Abstract
Breast cancer is one of the most prevalent types of cancer in women and contributes to 32% of all female cancer cases. Cathepsins, a family of proteins, are known to have a critical role in human cancers. However, previous studies on the systematic analysis of the role of cathepsin family members in breast cancer are limited. The aim of the present study was to identify biological markers to predict prognosis and treatment response of breast cancer patients, as well as to elucidate novel therapeutic targets. The present study analyzed the expression of six members of cathepsin family, including cathepsins B, G, D, K, L and V in 188 breast cancer tissue specimens using immunohistochemistry. The data showed that all members of the tested cathepsin families featured cytoplasmic staining. Notably, expression of cathepsin L was associated with advanced tumor stages, while cathepsins B and K expression levels were associated with positive estrogen receptor expression; in addition, cathepsin K expression was also demonstrated to be associated with progesterone receptor expression. Cathepsins V and D expression levels were found to be associated with breast cancer metastasis, while the expression levels of cathepsins B and D were associated with poor disease-free survival in breast cancer patients. In addition, univariate analysis demonstrated that breast cancer metastasis to the bone and the expression of cathepsin B protein were associated with poor disease-free survival. In conclusion, the results of the present study indicated that the altered expression of cathepsins, in particular cathepsins B and D, contributed to the progression of breast cancer and poor disease-free survival in breast cancer patients.
Keywords: breast cancer, cathepsins, prognosis, expression profile
Introduction
Breast cancer is the most prominent health problem for women worldwide, affecting one in eight women (1–3). Over the past three decades, there have been numerous advances for breast cancer patients, including earlier detection, increased treatment options and more prevention strategies. However, breast cancer remains to be the second highest cause of cancer-associated mortality in women (1,2). Breast cancer progression, such as metastasis to distant organs, is significantly associated with cancer-associated mortality (1,3). Thus, it is crucial to identify biomarkers to predict prognosis and treatment response, and elucidate novel therapeutic targets of breast cancer.
Proteolytic activity has been reported to be significantly associated with cancer metastasis and altered proteolytic activities was also found to cause a wide range of diseases, including cancer (4). To date, ~500–600 proteases have been identified in the human genome (5). Among them, up to 60 are lysosomal proteases and cathepsins; cathepsins are a group of lysosomal or cysteine proteases, which include 16 members in humans (6–9). Expression of cathepsins has been shown to be upregulated in various types of human cancers (10–12); in addition, altered expression of cathepsins has been associated with tumor angiogenesis, proliferation and invasion (13,14). For example, during cancer progression, translocated or secreted cathepsins were reported to promote tumor cell invasion and metastasis (14).
However, different cathepsins may have different roles in human cancers. Cathepsins B and D were often found to be overexpressed and associated with invasive and metastatic phenotypes of various types of human cancers (15–18). However, the role of each member of the cathepsin family in breast cancer remains to be fully elucidated and to date there have been a limited number of studies on the function of different members of the cathepsin family in breast cancer. A small number of previous studies have reported the altered expression of cathepsins D, B and E in breast carcinogenesis (19–23). In addition, previous studies have reported that cathepsin D was overexpressed in invasive ductal carcinoma compared with its expression in lobular carcinoma of the mammary glands (21,24,25). Furthermore, overexpression of cathepsin D was associated with lymph node metastasis of breast invasive ductal carcinoma (21).
Notably, the design of small molecular inhibitors targeting cathepsins is a strategy that has been used previously against human cancer, among other diseases (26). Each cathepsin has a different cleavage bond-specificity for substrate proteins, thus allowing for the development of cathepsin-specific inhibitors for targeting different family members (26,27). Such inhibitors include E-64, leupeptin and antipain (26,28,29). These inhibitors are able to selectively suppress cathepsin activity and inhibit tumor growth. For example, anti-cathepsin B and D have been demonstrated to have efficacy in cancer therapy (16,27,30).
Detailed comparison studies of the expression of different members of the cathepsin family in breast cancer are limited. Therefore, the aim of the present study was to analyze the expression of six important members of cathepsin family, including cathepsins B, D, G, K, L and V, in breast cancer tissue specimens. In addition, the expression of these cathepsins was compared with that of reversion-inducing cysteine-rich protein with Kazal motifs (RECK) and vascular endothelial growth factor (VEGF). The expression of these proteins was then analyzed for associations with the clinicopathological data of patients and breast cancer patient survival rates. These data may allow for the development of novel therapies for the treatment of breast cancer patients. In addition, the results of the present study may elucidate cathepsins that have a more dominant role in breast cancer compared with other family members, thus providing evidence for their use in predicting the prognosis of breast cancer patients as well as the development of novel cathepsin-specific treatment options.
Materials and methods
Patient specimens
In the present study, tissue specimens were retrospectively collected from 188 breast cancer patients who underwent treatment at the Liaoning Cancer Hospital and Institute (Liaoning, China). All patients were pathologically diagnosed with breast invasive ductal carcinoma. The present study was approved by the hospital review board of the Liaoning Cancer Hospital and Institute and each patient provided written informed consent for their participation in the study. In addition, follow-up data regarding survival and adverse effects were also collected for the present study at follow-up clinic visits or via phone interview. The detailed clinicopathological data, including age, gender, pathological type, clinical symptoms and stage, lymph node and distant metastasis, expression of estrogen receptor (ER), progesterone receptor (PR) and erythroblastic leukemia viral oncogene homolog 2 (erbB2), menstrual status and laboratory tests, were collected from the patients' medical records.
