Abstract
The present study aimed to retrospectively review the contribution of 18F-fluorodeoxygluose-positron emission tomography/computed tomography (18F-FDG PET/CT) in the assessment of biochemical recurrence in patients with a diagnosis of local-stage prostate cancer (PCa) who underwent radical prostatectomy (RP) or received external beam radiation therapy (EBRT). A total of 28 patients who underwent RP or received EBRT for PCa between July 2007 and April 2013, and who underwent 18F-FDG PET/CT scanning for re-staging due to biochemical recurrence were included in the present study. The mean age of the patients was 65.07 years and the standard deviation was 7.51 years (range, 51–82 years). Of the 28 patients, 23 (82.1%) underwent RP and 5 (17.9%) received definitive EBRT. Prior to scanning, all patients were required to fast for 6 h, and ~1 h after the intravenous injection of 555 MBq 18F-FDG, whole-body PET scans were performed from the skull base to the upper thighs. Whole-body CT scans were performed in the craniocaudal direction. 18F-FDG PET images were reconstructed using CT data for attenuation correction. Histopathology examination or clinical follow-up was used to confirm any suspicious recurrent or metastatic lesions. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 18F-FDG PET/CT were 61.6, 75.0, 61.6, 75.0 and 71.4%, respectively. 18F-FDG PET/CT can detect local and distant metastases with a high accuracy in the assessment of biochemical recurrence, thus detecting occult metastases and allowing the re-staging of PCa in the patients receiving definitive treatment. It is considered that 18F-FDG PET/CT may be useful in re-assessing the patients with PCa receiving definitive treatment.
Keywords: prostate cancer, positron emission tomography/computed tomography, restaging, occult metastases, biochemical recurrence
Introduction
Prostatic cancer (PCa) is currently being viewed as one of the most significant health problems experienced by the male population. PCa is the most frequently occurring solid neoplasm in men in Europe. With an incidence rate of 214 cases per 1,000 individuals, PCa cases outnumber lung and colorectal cancer cases. PCa accounts for 11% of all male cancers and 9% of cancer-associated mortalities, meaning that it ranks second in the cause of cancer-associated mortalities (1,2). The standard reference for the detection of PCa is the digital rectal examination (DRE). The common histology for PCa is adenocarcinoma. In 2005, the International Society of Urological Pathology (ISUP) determined applicable criteria for the Gleason score in the classification of PCa through core biopsy and surgical specimens for diagnosis of PCa. Ranging between 2 and 10, with a score of 2 being the least aggressive and 10 being the most aggressive, the Gleason score is calculated as the sum of the two most typical patterns (grades 1–5) of tumor growth observed. Furthermore, any pattern forming the score should be present at a rate of at least 5% in order to score the tumor specimen (3). The factors determining the risk of the development of clinical PCa are not known exactly. The clearest risk factor appears to be inheritance (4). The rate of autopsy-detected PCa is almost the same all over the world (4). Besides genetic risk factor, it is believed that external factors are also effective in the progression of latent PCa to clinical PCa and that they may be among the risk factors. These external factors are considered to include foods, sexual behavior patterns, consumption of alcohol, ultraviolet light exposure and chronic inflammation, and are believed to be significant in the disease etiology (5). The mortality trends of PCa differ widely between countries in the industrialized World (6). There is no evidence to suggest that prostate-specific antigen (PSA) screening reduces disease-specific mortality, although the disease-specific mortality rate has decreased (7). The age limit recommended for the first PSA screening is 40 years old. Re-screening is not recommended for 8 years if the PSA value detected in the first screening is <1 ng/ml. Furthermore, PSA screening is not recommended after the age of 75, as it has no clinical efficiency after that age (8). PSA is a serine protease of the kallikrein group, and is produced only by prostatic epithelial cells. PSA is organ-specific, but not disease-specific. A diagnosis of PCa is made in 34% of patients with PSA values ranging between 3 and 6 ng/ml in their 7-year cumulative follow-up, while this value has been found to be 44 and 71% for those individuals with PSA values of 6–10 ng/ml and >10 ng/ml, respectively. The disease is diagnosed by transrectal ultrasound (US)-guided biopsy and staged by DRE, PSA and bone scanning. The role of magnetic resonance imaging (MRI) and computed tomography (CT) in staging is controversial. The diagnostic tool of choice for the locally advanced stage of PCa is MRI (9). Men with localized PCa are treated with curative intent, however, a number will eventually develop biochemical recurrence and disease metastasis (10).
