Abstract
The aim of the present study is to investigate the effect of a recombinant plasmid adenovirus (pAd) expressing human interferon-λ1 (hIFN-λ1) on the proliferation of the gastric adenocarcinoma cell line SGC-7901. For this purpose, human gastric adenocarcinoma SGC-7901 cells were infected with recombinant pAd-hIFN-λ1, pAd-LacZ and phosphate-buffered saline (PBS), respectively, and the subsequent effects on the proliferation of the infected cells were compared. Cell proliferation was evaluated by MTT assay, while mRNA and protein expression of hIFN-λ1 were detected by reverse transcription-polymerase chain reaction analysis and immunofluorescence assay, respectively. In addition, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling assay and flow cytometry were conducted to analyze the rate of cell apoptosis. The results indicated that the proliferation of gastric adenocarcinoma SGC-7901 cells was significantly inhibited by pAd-hIFN-λ1. Furthermore, the apoptosis rate and the mRNA and protein expression levels of hIFN-λ1 were higher in pAd-hIFN-λ1-transfected cells, compared with the pAd-LacZ and PBS control groups. In conclusion, recombinant pAd-hIFN-λ1 induced the expression of hIFN-λ1 in gastric adenocarcinoma SGC-7901 cells, and significantly inhibited cell proliferation by promoting apoptosis in these cancer cells.
Keywords: gastric cancer, interferon-λ, recombinant adenovirus, apoptosis
Introduction
Gastric cancer is one of the most common types of cancer in the world (1). The incidence and mortality of gastric cancer vary geographically, with the highest rates reported in Eastern Asian countries (2). Although the incidence of gastric cancer has declined in recent years, it remains a remarkable burden for public health in China (3). Due to the lengthy time required to establish a definite diagnosis, a large number of patients with gastric cancer fail to be diagnosed prior to the optimal time for surgery, or tend to develop novel tumors or metastasis during the diagnostic phase (4,5). Post-surgical chemical therapy for gastric cancer causes resistance and significant side effects, leading to poor prognosis (6). In recent years, a large number of studies have focused on biological therapy for gastric cancer, among which, the interferon (IFN) family has exhibited anti-tumor abilities (7,8). IFN has been applied clinically as biological therapy for the treatment of hairy cell leukemia, chronic myelogenous leukemia, renal carcinoma and melanoma (9). Human (h)IFN-λ1, also known as interleukin (IL)-29, is a member of the IFN family, and has demonstrated anti-tumor effects in the treatment of lung cancer and colon carcinoma in previous studies (10,11). In order to examine the potential use of hIFN-λ1 for the treatment of stomach carcinoma, the present study investigated the anti-tumor mechanism of hIFN-λ1, and evaluated its effects on the stomach carcinoma cell line SGC-7901.
Materials and methods
Materials
The stomach carcinoma cell line SGC-7901 and 293A cells were provided by the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). The recombinant plasmid adenovirus (pAd)-hIFN-λ1 was generated with primers obtained from Sangon Biotech Co., Ltd. (Shanghai, China). Cell culture reagents were acquired from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA), while polymerase chain reaction (PCR) reagents and TRIzol were purchased from Toyobo Co., Ltd. (Osaka, Japan) and Invitrogen (Thermo Fisher Scientific, Inc.), respectively. The kits used for Annexin V (cat no. KGA105) and terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end-labeling (TUNEL) assays (cat no. KGA702) were obtained from KeyGen Biotech Co., Ltd. (Nanjing, China), and methyl thiazolyl tetrazolium (MTT; cat no. 0793) was obtained from Amresco LLC (Solon, OH, USA). Cyanine (Cy)3-labeled goat anti-rabbit immunoglobulin (Ig)G (cat no. KGAB019) was obtained from KeyGen Biotech Co., Ltd., rabbit anti-human polyclonal hIFN-λ1 antibody (cat no. ab38569) was obtained from Abcam (Cambridge, UK) and horseradish-peroxidase (HRP)-conjugated monoclonal mouse anti-GAPDH (cat no. kc5G5) was obtained from Kangcheng Biology Engineering Co., Ltd. (Shanghai, China). Furthermore, ECL (cat no. WBLUC0100) was obtained from EMD Millipore (Billerica, MA, USA) and the BCA kit (cat no. CW0013) was purchased from CWbio Co., Ltd. (Beijing, China).
