in cholesterol-fed rabbits. J Cell Biochem. 2001;83:484–493. doi: 10.1002/jcb.1233. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Wang SQ, Liu Y, Miao AD. Cryptotanshinone inhibits endothelin-1 expression and stimulates nitric oxide production in human vascular endothelial cells. Biochim Biophys Acta. 2006;1760:1–9. doi: 10.1016/j.bbagen.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Liu Y, Miao AD, Wang SQ. Salvianolic acid B attenuates plasminogen activator inhibitor type 1 production in TNF-alpha treated human umbilical vein endothelial cells. J Cell Biochem. 2005;96:109–116. doi: 10.1002/jcb.20567. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z, Liu Y, Miao AD, Wang SQ. Protocatechuic aldehyde suppresses TNF-alpha-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells. Eur J Pharmacol. 2005;513:1–8. doi: 10.1016/j.ejphar.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Song JX, Shen XC. Herbal remedies supply a novel prospect for the treatment of atherosclerosis: A review of current mechanism studies. Phytother Res. 2012;26:159–167. doi: 10.1002/ptr.3587. [DOI] [PubMed] [Google Scholar]
- 22.Lin TH, Hsieh CL. Pharmacological effects of Salvia miltiorrhiza (Danshen) on cerebral infarction. Chin Med. 2010;5:22. doi: 10.1186/1749-8546-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu WY, Wang YP. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol Sin. 2012;33:1119–1130. doi: 10.1038/aps.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faggiotto A, Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984;4:341–356. doi: 10.1161/01.ATV.4.4.323. [DOI] [PubMed] [Google Scholar]
- 25.Potts JR, Campbell ID. Structure and function of fibronectin modules. Matrix Biol. 1996;15:313–321. doi: 10.1016/S0945-053X(96)90133-X. [DOI] [PubMed] [Google Scholar]
- 26.Sechler JL, Corbett SA, Wenk MB, Schwarzbauer JE. Modulation of cell-extracellular matrix interactions. Ann NY Acad Sci. 1998;857:143–154. doi: 10.1111/j.1749-6632.1998.tb10114.x. [DOI] [PubMed] [Google Scholar]
- 27.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: A potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosmehl H, Berndt A, Katenkamp D. Molecular variants of fibronectin and laminin: Structure, physiological occurrence and histopathological aspects. Virchows Arch. 1996;429:311–322. doi: 10.1007/BF00198435. [DOI] [PubMed] [Google Scholar]
- 29.Powers RW, Majors AK, Cerula SL, Huber HA, Schmidt BP, Roberts JM. Changes in markers of vascular injury in response to transient hyperhomocysteinemia. Metabolism. 2003;52:501–507. doi: 10.1053/meta.2003.50081. [DOI] [PubMed] [Google Scholar]
- 30.Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr Opin Pharmacol. 2009;9:139–145. doi: 10.1016/j.coph.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Lee HB, Yu MR, Song JS, Ha H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 2004;65:1170–1179. doi: 10.1111/j.1523-1755.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24–27. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- 34.Baeuerle PA, Baltimore D. NF-kappaB: Ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 35.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/S0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 36.Jones WK, Brown M, Wilhide M, He S, Ren X. NF-kappaB in cardiovascular disease: Diverse and specific effects of a ‘general’ transcription factor? Cardiovasc Toxicol. 2005;5:183–202. doi: 10.1385/CT:5:2:183. [DOI] [PubMed] [Google Scholar]