There is axial bone metastases in 85% of patients who succumb to PCa. Bone scanning is used for detection of patients with bone metastases, but its false-positive rate is high. Bone metastases are very common in PCa, with th majority being sclerotic in nature. Lytic bone metastases are extremely rare in PCa. 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT has low sensitivity in the detection of the sclerotic metastatic lesions of PCa. However, in the detection of lytic PCa metastasis, 18F-FDG PET/CT is useful (11). 18F- or 11C-choline, 18F- or 11C-acetate, the synthetic L-leucine analog anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, 16β-18F-fluoro-5α-dihydrotestosterone targeted to the androgen receptor, and prostate-specific membrane antigen-based PET radiotracers are used for detecting PSA elevations following definitive treatment of metastatic PCa and hormone-refractory disease (12,13). An accumulation of evidence currently strongly suggests that 18F-FDG PET/CT may aid in the evaluation of imaging for men with metastatic PCa (14).
The main objective of the present study was to determine the role of 18F-FDG PET/CT in the re-staging of patients who received external beam radiation therapy (EBRT) or underwent radical prostatectomy (RP) for local-stage PCa (T1c, T2a-b or T3) with PSA relapse. Conventional methods are insufficient, as bone scanning has a high false-positivity rate in detecting the location of the metastases and bone metastases. 18F-FDG PET/CT may be useful due to the high sensitivity in the lytic bone lesions, the association of the biochemical recurrence with clinical disease and the possibility of early diagnosis. This increases the importance of 18F-FDG PET/CT in the verification of PSA relapse. Histology of the lesions (if available), or the clinical, laboratory and radiological investigations (PSA, bone scanning and MRI) were all used as reference standards.
Patients and methods
Patients
A total of 7,938 patients were assessed in the Department of Nuclear Medicine, School of Medicine, Sifa University (Izmir, Turkey) between July 2007 and April 2013, and a total of 10,553 18F-FDG PET/CT investigations were performed. From these patients, 28 who received RP or EBRT as definitive treatment for the diagnosis of local-stage prostatic adenocarcinoma were included in the present study. In this group of patients, 18F-FDG PET/CT was performed for re-staging upon development of PSA relapse following RP in 23 (82.1%) patients and following definitive EBRT in 5 (17.9%).
The mean age of the patients was 65.07 years and the standard deviation was 7.51 years (range, 51–82 years). The patients were in stages T1c, T2 and T3, defined as local stages according to the European Association of Urology's prostate cancer classification system (15). Based on Gleason's prostatic adenocarcinoma scoring system of the European Association of Urology and the ISUP (3), the Gleason score was <7 in 17 (60.7%) patients and ≥7 in 11 patients (39.3%). All patients underwent 18F-FDG PET/CT scanning for re-staging due to the suspicion of disease recurrence or biochemical recurrence during routine follow-ups. These patients were reviewed retrospectively and their pathology and 18F-FDG PET/CT results were recorded. Exclusion criteria included a history of any cancer other than PCa, active infection, active inflammatory conditions, poorly controlled diabetes mellitus, a recent or complicated non-healing fracture, and hip or knee arthroplasty.
Definition of PSA relapse
Treatment-specific definitions of PSA relapse by the American Urological Association and the American Society for Therapeutic Radiology and Oncology consensus were applied in those patients who had undergone either RP or EBRT, respectively. The definition by the American Urological Association classifies biochemical failure as an initial serum PSA level of ≥0.2 ng/ml, followed by a second confirmed rise in PSA (16). The definition by the American Society for Therapeutic Radiology and Oncology classifies biochemical failure as a rise in PSA level by ≥2 ng/ml above the nadir.