Immunohistochemistry
All tissue specimens were fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) and then dehydrated and embedded in paraffin (Sigma-Aldrich). For immunohistochemistry, the tissue blocks were cut into 4-cm-thick sections and placed in vertical plastic boxes until further use. The sections were dewaxed in analytical reagent (AR) grade xylene AR and rehydrated through graded alcohol series (AR grade; 100, 95, 90, 80 and 70%). For antigen retrieval, the sections were heated in a citrate buffer (0.01 M; pH 6.0) using a microwave oven (800 W; Haier, Qingdao, China) for 30 min at 93°C. Following washing three times in phosphate-buffered saline (PBS; Abcam, Cambridge, MA, USA) for 10 min each, the sections were incubated with 3% bovine serum albumin (Abcam) at 37°C for 30 min. Tissues were then incubated with primary antibodies, including antibodies against cathepsin B (rabbit anti-human polyclonal; 1:50 dilution; cat no. 12216-1-AP), cathepsin K (rabbit anti-human polyclonal; 1:100 dilution; cat no. 11239-1-AP), cathepsin D (mouse anti-human monoclonal; 1:200 dilution; cat no. 2926S), cathepsin G (rabbit anti-human polyclonal; 1:600 dilution; cat no. ab64891), cathepsin L (mouse anti-human monoclonal; 1:100 dilution; cat no. ab6314), cathepsin V (mouse anti-human monoclonal; 1:200 dilution; cat no. ab24508), VEGF (mouse anti-human monoclonal; 1:200 dilution; cat no. ab464154) and RECK (mouse anti-human monoclonal; 1:40 dilution; cat no. 611512) overnight at 4°C. Cathepsin B and K were obtained from Proteintech (Chicago, IL, USA); cathepsin D, G, L and V, and VEGF were obtained from Abcam; and RECK was obtained from Becton Dickinson (Franklin lakes, New Jersey, USA). The following day, the sections were washed three times with PBS and then further incubated with the corresponding secondary antibodies (Abcam) at 37°C for 30 min. For color reactions, the sections were washed again with PBS for three times of 5 min each and then stained with a diaminobenzidine solution (Abcam) for up to 5 min. Protein expression in tumor cells was assessed for proportion and intensity score independently by two experienced pathologists. Specifically, the intensity score was obtained by the average intensity of positive cells, which was scored as follows: 0, none; 1, weak; 2, intermediate; and 3, strong. The proportion score was determined according to the proportion of positive cells as follows: 0, none; 1, ≤10%; 2, 11–25%; 3, 26–50%; and 4, >50%. The final score for each protein was calculated by adding the scores for proportion and intensity, then statistically analyzed as low (total score, 0–2) vs. high (total score, 3–8) expression.
Statistical analysis
All statistical analyses were performed using the SPSS 17.0 statistical software package (SPSS Inc., Chicago, IL, USA). The Fisher exact test or χ2 test was used to analyze the difference between two groups, while the hazard ratios and 95% confidence intervals were used to calculate the difference between low and high expression groups. Cumulative survival curves were generated using the Kaplan-Meier method and analyzed using a Cox regression test. Univariate and multivariate analyses were performed to analyze the association between clinicopathological parameters and patient outcomes. P<0.05 was considered to indicate a statistically significant difference between values.
Results
Expression of cathepsins B, D, G, K, L and V as well as RECK and VEGF in breast cancer tissue specimens
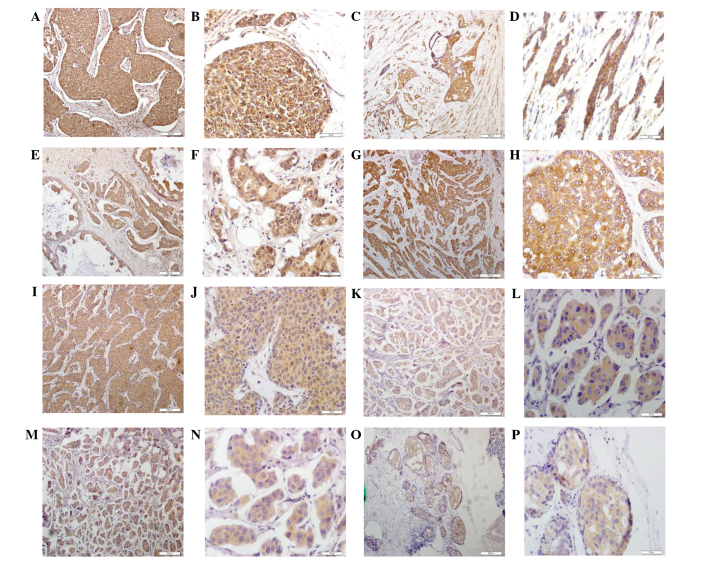
In the present study, the expression of cathepsins B, D, G, K, L and V was assessed using immunohistochemistry. In addition, cathepsin expression levels were compared with those of RECK, a metastasis suppressor with protease inhibitor-like domains, and VEGF, which is involved in the proliferation and metastasis of cancer cells. As shown in Fig. 1, all members of these cathepsin families as well as RECK and VEGF demonstrated positive cytoplasmic staining in these breast cancer tissues. Of note, cathepsin B was expressed in 109/142 (76.76%), cathepsin D in 91/155 (58.71%), cathepsin G in 99/171 (57.89%), cathepsin K in 97/143 (67.83%), cathepsin L in 95/166 (57.23%), cathepsin V in 45/164 (27.44%), RECK in 67/175 (38.29%) and VEGF in 100/164 (60.98%) samples of breast cancer tissues. Due to unsuccessful staining in certain samples, staining information could not be obtained from the total 188 samples.