Preparation of recombinant adenovirus
To produce the recombinant virus pAd-hIFN-λ1, a monolayer of 293A cells cultured in a 6-well plate was transfected with PacI-linearized plasmid pAd-hIFN-λ1 (4 µg/well; cat no. FD2204; Thermo Fisher Scientific, Inc.) using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc.). The viruses were propagated in 293A cells and purified by viral plaque three times. At 7–14 days post-transfection, when 90% of the cells displayed cytopathogenic alterations, the cell supernatants were collected by centrifugation at 10,000 × g for 10 min at room temperature (Jouan BR4i; Thermo Fisher Scientific, Inc.), and stored at −70°C for future use. The viral titer (T) was calculated according to the tissue culture infectious dose (TCID)50. SGC-7901 cells were infected with recombinant pAd-hIFN-λ1 viruses, with a multiplicity of infection (MOI) of 12.5, 25, 50, 100, 200, 400 and 800 pfu/cell, respectively. The optimal MOI was determined based on the morphology presented by the cells at 48 h post-infection.
Indirect immunofluorescence assay
SGC-7901 cells were cultured in a 24-well plate and infected with pAd-hIFN-λ1. The cells were collected at 48 h post-infection, fixed with cold 4% paraformaldehyde for 1 h at −20°C, and washed three times with phosphate-buffered saline (PBS). The fixed cells were incubated with rabbit anti-human hIFN-λ1 antibody for 1 h at 37°C in a humidified chamber, and then washed three times with PBS, followed by 1-h staining with Cy3-labeled goat anti-rabbit IgG at 37°C in a humidified chamber, and three washes with PBS. All antibodies were diluted to 1:300 and each washing step was performed for 5 min at room temperature. Subsequently, the cells were stained with Hoechst 33342. Photographs of the cells were then captured with a fluorescence microscope (Leica DFC300 FX, Leica Microsystems (UK) Ltd., Milton Keynes, UK).
Protein extraction and western blot analysis
At 48 h post-infection with pAd-hIFN-λ1, pAd-LacZ and PBS, respectively, 1×106 cells/group were lysed with 100 µl radioimmunoprecipitation assay buffer [50 mM Tris pH 7.4, 150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM dithiothreitol, 1 mM phenylmethanesulfonyl fluoride, 1 mM protease inhibitors and 1 mM sodium orthovanadate; CWbio Co., Ltd.]. The cell lysates were then centrifuged at 12,000 × g for 15 min at 4°C (Jouan BR4i; Thermo Fisher Scientific, Inc.), and the protein concentration in the supernatant was determined with a bicinchoninic acid protein assay kit (Bio-Rad Laboratories, Inc., Berkeley, CA, USA). Subsequently, the supernatant was mixed with loading buffer at a ratio of 1:1 (v:v), and heated at 100°C for 5 min. Next, 30 µg protein was loaded onto SDS-polyacrylamide gels and subjected to electrophoresis using a Mini-PROTEAN Tetra Cell system (Bio-Rad Laboratories, Inc.). A 10% gel and buffers for electrophoresis were prepared according to the manufacturer's protocol (Bio-Rad Laboratories, Inc.). Electrophoresis was performed at a constant voltage of 120 V for 90 min at room temperature to isolate the proteins. A Protein Molecular Weight Marker (cat no. 3452; Takara Bio, Inc., Otsu, Japan). Subsequently, the proteins were electrotransferred to polyvinylidene difluoride Immobilon membranes (EMD Millipore) with Mini Trans-Blot Cell (Bio-Rad Laboratories, Inc.) at a constant current of 350 mA for 90 min at 4°C. The membranes were then probed with rabbit anti-human hIFN-λ1 antibody, and washed with Tris-buffered saline pH 7.4 containing 1% Tween-20, followed by incubation with HRP-labeled goat anti-rabbit IgG (cat. no. CW0159, Beijing CoWin Biotech Co., Ltd., Beijing, China), and washed as indicated above. All antibodies were diluted to 1:300. Immunoblots were detected with enhanced chemiluminescence. Typhoon 9400 was used for scanning western blots (GE Healthcare Life Sciences, Little Chalfont, UK).