Imaging and interpretation of data
18F-FDG PET/CT scans were obtained after at least 6 h of fasting and when the blood glucose level was <150 mg/dl. Combined FDG PET/CT was performed using a Siemens HI-REZ biograph 6 (Siemens AG, Munich, Germany), which provides an in-plane spatial resolution of 4.8 mm, an axial field view of 16.2 cm and three-dimensional image acquisition. Prior to scanning, 6 h of fasting was required. At ~1 h after the intravenous injection of 555 MBq 18F-FDG, a whole-body PET scan from the skull base to the upper thighs was performed. The whole-body CT scan was performed in the craniocaudal direction, without intravenous contrast. Immediately after this, PET data were collected in the craniocaudal direction with the arms down. FDG PET images were reconstructed using CT data for attenuation correction.
A visual analysis of the PET scans and a semi-quantitative analysis using the maximum standard uptake value (SUVmax) were performed. The SUV was expressed in terms of body weight (g/ml). All recorded parameters, including the patient's weight (kg), height (cm), radioactivity during injection (MBq), injection start time, residual radioactivity (MBq) post-injection and radioisotope half-life (used as the standard 109.8 min for 18F-FDG), were automatically calculated by the software.
Two physicians who were experienced in nuclear medicine and were blinded to the study independently reviewed the hybrid 18F-FDG PET/CT scans and decided upon a positive or negative result for a primary tumor site. Each area of focal tracer uptake deviating from the normal physiological distribution was indicative of the disease. Background deviations and activity differences in the tissues surrounding the suspicious lesion were used to discriminate the benign lesions from the malignant lesions. For the patients receiving EBRT, an SUV value of 5.8 in the prostate was taken as the reference. The patients with an SUV value of >5.8 were considered as positive. No specific SUV value was used for the patients undergoing RP.
Statistical analysis
All statistical data analyses were calculated using SPSS statistics software (version 16.0; SPSS, Inc., Chicago, IL, USA). All statistical data analyses were calculated using Fisher's Exact test. P<0.05 was considered to indicate a statistically significant difference.
Ethics
All procedures followed were in accordance with the ethical standards of the responsible Committee on Human Experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
Results
18F-FDG PET/CT imaging was negative in 16 (57.1%) patients and positive in 12 (42.9%). Local recurrence and bone metastases were found in 2 (7.1%) patients, bone metastases only in 7 (25.0%) patients (mean SUVmax, 9.8), local recurrence only in 1 (3.6%) patient, local recurrence and bone or lymph node metastasis in 1 (3.6%) patient, and recurrence in the prostate in 1 (3.6%) patient. The last patient had received definitive EBRT and the SUVmax value was 21.8. The patients were divided in two groups based on their Gleason scores of <7 or ≥7. The 18F-FDG PET/CT results of the patients with Gleason scores of <7 and ≥7 are shown in Table I. Patients with Gleason scores of >7 on PET/CT exhibit increased visualization. Increased visualization may be a result of increased glucose metabolism, which is associated with cancer tissues. A total of 4 (14.3%) and 7 (28.6%) patients with Gleason scores of <7 and ≥7, respectively, were positive on PET/CT.
Table I.
PET/CT results by Gleason score of the primary tumor.
| Gleason score | Positive on PET/CT, n (%) | Negative on PET/CT, n (%) |
|---|---|---|
| <7 | 4 (14.3) | 13 (46.4) |
| ≥7 | 8 (28.6) | 3 (10.7) |
PET/CT, positron emission tomography/computed tomography.
18F-FDG PET/CT results correlated with histological subtype in probability charts (P=0.0189). The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 18F-FDG PET/CT were 61.6%, 75.0%, 61.6%, 75.0% and 71.4%, respectively.
Discussion
Definitive therapeutic modalities of local-stage PCa have been standardized as RP and EBRT, and the strategy to follow patients treated with RP or EBRT is based on PSA relapse. PSA relapse does not always mean clinical recurrence, as the condition may occasionally be diagnosed as biochemical relapse and the disease will not manifest. Thus, correctly diagnosing patients and monitoring their response to the treatment is of vital importance (16).