Figure 1.
Detection of cathepsins B, D, G, K, L and V as well as RECK and VEGF proteins in breast cancer tissue specimens using immunohistochemistry. Representative images were selected to reflect the expression of (A and B) cathepsin B, (C and D) cathepsin C, (E and F) cathepsin D, (G and H) cathepsin K, (I and J) cathepsin L, (K and L) RECK, (M and N) cathepsin V expression and (O and P) VEGF in breast cancer tissues. Magnification, ×200. RECK, reversion-inducing cysteine-rich protein with Kazal motifs; VEGF, vascular endothelial growth factor.
Associations between cathepsins B, D, G, K, L and V as well as RECK and VEGF expression in breast cancer tissue specimens
The expression of certain proteins may have an effect on the expression of other proteins in the same family. In order to determine whether cathepsins interact to effect each others expression, the dependence in expression of different members of the cathepsin family, as well as RECK and VEGF, was analyzed. The results revealed that the expression of cathepsin B was associated with the expression of cathepsins D (P=0.036) and L (P=0.002), while cathepsin D expression was associated with the expression of cathepsins G (P=0.016), L (P=0.003) and V (P=0.006) (Table I). In addition, cathepsin G expression was associated with the expression of cathepsins K (P=0.023), L (P=0.035) and V (P=0.025) (Table I). Furthermore, cathepsin L expression was associated with that of VEGF (P=0.032), while VEGF expression was associated with RECK expression (P=0.001) (Table I).
Table I.
Association of these protein expressions in breast cancer tissues.
| Cathepsin D | Cathepsin G | Cathepsin K | Cathepsin L | Cathepsin V | VEGF | RECK | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | L | H | P | L | H | P | L | H | P | L | H | P | L | H | P | L | H | P | L | H | P |
| Cathepsin B | |||||||||||||||||||||
| − | 55 | 8 | 0.036 | 49 | 17 | 0.105 | 36 | 16 | 0.830 | 52 | 14 | 0.002 | 42 | 18 | 0.439 | 30 | 38 | 0.100 | 43 | 29 | 0.625 |
| + | 41 | 16 | 39 | 25 | 37 | 15 | 33 | 29 | 48 | 15 | 17 | 40 | 35 | 28 | |||||||
| Cathepsin D | |||||||||||||||||||||
| − | – | – | – | 89 | 28 | 0.016 | 67 | 26 | 0.969 | 82 | 30 | 0.003 | 85 | 24 | 0.006 | 45 | 68 | 0.152 | 80 | 37 | 0.248 |
| + | – | – | – | 18 | 15 | 21 | 8 | 15 | 18 | 17 | 15 | 8 | 23 | 18 | 14 | ||||||
| Cathepsin G | |||||||||||||||||||||
| − | – | – | – | – | – | – | 75 | 21 | 0.023 | 81 | 30 | 0.035 | 84 | 25 | 0.025 | 41 | 69 | 0.925 | 78 | 31 | 0.073 |
| + | – | – | – | – | – | – | 21 | 15 | 25 | 20 | 26 | 18 | 16 | 26 | 22 | 21 | |||||
| Cathepsin K | |||||||||||||||||||||
| − | – | – | – | – | – | – | – | – | – | 68 | 26 | 0.124 | 69 | 23 | 0.747 | 36 | 55 | 0.180 | 66 | 33 | 0.259 |
| + | – | – | – | – | – | – | – | – | – | 21 | 15 | 26 | 10 | 10 | 27 | 22 | 17 | ||||
| Cathepsin L | |||||||||||||||||||||
| − | – | – | – | – | – | – | – | – | – | – | – | – | 78 | 27 | 0.292 | 44 | 58 | 0.032 | 72 | 37 | 0.111 |
| + | – | – | – | – | – | – | – | – | – | – | – | – | 31 | 16 | 12 | 36 | 27 | 24 | |||
| Cathepsin V | |||||||||||||||||||||
| − | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 45 | 62 | 0.059 | 70 | 45 | 0.673 |
| + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 11 | 32 | 24 | 18 | ||
| VEGF | |||||||||||||||||||||
| − | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 47 | 12 | 0.001 |
| + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 54 | 46 | |
L, low expression; H, high expression; VEGF, vascular endothelial growth factor; RECK, reversion-inducing cysteine-rich protein with Kazal motifs.
Association of cathepsins B, D, G, K, L and V as well as RECK and VEGF expression with clinicopathological parameters
The expression of cathepsins B, D, G, K, L and V as well as RECK and VEGF were subsequently evaluated for their associations with the clinicopathological parameters of breast cancer patients. The results demonstrated that cathepsin L expression was associated with advanced tumor stage, while the expression of cathepsins B (P=0.033) and K (P=0.038) was associated with ER expression (P=0.022) (Table II). In addition, cathepsin K expression was found to be associated with PR expression (P=0.022) and it was revealed that RECK expression was associated with ErbB2 expression (P=0.023) as well as lymph node metastasis (P=0.023) (Table II). Furthermore, cathepsin V expression was associated with distant metastasis (P=0.035), while cathepsin D expression was associated with breast cancer metastasis to the chest (P=0.041) (Table II). However, there was no association identified between protein expression and patient age, tumor size, histological grade, menopausal status, bone metastasis, lung metastasis, hepatic metastasis, brain metastasis or opposite breast metastasis (Table II).
Table II.