Reverse transcription (RT)-PCR analysis
SGC-7901 cells were infected with pAd-hIFN-λ1, pAd-LacZ and PBS, respectively, and total RNA was extracted 24 h later with TRIzol, following the manufacturers protocol. The RNA was converted into complementary (c)DNA via RT, using SuperScript III First-Strand Synthesis System (Invitrogen, Thermo Fisher Scientific, Inc.). RT was conducted with oligo(dT) primers in 25 µl reactions, which were incubated at 50°C for 50 min, and terminated at 85°C for 5 min. The cDNA obtained was stored at −20°C until further use.
Amplification of hIFN-λ1 cDNA was performed using a MasterCycler Gradient Thermal Cycler (Eppendorf, Hamburg, Germany) with primers 5-TATCCAGCCTCAGCCCACAGCA-3 (sense) and 5-ACAGGTTCCCATCGGCCACATA-3 (anti-sense), under the following PCR conditions: 4 min at 95°C for pre-denaturation, 40 sec at 94°C for denaturation, 40 sec at 61°C for primer annealing, and 35 sec at 72°C for elongation during 35 cycles of amplification, followed by 10-min incubation at 72°C for final extension. To identify the PCR products, 5 µl of the reaction was loaded onto 1% agarose gels, and subjected to electrophoretic analysis. DL2000 DNA Marker (Clontech Laboratories, Inc., Mountainview, CA, USA) was used as DNA molecular size marker.
MTT proliferation assay
SGC-7901 cells at logarithmic growth phase were seeded in 96-well plates at a density of 5×104 cells/ml in the presence of 200 µl culture medium supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Inc.), and incubated for 24 h. Next, the cells were placed in quintuplicate wells in the presence of 200 µl pAd-hIFN-λ1 (6.3×106 pfu/ml), pAd-LacZ and PBS, respectively. MTT solution (5 mg/ml) was prepared by dissolving MTT in PBS, and subsequently filter-sterilizing the solution using a filter paper (EMD Millipore) of 0.2-µm pores. MTT (20 µl) was then added to each well, and the plate was incubated for 4 h at 37°C, prior to reading the absorbance at 490 nm using an iMARK microplate absorbance reader (Bio-Rad Laboratories, Inc.). Quintuplicate samples/group were evaluated.
TUNEL assay
A TUNEL assay kit was used to assess the presence of apoptotic cells in SGC-7901 cells infected with pAd-hIFN-λ1, pAd-LacZ and PBS, respectively. SGC-7901 cells were fixed in 4% formaldehyde (pH 7.4) for 48 h at room temperature, rinsed in PBS, and permeabilized in PBS with 0.1% Triton X-100 and 0.1% sodium citrate at 4°C for 2 min. Next, the cells were stained following the protocol provided by the manufacturer, observed under a light microsocope, and photographed. The number of positive cells/100 cells was counted, and the average ratio of positive cells was defined as the apoptotic index.