Re-staging of the disease, minimizing the false-positive and false-negative results, and diagnosing the local recurrences and metastatic disease are the most important and most realistic focuses of the entire therapeutic strategy (16). For the patients with PSA relapse, it is not always possible to know the anatomical location and volume of the disease, further increasing the importance of anatomical and functional imaging. PET is an ideal non-invasive tool for imaging of the underlying tumor biology. 18F-FDG PET/CT is one of the most important diagnostic tools in which particularly anatomical and functional images are processed. PSA relapse, transrectal US-guided re-biopsy, contrast-enhanced CT, MRI, bone scanning and 18F-FDG PET/CT may be used to detect metastatic disease following RP or EBRT, and to monitor the response to treatment (16).
PSA is an independent prognostic factor in the early diagnosis of PCa, and in determining recurrences and metastases. Locally recurrent cancer is ultimately detected in 25–35% of men with biochemical failure, while metastatic disease is only found in 20-25%, and local recurrence and metastatic disease together is detected in 45–55% (17). When 18F- and 11C-choline were used in patients with PCa with biochemical recurrences in the screening series of patients of European and Japanese origin, a sensitivity ranging between 38 and 98% was found in the detection of local recurrences and metastatic disease.
One of the most significant features of cancer is that the metabolic utilization of glucose in higher in the cancer compared to the normal tissues (Warburg's effect) (18). PET imaging with 18F-FDG, an analog of glucose, tracks the glucose metabolism of tissues. The use of 18F-FDG PET is therefore fundamental to oncology studies (19). Elevated glucose metabolism in malignant tissues due to increased of cellular membrane glucose transporter (GLUT) expression, mainly GLUT-1, and enhanced hexokinase (HK-II) enzymatic activity in tumors, is the basis of cancer detection by 18F-FDG PET (20). The uptake of 18F-FDG in PCa is dependent on the differentiation of the tumor, with low uptake found in well-differentiated tumors and high uptake found in aggressive poorly-differentiated tumors (14). Patients with advanced disease may be evaluated by 18F-FDG PET/CT through the detection of active osseous and soft-tissue metastases. The technique may also be useful for evaluating the hormonal treatment response (16). In an evaluation of the mRNA expression of GLUT1 in hormone-dependent and hormone-independent tissues of PCa, Effert et al found that the hormone-resistant cells with poor prognosis exhibited a much higher level of GLUT1 compared with the well-differentiated hormone-sensitive cells (21). Considering these findings as the basic principle for the use of 18F-FDG PET in PCa, there are other studies in the literature supporting these results. The most important factor in commenting on the activity of FDG in PCa is that the tissue of benign prostatic hyperplasia (BPH) has also measurable FDG activity. Stewart et al (22), however, found that the gene expression of GLUT1 was higher in PCa than in BPH. Kukuk et al (23) demonstrated that there was a large amount of FDG accumulation in the xenograft mouse models of hormone-resistant PCa, and that this FDG activity decreased following androgen ablation. Primarily staging PCa with 18F-FDG PET is quite difficult due to the physiological urinary activity of FDG. False-positive FDG activity in the residual urine, and in the bladder and urethra due to the anatomical neighborhood of these anatomical structures limits the value of this method. Additionally, the false-positive FDG activity of the normal prostatic tissues and BPH also limits the procedure; it also fails in distinguishing low-volume foci of PCa. Thus, 18F-FDG PET/CT has a limited role in the primary staging of PCa. Nonetheless, FDG-PET application has not yet been full developed for PCa (24).
A recent study from Japan used a time-of-flight PET/CT scanner to assess for the visual identification of primary PCa with FDG (25). For the differentiation between biopsy specimens with a summed Gleason score of ≤5 and those specimens with a summed Gleason score of ≤6, the cut-off SUVmax, sensitivity and specificity were 2.8, 61.7 and 80%, respectively. In a study by Minamimoto et al (26), patients with Gleason scores of ≥7 exhibited sensitivity and positive predictive values of 80 and 87%, respectively (26). 18F-FDG PET/CT may be useful in the imaging evaluation of men with biochemical failure following definitive therapy for primary PCa. The main objective of the present study was to assess the efficiency of 18F-FDG PET/CT in re-staging following definitive treatment.