Association of cathepsins B, D, C, K, L and V as well as RECK and VEGF expression with clinicopathological parameters from breast cancer patients.
| Cathepsin B | Cathepsin D | Cathepsin G | Cathepsin K | Cathepsin L | Cathepsin V | RECK | VEGF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Low | High | Low | High | Low | High | Low | High | Low | High | Low | High | Low | High | Low | High |
| Age | ||||||||||||||||
| <50 | 41 | 37 | 66 | 21 | 68 | 26 | 59 | 19 | 60 | 31 | 63 | 31 | 62 | 35 | 33 | 59 |
| >50 | 36 | 28 | 56 | 12 | 55 | 21 | 44 | 21 | 55 | 20 | 55 | 15 | 46 | 31 | 29 | 44 |
| P-value | 0.661 | 0.327 | 0.997 | 0.292 | 0.304 | 0.103 | 0.573 | 0.611 | ||||||||
| Tumor size | ||||||||||||||||
| <2.0 cm | 21 | 14 | 26 | 5 | 25 | 12 | 26 | 7 | 33 | 6 | 24 | 12 | 12 | 26 | 19 | 18 |
| 2.0–5.0 cm | 42 | 40 | 76 | 22 | 79 | 27 | 56 | 27 | 66 | 36 | 75 | 24 | 38 | 60 | 71 | 38 |
| >5.0 cm | 14 | 11 | 20 | 6 | 19 | 8 | 21 | 6 | 17 | 9 | 19 | 10 | 12 | 17 | 18 | 11 |
| P-value | 0.670 | 0.733 | 0.695 | 0.359 | 0.075 | 0.406 | 0.663 | 0.298 | ||||||||
| Histological grade | ||||||||||||||||
| Grade 1 | 18 | 19 | 31 | 10 | 32 | 12 | 26 | 7 | 32 | 13 | 28 | 15 | 33 | 12 | 18 | 27 |
| Grade 2 | 46 | 38 | 71 | 18 | 69 | 29 | 58 | 30 | 66 | 30 | 68 | 25 | 55 | 46 | 33 | 61 |
| Grade 3 | 13 | 8 | 20 | 5 | 22 | 6 | 19 | 3 | 17 | 8 | 22 | 6 | 20 | 8 | 11 | 15 |
| P-value | 0.615 | 0.852 | 0.694 | 0.099 | 0.950 | 0.435 | 0.051 | 0.739 | ||||||||
| Tumor stage | ||||||||||||||||
| 0/I | 11 | 6 | 14 | 1 | 15 | 4 | 14 | 3 | 18 | 0 | 12 | 6 | 7 | 12 | 9 | 9 |
| IIA/IIB | 42 | 41 | 72 | 23 | 77 | 29 | 59 | 23 | 61 | 39 | 69 | 30 | 35 | 64 | 69 | 37 |
| III/IV | 23 | 16 | 33 | 8 | 29 | 13 | 28 | 13 | 32 | 12 | 34 | 10 | 20 | 25 | 27 | 19 |
| P-value | 0.464 | 0.289 | 0.723 | 0.553 | 0.004 | 0.582 | 0.578 | 0.420 | ||||||||
| ER | ||||||||||||||||
| − | 29 | 14 | 43 | 9 | 44 | 13 | 39 | 8 | 35 | 15 | 42 | 10 | 40 | 17 | 24 | 28 |
| + | 47 | 51 | 78 | 24 | 78 | 34 | 63 | 32 | 79 | 36 | 75 | 36 | 68 | 48 | 37 | 75 |
| P-value | 0.033 | 0.374 | 0.300 | 0.038 | 0.868 | 0.081 | 0.140 | 0.106 | ||||||||
| PR | ||||||||||||||||
| − | 22 | 17 | 46 | 8 | 45 | 15 | 44 | 9 | 36 | 17 | 43 | 13 | 41 | 18 | 23 | 31 |
| + | 54 | 48 | 75 | 25 | 77 | 32 | 58 | 31 | 78 | 34 | 74 | 33 | 67 | 47 | 38 | 72 |
| P-value | 0.712 | 0.142 | 0.545 | 0.022 | 0.824 | 0.304 | 0.168 | 0.316 | ||||||||
| ErbB2 | ||||||||||||||||
| − | 15 | 21 | 35 | 11 | 37 | 14 | 31 | 13 | 37 | 21 | 34 | 14 | 37 | 12 | 21 | 27 |
| + | 61 | 43 | 86 | 21 | 84 | 33 | 72 | 27 | 76 | 39 | 82 | 32 | 70 | 53 | 40 | 75 |
| P-value | 0.078 | 0.550 | 0.920 | 0.835 | 0.233 | 0.888 | 0.023 | 0.281 | ||||||||
| Lymph node | ||||||||||||||||
| Negative | 35 | 25 | 49 | 13 | 58 | 15 | 44 | 14 | 50 | 19 | 46 | 24 | 39 | 35 | 24 | 45 |
| Positive | 39 | 39 | 70 | 20 | 63 | 31 | 55 | 26 | 62 | 31 | 70 | 21 | 67 | 29 | 36 | 56 |
| P-value | 0.330 | 0.854 | 0.074 | 0.307 | 0.430 | 0.116 | 0.023 | 0.572 | ||||||||
| Menopausal status | ||||||||||||||||