Analysis of apoptosis by flow cytometry
Apoptosis in SGC-7901 cells infected with pAd-hIFN-λ1, pAd-LacZ and PBS, respectively, was determined with an Annexin V-fluorescein isothiocyanate (FITC) staining kit, according to the manufacturers protocol. Propidium iodide (PI) was used to differentiate apoptotic cells with membrane integrity (Annexin V+/PI−) from necrotic cells that had lost their membrane integrity (Annexin V+/PI+). The percentage of apoptotic cells was calculated using CellQuest Pro Software (BD Biosciences, Franklin Lakes, NJ, USA), and the data were analyzed with WinMDI version 2.9 software (http://en.bio-soft.net/other/WinMDI.html).
Statistical analysis
Data are expressed as the mean ± standard deviation, unless otherwise stated. Statistically significant differences between the cells in the pAd-hIFN-λ1 group and the cells in the control groups were determined by paired t-test or one-way analysis of variance. SPSS software (version 14.0; SPSS, Inc., Chicago, IL, USA) was used for all statistical analysis (ANOVA with Fisher's Least Significant Difference post hoc) and P<0.05 was considered to indicate a statistical significant difference.
Results
Viral T of adenovirus
The viral T of pAd-hIFN-λ1 was calculated as follows: T=101+(1+1+1+1+1+1+1+1–0.5)=108.5/100 µl, which equates to 109.5 TCID50/ml or 6.3×108 pfu/ml. Thus, there is an exponential association between T and the MOI. For pAd-LacZ, T=4×108 pfu/ml was observed.
MOI of recombinant virus for infection of SGC-7901 cells
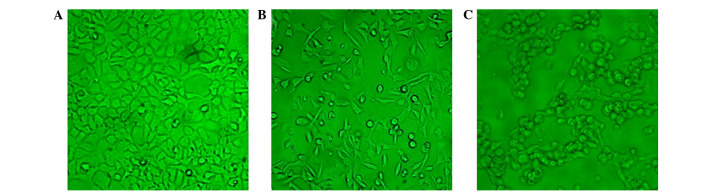
SGC-7901 cells were seeded on 24-well plates at a density of 5×104 cells/well, and incubated overnight at 37°C with 5% CO2, prior to be infected with pAd-hIFN-λ1 or pAd-LacZ viruses with variable MOI (12.5, 25, 50, 100, 200, 400 and 800 pfu/cell, respectively). At 48 h post-infection, the cells were monitored under the microscope. As presented in Fig. 1, the number of cells infected with pAd-hIFN-λ1 increased with increasing MOI, until MOI = 200, while at higher MOI values (MOI = 400 and 800), the cells became necrotic and their size reduced, indicating that the optimal MOI was 200.
Figure 1.
Microscopic images of 293A cells transfected with a recombinant plasmid adenovirus coding for human interferon-λ1 (magnification, ×200). (A) Non-transfected 293A cells. Transfected 293A cells at (B) 1 and (C) 2 days post-transfection.
Messenger (m)RNA and protein expression levels of hIFN-λ1 in SGC-7901 cells
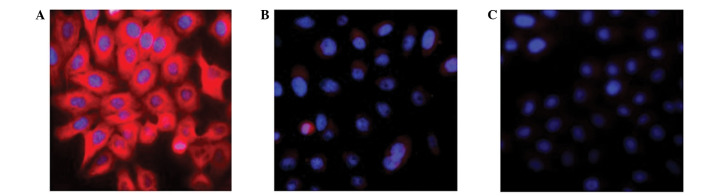
IFN-λs have been recently identified as cytokines whose target cells remain to be elucidated (12,13). Immunofluorescence was used to detect the expression of hIFN-λ1 in SGC-7901 cells infected with pAd-hIFN-λ1, pAd-LacZ and PBS, respectively. The results are presented in Fig. 2. The pAd-LacZ and PBS control groups did not exhibit any red fluorescence, while the pAd-hIFN-λ1 group markedly displayed red fluorescence, indicating expression of hIFN-λ1 protein in these cells.
Figure 2.