The present study showed that this method was able to detect biochemical recurrence and recurrence at the prostatic location despite its anatomical and physiological disadvantages (Fig. 1). However, we believe that this was due to the high SUVmax value (21.8). The fact that the Gleason score was >7 and the PSA value was 28 ng/ml may have increased the visualization due to the high activity of glucose metabolism. The detection of recurrence in the prostate remains a big problem for 18F-FDG PET/CT in cases of low-volume disease, relapses with low PSA levels and Gleason scores of <6.
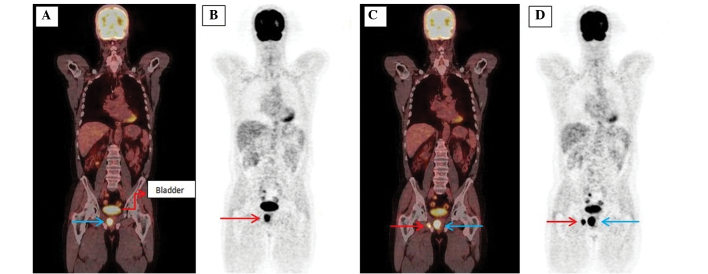
Figure 1.
Images of a 59-year-old male with biochemically recurrent prostate cancer post-external beam radiation therapy (prostate-specific antigen, 28.0 ng/ml; Gleason score, 4+4). (A) The coronal 18F-FDG PET/CT scans show an ill-defined recurrent mass (blue arrow) 4×3 cm in size compressing the adjacent tissues in the right half of the prostate (SUVmax, 21.8). (B) MIP images of a patient with local recurrence (arrow). (C) The coronal 18F-FDG PET/CT scans show local recurrence (blue arrow; SUVmax, 21.8) and metastasis on the right pubic bone (red arrow; SUVmax, 20.6). (D) MIP images of a patient with local recurrence (blue arrow) and metastasis of the pubic bone (red arrow).18F-FDG PET/CT, 18F-fluorodeoxygluose-positron emission tomography/computed tomography; MIP, maximum intensity projection.
In a study by Schöder et al (27) on 91 men with PSA relapse following prostatectomy, the mean PSA levels were higher in the FDG PET-positive patients than in the FDG PET-negative patients (9.5±2.2 ng/ml vs. 2.1±3.3 ng/ml). In the study, either local recurrence or systemic metastases were found in 31% of the patients undergoing FDG-PET due to PSA recurrence. In another study on 24 patients with verification of the pelvic lymph nodes, Chang et al (28) found a sensitivity, specificity, positive predictive value and negative predictive value of 75, 100, 100 and 67.7%, respectively. In the study, it was demonstrated that FDG-PET could detect metastatic disease in ~31% of the patients with PSA recurrence following RP. It was stated that the technique would be more useful when it was used in patients with a PSA level of >2.4 ng/ml or a PSA doubling time of >1.3 ng/ml/year, but that this had to be supported by prospective studies. Jadvar et al found that the method gave usable data in a study which evaluated 18F-NaF and 18F-FDG PET/CT for the detection of occult metastases in patients with the biochemical recurrence of PCa (14). The study consisted of 37 patients (26 undergoing RP and 11 receiving EBRT). A sensitivity, specificity, positive predictive value, negative predictive value and accuracy rate of 50, 82, 64, 73 and 70%, respectively, were found. Combined imaging using simultaneous 18F-FDG and 18F-NaF injection has also been reported. However, there is currently a lack of evidence to support its use in routine clinical practice.
In the current study, a sensitivity and positive predictive value of 61%, a specificity and negative predictive value of 75%, and an accuracy of 71% were found, all being consistent with the values reported in the literature. Within the limitations of the present observational study, these data indicate that PET/CT may be useful for the process of making clinical decisions. PET and PET/CT studies from the literature are summarized in the Table II.