| Pre-menopausal | 43 | 40 | 67 | 23 | 74 | 27 | 61 | 21 | 63 | 34 | 66 | 33 | 66 | 36 | 33 | 63 |
| Post-menopausal | 33 | 25 | 55 | 10 | 49 | 20 | 42 | 19 | 52 | 17 | 52 | 13 | 41 | 30 | 29 | 39 |
| P-value | 0.551 | 0.127 | 0.747 | 0.466 | 0.152 | 0.063 | 0.354 | 0.282 | ||||||||
| Distant metastasis | ||||||||||||||||
| − | 56 | 39 | 71 | 23 | 79 | 28 | 58 | 25 | 73 | 33 | 69 | 35 | 39 | 68 | 69 | 42 |
| + | 21 | 26 | 51 | 10 | 44 | 19 | 45 | 15 | 42 | 18 | 49 | 11 | 23 | 35 | 39 | 24 |
| P-value | 0.108 | 0.230 | 0.574 | 0.501 | 0.829 | 0.035 | 0.685 | 0.973 | ||||||||
| Bone metastasis | ||||||||||||||||
| − | 62 | 50 | 94 | 27 | 96 | 37 | 75 | 32 | 87 | 44 | 89 | 39 | 50 | 82 | 88 | 50 |
| + | 15 | 15 | 28 | 6 | 27 | 10 | 28 | 8 | 28 | 7 | 29 | 7 | 12 | 21 | 20 | 16 |
| P-value | 0.601 | 0.557 | 0.924 | 0.374 | 0.122 | 0.193 | 0.872 | 0.366 | ||||||||
| Lung metastasis | ||||||||||||||||
| − | 69 | 52 | 99 | 28 | 103 | 39 | 82 | 34 | 97 | 42 | 95 | 41 | 51 | 89 | 91 | 54 |
| + | 8 | 13 | 23 | 5 | 20 | 8 | 21 | 6 | 18 | 9 | 23 | 5 | 11 | 14 | 17 | 12 |
| P-value | 0.108 | 0.624 | 0.905 | 0.460 | 0.748 | 0.817 | 0.472 | 0.675 | ||||||||
| Hepatic metastasis | ||||||||||||||||
| − | 71 | 55 | 106 | 31 | 109 | 42 | 88 | 36 | 104 | 45 | 103 | 43 | 57 | 91 | 100 | 56 |
| + | 6 | 10 | 16 | 2 | 14 | 5 | 15 | 4 | 11 | 6 | 15 | 3 | 5 | 12 | 8 | 10 |
| P-value | 0.154 | 0.262 | 0.891 | 0.471 | 0.666 | 0.255 | 0.463 | 0.104 | ||||||||
| Brain metastasis | ||||||||||||||||
| − | 77 | 65 | 120 | 33 | 120 | 47 | 100 | 40 | 113 | 51 | 116 | 46 | 62 | 101 | 109 | 64 |
| + | 1 | 0 | 2 | 0 | 3 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 2 |
| P-value | 0.357 | 0.459 | 0.280 | 0.275 | 0.343 | 0.374 | 0.270 | 0.069 | ||||||||
| Chest metastasis | ||||||||||||||||
| − | 74 | 58 | 108 | 141 | 111 | 44 | 91 | 37 | 104 | 48 | 107 | 43 | 56 | 94 | 98 | 61 |
| + | 3 | 7 | 14 | 0 | 12 | 3 | 12 | 3 | 11 | 3 | 11 | 3 | 6 | 9 | 10 | 5 |
| P-value | 0.111 | 0.041 | 0.488 | 0.467 | 0.431 | 0.564 | 0.839 | 0.701 | ||||||||
| Opposite breast | ||||||||||||||||
| − | 77 | 63 | 117 | 32 | 119 | 45 | 99 | 39 | 111 | 49 | 113 | 46 | 60 | 99 | 103 | 65 |
| + | 0 | 2 | 5 | 1 | 4 | 2 | 4 | 1 | 4 | 2 | 5 | 01 | 2 | 4 | 5 | 1 |
| P-value | 0.121 | 0.778 | 0.751 | 0.686 | 0.888 | 0.156 | 0.827 | 0.275 | ||||||||
VEGF, vascular endothelial growth factor; RECK, reversion-inducing cysteine-rich protein with Kazal motifs; ER, estrogen receptor; PR, progesterone receptor; ErbB2, erythroblastic leukemia viral oncogene homolog 2.
Association of cathepsins B, D, G, K, L and V as well as RECK and VEGF expression with the prognosis of breast cancer patients
Associations of the expression of cathepsins B, D, G, K, L and V as well as RECK and VEGF proteins with breast cancer patient prognosis were determined. The results revealed that high expression levels of cathepsin D and B and VEGF were associated with poor disease-free survival in breast cancer patients (Fig. 2). However, the expression of other proteins did not show any association with disease-free survival of these patients (data not shown).
Figure 2.