(A) Expression of hIFN-λ1 was detected by the fluorescence microscopy in SGC-7901 cells infected with pAd-hIFN-λ1, compared with control cells infected with (B) pAd-LacZ and (C) phosphate-buffered saline (magnification, ×400). hIFN-λ1, human interferon-λ1; pAd, plasmid adenovirus.
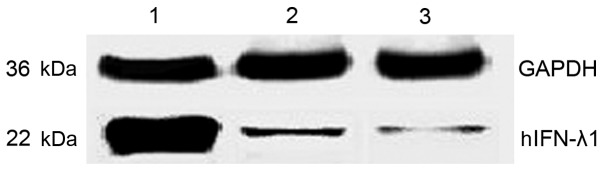
To quantify the protein expression levels of hIFN-λ1 in the pAd-hIFN-λ1, pAd-LacZ and PBS groups, western blot assay was performed. The results are expressed as relative to the protein levels of glyceraldehyde 3-phosphate dehydrogenase measured in these cells. As presented in Fig 3, the protein expression levels of hIFN-λ1 were higher in the pAd-hIFN-λ1 group, compared with the control groups, suggesting that hIFN-λ1 was overexpressed in these cells.
Figure 3.
Expression of hIFN-λ1 in SGC-7901 cells infected with pAd-hIFN-λ1 was detected by western blotting. Lane 1, cells infected with pAd-hIFN-λ1; lane 2, cells infected with pAd-LacZ; lane 3, cells infected with phosphate-buffered saline. hIFN-λ1, human interferon-λ1; pAd, plasmid adenovirus; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
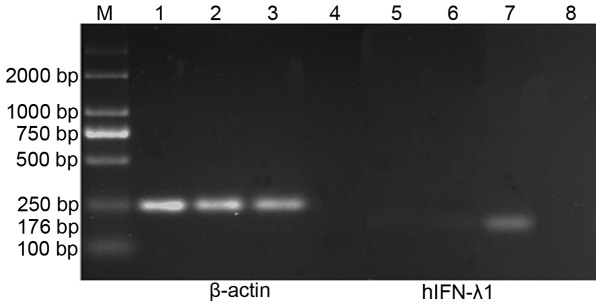
In addition, RT-PCR was used to evaluate the mRNA expression levels of hIFN-λ1 in each group. mRNA was isolated from SGC-7901 cells infected with pAd-hIFN-λ1, pAd-LacZ and PBS, respectively, and the levels of hIFN-λ1 transcript present in these mRNA samples were determined by RT-PCR. The results demonstrated the presence of high mRNA expression levels of hIFN-λ1 in the pAd-hIFN-λ1 group, but not in the controls (Fig. 4).
Figure 4.
Reverse transcription-polymerase chain reaction analysis of the expression of hIFN-λ1 in SGC-7901 cells. Lane M, DL2000 DNA Marker; lanes 1 and 5, cells infected with phosphate-buffered saline; lanes 2 and 6, cells infected with pAd-LacZ; lanes 3 and 7, cells infected with pAd-hIFN-λ1; lanes 4 and 8, negative controls absent of template. hIFN-λ1, human interferon-λ1; M, marker; pAd, plasmid adenovirus.
Apoptosis induced by infection with pAd-hIFN-λ1
To assess whether the hIFN-λ1-induced anti-proliferative effects observed in SGC-7901 cells infected with pAd-hIFN-λ1 may be due to the induction of apoptosis, MTT assay was performed. The results obtained confirmed the initial hypothesis. Thus, pAd-hIFN-λ1 significantly inhibited cell proliferation in SGC-7901 cells (43.28±1.65% inhibition vs. 3.12±2.91% inhibition for the pAd-LacZ control) (data not shown).