Table II.
Diagnostic performance of PET and PET/CT studies in the literature.
| First author (ref.) | Modality | n | Sensitivity, % | Specifity, % | PPV, % | NPV, % | Accurary, % |
|---|---|---|---|---|---|---|---|
| Chang et al (28) | FDG-PET | 24 | 75 | 100 | 100 | 68 | – |
| Schöder et al (27) | FDG-PET | 91 | |||||
| PSA>2.4a | 80 | 73 | – | – | – | ||
| PSA-DT>1.3b | 71 | 77 | – | – | – | ||
| Jadvar et al (29) | FDG-PET/CT | 37 | 50 | 82 | 64 | 73 | 70 |
| Present study | FDG-PET/CT | 28 | 61 | 75 | 61 | 75 | 71 |
ng/ml;
ng/ml/year. PET, positron emission tomography; CT, computed tomography; FDG, fluorodeoxyglucose; PPV, positive predictive value; NPV, negative predictive value; PSA-DT, prostate-specific antigen doubling time.
18F-FDG PET/CT may be particularly useful in the treatment response evaluation of metastatic PCa. FDG accumulation at metastatic sites tends to decrease with successful chemohormonal therapy (29). There may be differences in imaging-based assessment using various response criteria [e.g., Response Evaluation Criteria in Solid Tumors (RECIST) (30), European Organization for Research and Treatment of Cancer, PET Response Criteria in Solid Tumors and National Oncology PET Registry (31)] and the PSA-based response criteria (32). Hwang et al (33) performed an analysis of 18F-FDG PET/CT images in 12,037 subjects who showed abnormal hypermetabolism in the prostate. While 120 patients exhibited abnormal 18F-FDG PET/CT signaling, 38 of these subsequently underwent investigation by prostate biopsy as a result of an abnormal total PSA serum level and/or the clinical suspicion of cancer upon DRE. Minamimoto et al (34) found positive results indicating the possibility of cancer in 16,955 cases (10.9%). Although only 1,912 cancers were actually detected, PCa was found in 165 patients, with a FDG-PET sensitivity of 37.0%. From this it may be concluded that altered PSA levels may be exhibited in approximately one-third of PCa patients with positive FDG-PET findings. Confounding factors for a decreased PSA level in patients with PCa may include the concomitant use of medical therapies, such as statins, non-steroidal anti-inflammatory drugs and hormones, and/or a higher body mass index (BMI). In the current study, the BMIs of the patients were not known. So, no comparison was made with the literature on this basis. In the study by Wright et al (35), obese males exhibited lower age-adjusted PSA levels compared with those of normal weight. Koochekpour et al (36) also recently demonstrated a possible characterization of PCa cells in association with their metabolism. Glutamate receptor 1 antagonists may alter the metabolism of aggressive cancers, with the subsequent development of the Warburg effect in cases where hypoxia is induced by the tumor. In the study, a significant difference was found in the serum levels of glutamate between the patients with a Gleason score of <7 and those with a score of >8. In the future, an increased and altered glutamate metabolism is being considered as a biological marker for patients with a Gleason score >8, i.e., for those with aggressive PCa. In aggressive PCa, increased glutamate metabolism increases the rate of positive subjects and also affects sensitivity due to increased FDG-PET activity (36). A strong correlation exists between the Warburg effect and the mitochondrial metabolism of the tumor cells, even in the patients with normal PSA values, suggesting that 18F-FDG PET/CT may be used in such patients (37).