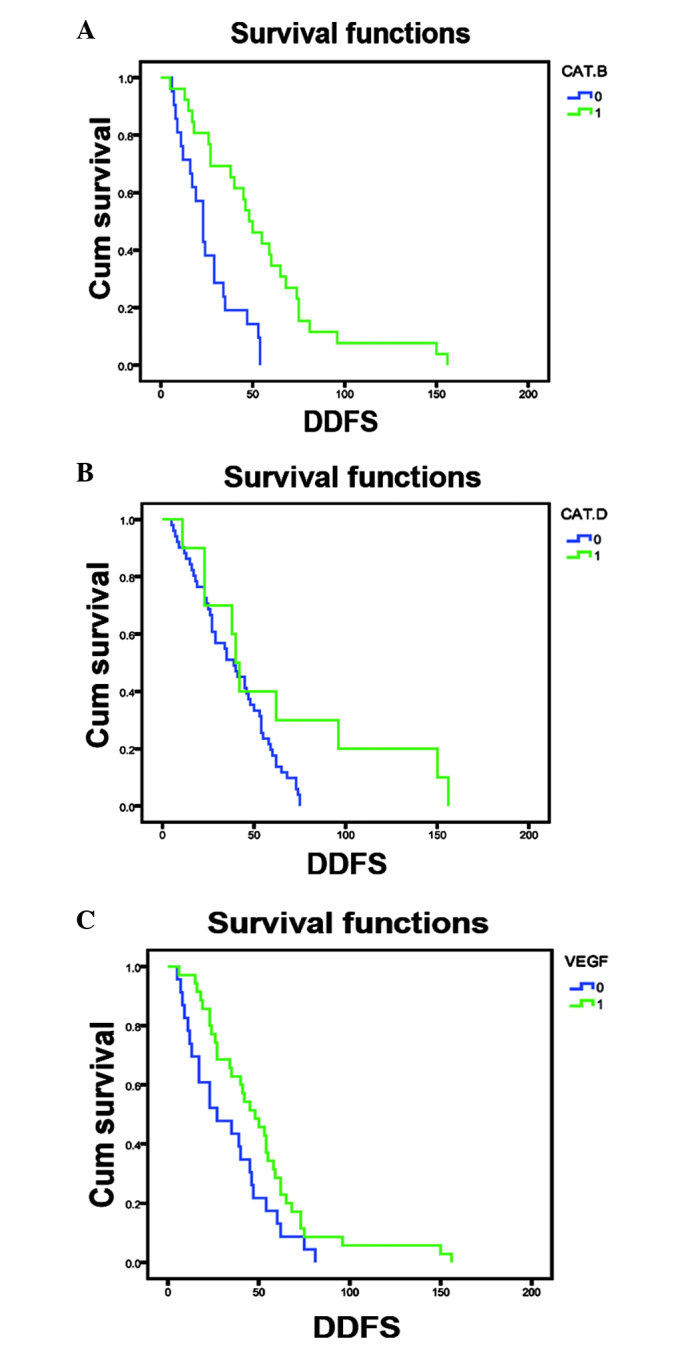
Association of cathepsin B, cathepsin D and VEGF expression in breast cancer tissues with disease-free survival of breast cancer patients. Kaplan-Meier curve of survival of patients according to (A) cathepsin B expression (P<0.001), (B) cathepsin D expression (P=0.050) and (C) VEGF expression (P=0.041). VEGF, vascular endothelial growth factor; Cum survival, cumulative survival; DDFS, distant disease-free survival; Cat., cathepsin; 0, low expression; 1, high expression.
Furthermore, univariate analysis data indicated that breast cancer metastasis to the bone and the expression of cathepsin B proteins were associated with poor disease-free survival. In addition, multivariate analysis demonstrated that PR expression was associated with poor disease-free survival and cathepsin B expression was only marginally associated with poor disease-free survival (P=0.058) (Table III).
Table III.
Cox regression analysis of disease-free survival according to clinicopathological parameters and protein expression.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Exp (B) | 95% CI | P-value | Exp (B) | 95% CI | P-value | |
| pTNM | 1.496 | 0.939–2.384 | 0.090 | 1.652 | 0.816–3.341 | 0.163 |
| Bone metastasis | 1.780 | 1.067–2.970 | 0.027 | 2.069 | 0.930–4.606 | 0.75 |
| Cathepsin B | 0.280 | 0.140–0.561 | 0.000 | 0.471 | 0.216–1.026 | 0.058 |
| ER | 0.625 | 0.370–1.055 | 0.078 | 0.613 | 0.246–1.529 | 0.294 |
| PR | 0.641 | 0.384–1.070 | 0.089 | 0.470 | 0.224–0.985 | 0.046 |
| Cathepsin D | 0.461 | 0.205–1.035 | 0.061 | 0.770 | 0.187–3.165 | 0.717 |
| VEGF | 0.580 | 0.338–0.995 | 0.048 | 0.429 | 0.209–0.882 | 0.021 |
Exp (B), relative risk ratio; CI, confidence interval; pTNM, pathological tumor-node-metastasis; ER, estrogen receptor; PR, progesterone receptor; VEGF, vascular endothelial growth factor.
Discussion
The development of breast cancer involves a progressive multistage process, from premalignant lesion, to ductal carcinoma in situ, to invasive carcinoma and then to metastatic disease (19). Given the variability in clinical progression and its high incidence and mortality rates, the identification of biomarkers that are able to predict tumor behavior may be particularly important in breast cancer (19). Therefore, the current study aimed to detected the expression of different cathepsins as well as RECK and VEGF proteins in breast cancer tissue specimens. The results demonstrated that cathepsin B was expressed in 109/142 (76.76%), cathepsin D in 91/155 (58.71%), cathepsin G in 99/171 (57.89%), cathepsin K in 97/143 (67.83%), cathepsin L in 95/166 (57.23%), cathepsin V in 45/164 (27.44%), RECK in 67/175 (38.29%) and VEGF in 100/164 (60.98%) samples of breast cancer tissues. It was also determined that the expression of cathepsin L was associated with advanced tumor stage, the expression of cathepsins B and K was associated with positive ER expression and that of cathepsin K was associated with PR expression. In addition, the expression of cathepsins V and D was associated with breast cancer metastasis. Furthermore, the expression of cathepsins B and D as well as that of VEGF was associated with poor disease-free survival in breast cancer patients. Univariate analysis revealed that breast cancer metastasis to the bone as well as the expression of cathepsin B and VEGF proteins were associated with poor disease-free survival. In addition, multivariate analysis demonstrated that PR and VEGF expression were significantly associated with poor disease-free survival of the patients, while cathepsin B expression was only marginally associated with poor disease-free survival. Therefore, the present results indicated that targeting cathepsin B and D may have a potential therapeutic benefit for breast cancer patients.