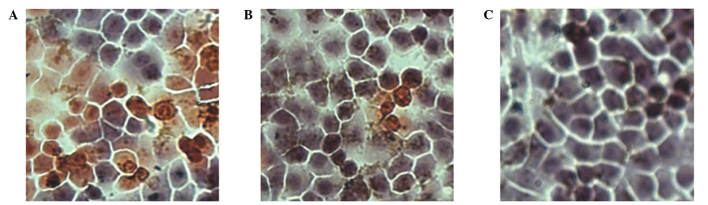
These results were in agreement with the findings of TUNEL assay. As presented in Fig. 5, the pAd-hIFN-λ1 group exhibited a higher number of apoptotic cells that the pAd-LacZ control group.
Figure 5.
The apoptotic index of SGC-7901 cells infected with (A) pAd-human interferon-λ1, (B) pAd-LacZ or (C) phosphate-buffered saline was determined by immunocytochemistry (magnification, ×200). pAd, plasmid adenovirus.
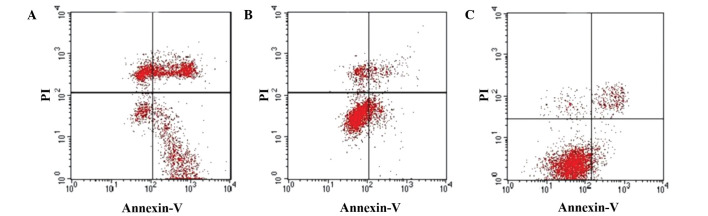
These observations were further confirmed by flow cytometry using Annexin V and PI staining. As depicted in Fig. 6, the apoptotic index significantly increased in SGC-7901 cells infected with pAd-hIFN-λ1, whereas a slight increase in the apoptotic index was observed in pAd-hIFN-λ1-infected cells, compared with the PBS control.
Figure 6.
Apoptosis in SGC-7901 cells infected with (A) pAd-human interferon-λ1 (39.32%), (B) pAd-LacZ (11.41%) or (C) phosphate-buffered saline (5.93%) was detected by flow cytometry. pAd, plasmid adenovirus; PI, propidium iodide.
Discussion
Members of the type III IFN-λ family include IFN-λ1, 2 and 3, also termed IL-29, −28A and −28B, respectively (14). IFN-λs exhibit similar effects to type I IFNs, which are involved in signal transduction pathways, anti-virus, anti-proliferation and immunological regulation (15–17). Gene therapy is a highly effective and specific type of therapy that enables the translation of a gene of interest in the corresponding target cells via a vector (18). The success of gene therapy mainly depends on the selection of an adequate vector (19). Since viruses are able to translate independently their genetic material inside the host cell, and their DNA does not integrate into the host cell chromosome, virus-derived vectors such as adenoviruses are considered safer than other type of vectors that require integration of their genetic material in the DNA of the host (20). Therefore, virus-derived vectors, and in particular adenoviruses, have become the most widely used type of vector in gene therapy (21).
In the present study, pAd-hIFN-λ1 was infected into the human gastric cell line SGC-7901, and the genetic material carried by the vector was efficiently transcribed inside these cells, as demonstrated by the high mRNA expression levels of hIFN-λ1 detected by RT-PCR. In addition, immunofluorescence and western blot analyses revealed high protein expression levels of hIFN-λ1 in pAd-hIFN-λ1-infected SGC-7901 cells, indicating that hIFN-λ1 was also efficiently translated inside these tumor cells. Previous studies have demonstrated that hIFN-λ1, a recently identified member of the IFN-λs family, exhibits anti-tumor effects in lung carcinoma (11), colon cancer (10) and esophageal cancer (22). The results of the MTT assay conducted in the present study confirmed that pAd-hIFN-λ1 is effective in inhibiting the proliferation of SGC-7901 cells, which is in agreement with the anti-tumor effects of hIFN-λ1 reported in previous studies. Therefore, hIFN-λ1 may be a potential target for biological therapy of stomach carcinoma. hIFN-λ1 may exert its anti-tumor effects by inhibiting tumor replication (23), inducing cell apoptosis (24,25), inhibiting cell proliferation (16,26), regulating the immune system in order to kill tumor cells (27), targeting tumor matrix cells, and promoting anti-angiogenesis (28).