Bone metastasis is a potential consequence of progressive PCa. Accurate detection of the bone metastases through imaging-based analysis is absolutely required for effective treatment. Moreover, even though novel bone-targeted therapies are being developed, the current standard diagnostic imaging techniques, including 99mTc-based bone scintigraphy and CT, are not adequate for the accurate measurement of tumor burden. One study has reported an attempt to semi-quantitatively measure bone metastases on 99mTc-based bone scintigraphy (bone scan index). However, the fundamental limitations of bone scintigraphy, including indirect imaging of tumor presence, low specificity (false-positives with benign conditions), and low sensitivity (gross underestimation of the true prevalence of bone metastases), are restrictive, and this technique has not been widely used (38). When considering the inclination of PCa to metastasize to the bones and the limitations of current imaging tools for assessing these bone metastases, the quantitative evaluation a response has proved difficult. Obstacles include the inability to use response criteria, such as RECIST, for bone metastasis assessment by CT, the confounding effect of the flare phenomenon on standard bone scintigraphy, and the ambiguity associated with the clinical significance of serum PSA level changes (39,40). 18F-FDG PET/CT will be particularly useful in PCa patients with lytic skeletal metastasis (11). Although bone metastases are extremely common in PCa, lytic metastases are rare. This type of metastasis probably results from the overproduction of parathyroid hormone-related peptide by PCa cells in vivo (41). 18F-FDG PET is less sensitive than bone scanning for defining sclerotic bone metastases. By contrast, 18F-FDG PET-CT is superior to bone scintigraphy for the detection of lytic PCa metastases. In the present study, osteoblastic bone metastases could be detected by 18F-FDG PET-CT. We believe that this apparent activity could be detected due to an increased Warburg's effect as a result of a high level of PSA, a high tumor volume and a high Gleason score creating a high SUV on the detector (Fig. 2).
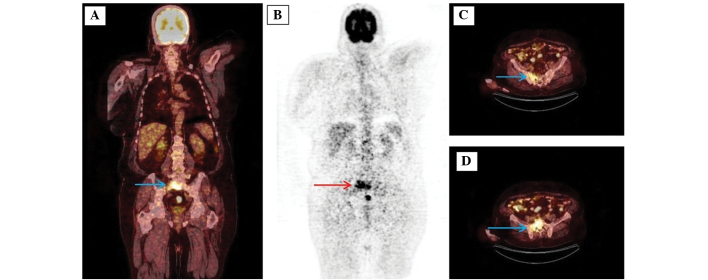
Figure 2.
Images of a 66-year-old male with biochemically recurrent prostate cancer post-radical prostatectomy (prostate-specific antigen, 35.0 ng/ml; Gleason score, 4+5). (A) The coronal 18F-FDG PET/CT scans show osteoblastic sacral metastasis (arrow; SUVmax, 14.4). (B) Maximum intensity projection images of a patient with sacral metastasis (arrow). (C and D) The axial 18F-FDG PET/CT scans show osteoblastic sacral metastasis (arrow; SUVmax, 14.4). 18F-FDG PET/CT, 18F-fluorodeoxygluose-positron emission tomography/computed tomography.
Other studies have also shown a potential prognostic utility for FDG PET, with generally higher tumor SUVs indicating a poorer prognosis than lower SUVs, which is similar to the general experience with other cancer types (42). The prognostic utility of bone scintigraphy and 18F-FDG PET was analyzed in a study by Meirelles et al (43), which evaluated 39 patients with castration-resistant disease and 12 patients that did not require medical or surgical castration. These patients were followed up for a minimum of 5 years or until they succumbed. The SUVmax of the most active bone lesion was used as the outcome measure for the 18F-FDG PET studies. An inverse association was determined between bone scan index and SUVmax. The median survival time of 32.8 months for an SUVmax of <6.10 was significantly longer than the 14.4 months for an SUVmax of >6.10. Moreover, upon multivariate analysis, the SUVmax was found to be an independent prognostic factor (41). In the present study, bone metastases were found in 25.0% (n=7) of the patients. The mean SUVmax of these metastases was 9.8, but no statistical correlation was made as the survival data were inadequate. The 18F-FDG uptake in PCa cells is modulated by androgens (44). The benefits of 18F-FDG PET/CT in monitoring the response to anti-androgen treatment remains controversial. The technique is, however, usually useful in distinguishing the healed lesions from the active metastases (45). It has been demonstrated that PET/CT with 18F-FDG may aid in the direct imaging of metastatic PCa (13). Recently, a study by Jadvar et al indicated the utilization of 18F-FDG PET/CT in castration-resistant metastatic PCa. In the series of 87 patients, a negative correlation was found between the SUV value and disease-specific survival. 18F-FDG PET/CT is a useful imaging biomarker for the prediction of overall survival in men with castration-resistant metastatic PCa (46).