Cathepsins are a superfamily of proteins that are highly expressed in various types of human cancers and have been associated with cancer metastasis (6). Each cathepsin member has relatively different functions and thus, has a different role under normal as well as disease conditions. In cancer, cathepsins have been reported to have various functions, including the regulation of tissue remodeling, cell proliferation and angiogenesis, as well as cancer progression and metastasis (6). Several members of the cathepsin family have previously been studied in breast cancer (31). The results of the present study indicated that the expression of cathepsin V was associated with distant breast cancer metastasis and expression of cathepsin D was associated with breast cancer metastasis to the chest. These results were consistent with those of previously published studies suggesting that members of the cathepsin family were highly expressed in metastatic tumors (10,11,14,19). Cathepsin D protein has been reported to be overexpressed in breast cancer and hyper-secreted by epithelial breast cancer cells (21,32,33). Other previous studies have suggested that cathepsin D expression may be an independent predictor for poor prognosis of breast cancer patients as cathepsin D expression was associated with a high incidence of cancer metastasis (20,34). Consistent with these previous findings, the current study also demonstrated that high expression of cathepsin D protein was associated with poor prognosis of breast cancer patients; however, multivariate analysis failed to indicate that this was an independent predictor of disease-free survival. In addition, numerous studies have suggested that cathepsin B overexpression was associated with the invasive and metastatic phenotypes of various cancers (15,35). However, the role of cathepsin B in breast cancer remains to be fully elucidated (23,36). In the current study, expression of cathepsin B showed a trend (P=0.058) to independently predict breast cancer disease-free survival; however, this results was not statistically significant. Thus, further studies are required with a larger sample size in order to verify the role of cathepsins in breast cancer and their potential use as biomarkers for disease progression.
Numerous previous efforts have been made to develop specific inhibitors to target the activity of each cathepsin family member (26,37,38). For example, intraperitoneal administration of a highly selective cathepsin B inhibitor, such as CA-074, was reported to reduce the metastatic potential of breast cancer cells in nude mice (39). In addition, inhibitors of the other cysteine cathepsins, including S, L, C and K, are also available (40).
In conclusion, the present study demonstrated that the altered expression of cathepsins, in particular cathepsins B and D, was associated with breast cancer progression and poor disease-free survival. However, future studies are required in order to investigate whether the targeting of these two cathepsins, B and D, may have potential therapeutic use for attenuating the progression of breast cancer.
Acknowledgements
This study was supported by the Cooperation Project of China and Slovenia (grant no. 10–15 to Tao Sun).
Glossary
Abbreviations
- ER
estrogen receptor
- PR
progesterone-receptor
- RECK
reversion-inducing cysteine-rich protein with Kazal motifs
- VEGF
vascular endothelial growth factor
References
- 1.Figueroa-Magalhaes MC, Jelovac D, Connolly RM, Wolff AC. Treatment of HER2-positive breast cancer. Breast. 2014;23:128–136. doi: 10.1016/j.breast.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herold CI, Anders CK. New targets for triple-negative breast cancer. Oncology (Williston Park) 2013;27:846–854. [PubMed] [Google Scholar]
- 3.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 4.Turk B, Turk D, Turk V. Lysosomal cysteine proteases: More than scavengers. Biochim Biophys Acta. 2000;1477:98–111. doi: 10.1016/S0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- 5.López-Otín C, Overall CM. Protease degradomics: A new challenge for proteomics. Nat Rev Mol Cell Biol. 2002;3:509–519. doi: 10.1038/nrm858. [DOI] [PubMed] [Google Scholar]
- 6.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–64. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 7.Chen JM, Dando PM, Rawlings ND, et al. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem. 1997;272:8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- 8.Chen JM, Rawlings ND, Stevens RA, Barrett AJ. Identification of the active site of legumain links it to caspases, clostripain and gingipains in a new clan of cysteine endopeptidases. FEBS Lett. 1998;441:361–365. doi: 10.1016/S0014-5793(98)01574-9. [DOI] [PubMed] [Google Scholar]
- 9.Im E, Kazlauskas A. The role of cathepsins in ocular physiology and pathology. Exp Eye Res. 2007;84:383–388. doi: 10.1016/j.exer.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Tan GJ, Peng ZK, Lu JP, Tang FQ. Cathepsins mediate tumor metastasis. World J Biol Chem. 2013;4:91–101. doi: 10.4331/wjbc.v4.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed MM, Sloane BF. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 12.Jedeszko C, Sloane BF. Cysteine cathepsins in human cancer. Biol Chem. 2004;385:1017–1027. doi: 10.1515/BC.2004.132. [DOI] [PubMed] [Google Scholar]
- 13.Krepela E. Cysteine proteinases in tumor cell growth and apoptosis. Neoplasma. 2001;48:332–349. [PubMed] [Google Scholar]