In the present study, the results of TUNEL assay and Annexin V-FITC/PI flow cytometry revealed that the apoptosis rate was higher in the pAd-hIFN-λ1 group than in the pAd-LacZ and PBS control groups. Previous studies using IFN-λs to regulate the apoptosis of colon carcinoma cells have demonstrated that IFN-λs are able to arrest cells in the G1/G0 phase of the cell cycle, leading to the extracellular translocation of phosphatidylserine from the inner side of the cell membrane, DNA damage and activation of caspase-3, −8 and −9, which eventually results in apoptosis (28,29). Therefore, one of the possible mechanisms of inhibition of cell proliferation in gastric carcinoma cells is the induction of apoptosis via phosphatidylserine (30).
In conclusion, the results of the present study confirm that pAd-hIFN-λ1 is capable of inhibiting proliferation and inducing apoptosis in stomach cancer cells, which provides evidence for future studies on the use of pAd-hIFN-λ1 as biological therapy for the treatment of stomach cancer. Compared with type I IFNs and cytotoxic drugs, pAd-hIFN-λ1-based therapy does not appear to produce any side effects. However, whether the combination of pAd-hIFN-λ1 with a reduced dosage of cytotoxic drugs may enhance its anti-tumor effects or reduce the side effects caused by the cytotoxic drugs requires further investigation.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu Province (grant no. BK20151333).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Pourhoseingholi MA, Vahedi M, Baghestani AR. Burdenof gastrointestinal cancer in Asia; an overview. Gastroenterol Hepatol Bed Bench. 2015;8:19–27. [PMC free article] [PubMed] [Google Scholar]
- 3.Tänzer M, Liebl M, Quante M. Molecular biomarkers in esophageal, gastric, and colorectal adenocarcinoma. Pharmacol Ther. 2013;140:133–147. doi: 10.1016/j.pharmthera.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Liang H, Kim YH. Identifying molecular drivers of gastric cancer through next-generation sequencing. Cancer Lett. 2013;340:241–26. doi: 10.1016/j.canlet.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González CA, Agudo A. Carcinogenesis, prevention and early detection of gastric cancer: Where we are and where we should go. Int J Cancer. 2012;130:745–753. doi: 10.1002/ijc.26430. [DOI] [PubMed] [Google Scholar]
- 6.Yared JA, Tkaczuk KH. Update on taxane development: New analogs and new formulations. Drug Des Devel Ther. 2012;6:371–384. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu XF, Zhang J, Jia LJ, Liang B, Zhang J, Liu Y, Yan YL. Effect of human interferon-λ1 recombinant adenovirus on a gastric cancer orthotopic transplantation model. Exp Ther Med. 2014;8:1115–1122. doi: 10.3892/etm.2014.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Z, Zhu M, Wu Y, Gao P, Qin Z, Wang H. Interferon-λ1 induces G1 phase cell cycle arrest and apoptosis in gastric carcinoma cells in vitro. Oncol Rep. 2014;32:199–204. doi: 10.3892/or.2014.3185. [DOI] [PubMed] [Google Scholar]
- 9.Ferrantini M, Capone I, Belardelli F. Interferon-α and cancer: Mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89:884–893. doi: 10.1016/j.biochi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Hui X, Chen H, Zhang S, Ma X, Wang X, Huang B. Antitumor activities of recombinant human interferon (IFN)-λ1 in vitro and in xenograft models in vivo for colon cancer. Cancer Lett. 2011;311:141–151. doi: 10.1016/j.canlet.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Yan Y, Zhang J, Liu Y, Zhu T, Yuan L, Ge Y, Ding H, Bu X. Inhibition of lung adenocarcinoma transfected with interleukin 28A recombinant adenovirus (Ad-mIFN-λ2) in vivo. Cancer Biother Radiopharm. 2013;28:124–130. doi: 10.1089/cbr.2012.1247. [DOI] [PubMed] [Google Scholar]
- 12.Lopušná K, Režuchová I, Betáková T, et al. Interferons lambda, new cytokines with antiviral activity. Acta Virol. 2013;57:171–179. doi: 10.4149/av_2013_02_171. [DOI] [PubMed] [Google Scholar]
- 13.Steen HC, Gamero AM. Interferon-lambda as a potential therapeutic agent in cancer treatment. J Interferon Cytokine Res. 2010;30:597–602. doi: 10.1089/jir.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly RP, Kotenko SV. Interferon-lambda: A new addition to an old family. J Interferon Cytokine Res. 2010;30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins −28 and −29: Comparison with type I interferons. Cytokine. 2005;31:109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-λ1: Similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 17.Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, et al. Characterization of the mouse IFN-lambdas ligand-receptor system: IFN-λs exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- 18.Khalighinejad N, Hariri H, Behnamfar O, Yousefi A, Momeni A. Adenoviral gene therapy in gastric cancer: A review. World J Gastroenterol. 2008;14:180–184. doi: 10.3748/wjg.14.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rincon MY, VandenDriessche T, Chuah MK. Gene therapy for cardiovascular disease: Advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res. 2015;108:4–20. doi: 10.1093/cvr/cvv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang I, Huang I. Adenovirus technology for gene manipulation and functional studies. Drug Discov Today. 2000;5:10–16. doi: 10.1016/S1359-6446(99)01433-6. [DOI] [PubMed] [Google Scholar]
- 21.Büning H, Huber A, Zhang L, Meumann N, Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr Opin Pharmacol. 2015;24:94–104. doi: 10.1016/j.coph.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Kawamura K, Okamoto S, Fujie H, Numasaki M, Namba M, Nagata M, Shimada H, Kobayashi H, Tagawa M. Adenoviruses-mediated transduction of human oesophageal carcinoma cells with the interferon-λ genes produced anti-tumour effects. Br J Cancer. 2011;105:1302–1312. doi: 10.1038/bjc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly C, Klenerman P, Barnes E. Interferon-lambdas: The next cytokine storm. Gut. 2011;60:1284–1293. doi: 10.1136/gut.2010.222976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagawa M, Kawamura K, Li Q, Tada Y, Hiroshima K, Shimada H. A possible anticancer agent, type III interferon, activates cell death pathways and produces antitumor effects. Clin Dev Immunol. 2011;2011:479013. doi: 10.1155/2011/479013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-λ in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 26.Maher SG, Sheikh F, Scarzello AJ, Romero-Weaver AL, Baker DP, Donnelly RP, Gamero AM. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther. 2008;7:1109–1115. doi: 10.4161/cbt.7.7.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Liu X, Zhou Y, Su SB. Interferon-lambdas: The modulators of antivirus, antitumor, and immune responses. J Leukoc Biol. 2009;86:23–32. doi: 10.1189/jlb.1208761. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Lewis-Antes A, Huang J, Balan M, Kotenko SV. Regulation of apoptosis by type III interferons. Cell Prolif. 2008;41:960–979. doi: 10.1111/j.1365-2184.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Kawamura K, Tada Y, Shimada H, Hiroshima K, Tagawa M. Novel type III interferons produce anti-tumor effects through multiple functions. Front Biosci (Landmark Ed) 2013;18:909–918. doi: 10.2741/4152. [DOI] [PubMed] [Google Scholar]
- 30.Woehlecke H, Pohl A, Alder-Baerens N, Lage H, Herrmann A. Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2) Biochem J. 2003;376:489–495. doi: 10.1042/bj20030886. [DOI] [PMC free article] [PubMed] [Google Scholar]