- 14.Joyce JA, Hanahan D. Multiple roles for cysteine cathepsins in cancer. Cell Cycle. 2004;3:1516–1619. doi: 10.4161/cc.3.12.1289. [DOI] [PubMed] [Google Scholar]
- 15.Gondi CS, Rao JS. Cathepsin B as a cancer target. Expert Opin Ther Targets. 2013;17:281–291. doi: 10.1517/14728222.2013.740461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frlan R, Gobec S. Inhibitors of cathepsin B. Curr Med Chem. 2006;13:2309–2327. doi: 10.2174/092986706777935122. [DOI] [PubMed] [Google Scholar]
- 17.Masson O, Bach AS, Derocq D, et al. Pathophysiological functions of cathepsin D: Targeting its catalytic activity versus its protein binding activity? Biochimie. 2010;92:1635–1643. doi: 10.1016/j.biochi.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Benes P, Vetvicka V, Fusek M. Cathepsin D-many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clezardin P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011;13:207. doi: 10.1186/bcr2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Liu Z, Chen S, Liu Y, Shao Z. A prognostic model for triple-negative breast cancer patients based on node status, cathepsin-D and Ki-67 index. PLoS One. 2013;8:e83081. doi: 10.1371/journal.pone.0083081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dian D, Heublein S, Wiest I, et al. Significance of the tumor protease cathepsin D for the biology of breast cancer. Histol Histopathol. 2014;29:433–438. doi: 10.14670/HH-29.10.433. [DOI] [PubMed] [Google Scholar]
- 22.Kawakubo T, Yasukochi A, Toyama T, et al. Repression of cathepsin E expression increases the risk of mammary carcinogenesis and links to poor prognosis in breast cancer. Carcinogenesis. 2014;35:714–726. doi: 10.1093/carcin/bgt373. [DOI] [PubMed] [Google Scholar]
- 23.Bengsch F, Buck A, Günther SC, et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene. 2014;33:4474–4484. doi: 10.1038/onc.2013.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteva FJ, Hortobagyi GN. Prognostic molecular markers in early breast cancer. Breast Cancer Res. 2004;6:109–118. doi: 10.1186/bcr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rochefort H, Garcia M, Glondu M, et al. Cathepsin D in breast cancer: Mechanisms and clinical applications, a 1999 overview. Clin Chim Acta. 2000;291:157–170. doi: 10.1016/S0009-8981(99)00226-0. [DOI] [PubMed] [Google Scholar]
- 26.Tomoo K. Development of cathepsin inhibitors and structure-based design of cathepsin B-specific inhibitor. Curr Top Med Chem. 2010;10:696–707. doi: 10.2174/156802610791113441. [DOI] [PubMed] [Google Scholar]
- 27.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashida S, Kominami E, Katunuma N. Inhibitions of cathepsin B and cathepsin L by E-64 in vivo. II. Incorporation of [3H] E-64 into rat liver lysosomes in vivo. J Biochem. 1982;91:1373–1380. doi: 10.1093/oxfordjournals.jbchem.a133825. [DOI] [PubMed] [Google Scholar]
- 29.Hara K, Kominami E, Katunuma N. Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Lett. 1988;231:229–231. doi: 10.1016/0014-5793(88)80737-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsukuba T, Okamoto K, Yasuda Y, et al. New functional aspects of cathepsin D and cathepsin E. Mol Cells. 2000;10:601–611. doi: 10.1007/s10059-000-0601-8. [DOI] [PubMed] [Google Scholar]
- 31.Garcia M, Platet N, Liaudet E, et al. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells. 1996;14:642–650. doi: 10.1002/stem.140642. [DOI] [PubMed] [Google Scholar]
- 32.Vetvicka V, Fusek M. Procathepsin D as a tumor marker, anti-cancer drug or screening agent. Anticancer Agents Med Chem. 2012;12:172–175. doi: 10.2174/187152012799014904. [DOI] [PubMed] [Google Scholar]
- 33.Nicotra G, Castino R, Follo C, Peracchio C, Valente G, Isidoro C. The dilemma: Does tissue expression of cathepsin D reflect tumor malignancy? The question: Does the assay truly mirror cathepsin D mis-function in the tumor? Cancer Biomark. 2010;7:47–64. doi: 10.3233/CBM-2010-0143. [DOI] [PubMed] [Google Scholar]
- 34.Markićević M, Kanjer K, Mandušić V, et al. Cathepsin D as an indicator of clinical outcome in early breast carcinoma during the first 3 years of follow-up. Biomark Med. 2013;7:747–758. doi: 10.2217/bmm.13.62. [DOI] [PubMed] [Google Scholar]
- 35.Zhong YJ, Shao LH, Li Y. Cathepsin B-cleavable doxorubicin prodrugs for targeted cancer therapy (Review) Int J Oncol. 2013;42:373–383. doi: 10.3892/ijo.2012.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nouh MA, Mohamed MM, El-Shinawi M, et al. A potential prognostic marker for inflammatory breast cancer. J Transl Med. 2011;9:1. doi: 10.1186/1479-5876-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deaton DN, Tavares FX. Design of cathepsin K inhibitors for osteoporosis. Curr Top Med Chem. 2005;5:1639–1675. doi: 10.2174/156802605775009676. [DOI] [PubMed] [Google Scholar]
- 38.Hoegl L, Korting HC, Klebe G. Inhibitors of aspartic proteases in human diseases: Molecular modeling comes of age. Pharmazie. 1999;54:319–329. [PubMed] [Google Scholar]
- 39.Withana NP, Blum G, Sameni M, et al. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 2012;72:1199–1209. doi: 10.1158/0008-5472.CAN-11-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frizler M, Stirnberg M, Sisay MT, Gutschow M. Development of nitrile-based peptidic inhibitors of cysteine cathepsins. Curr Top Med Chem. 2010;10:294–322. doi: 10.2174/156802610790725452. [DOI] [PubMed] [Google Scholar]