The metabolic rate of PCa with a low Gleason score is similar to that of normal tissues. The increased glucose metabolism in PCa patients with a high PSA level and a high Gleason score allows visualization of the lesions due to an increase in FDG uptake.
False-positive or false-negative FDG uptake results cannot be explained solely by the glucose metabolism of tumor tissue. Studies have demonstrated that 18F-FDG PET/CT scans can provide information only in the presence of a certain increased number of tumor cells with abnormal glucose metabolism (104-107 cells). Such diagnostic failures are particularly significant in metastases of solid organs, such as the lungs and liver. Generally, 18F-FDG PET/CT is unable to accurately evaluate metastases that are <5 mm in diameter. It is not known why high SUVs are not produced in lung lesions below this threshold. Inaccurate evaluation can be caused by motion artifacts and the low metabolic activity of metastatic lesions. Certain techniques can result in a reduction of motion artifacts, which achieves better spatial resolution and determines higher cut-off SUV values for such lesions, thus increasing the accuracy of the diagnostic process (47).
It is essential that the PET/CT findings be verified by histopathological work-up so that disease recurrence can be confirmed. Theoretically, this technique remains the gold standard. Unfortunately, in daily practice, clinical reasons, procedure feasibility and the effective advantages of this approach in the absence of radical surgical intent mean that this is seldom possible. In the present study, confirmation by histological examination was possible in 9 patients, while all others were compared using clinical plus radiological findings.
The main limitation of the present study is its retrospective nature. A certain amount of selection bias may have been present, as it is likely that only those PCa patients with metastatic disease in biochemical recurrence who were suspected to have recurrence were referred for PET/CT.
In the current study, the contribution of 18F-FDG PET/CT in detecting local recurrences and metastases in the patients with biochemical recurrence following RP or EBRT treatment for the diagnosis of local-stage PCa was reviewed retrospectively and found to be consistent with the literature findings. A possible limitation of the study was the lack of histological verification for the lesions in view of constraints imposed by practical, economic and ethical issues. It may be indicated that 18F-FDG PET/CT would be useful in diagnosing biochemical recurrence with a high accuracy (70%) in patients with PCa.
References
- 1.Boyle P, Ferlay J. Cancer incidence and mortality in Europe 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. ISUP grading committee. The 2005 International Society of Urologic Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 4.Hemminki K. Familial risk and familial survival in prostate cancer. World J Urol. 2012;30:143–148. doi: 10.1007/s00345-011-0801-1. [DOI] [PubMed] [Google Scholar]
- 5.Leitzmann MF, Rohrmann S. Risk factors for the onset of prostatic cancer: Age, location and behavioral correlates. Clin Epidemiol. 2012;4:1–11. doi: 10.2147/CLEP.S16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver SE, May MT, Gunnell D. International trends in prostate-cancer mortality in the ‘PSA-ERA’. Int J Cancer. 2001;92:893–898. doi: 10.1002/ijc.1260. [DOI] [PubMed] [Google Scholar]
- 7.Ilic D, O'Connor D, Green S, Wilt T. Screening for prostate cancer: A Cochrane systematic review. Cancer Causes Control. 2007;18:279–285. doi: 10.1007/s10552-006-0087-6. [DOI] [PubMed] [Google Scholar]
- 8.Roobol MJ, Roobol DW, Schröder FH. Is additional testing necessary in men with prostate-specific antigen levels of 1.0 ng/ml or less in a population-based screening setting? (ERSPC, section Rotterdam) Urology. 2005;65:343–346. doi: 10.1016/j.urology.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Heenan SD. Magnetic resonance imaging in prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:282–288. doi: 10.1038/sj.pcan.4500767. [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, Karunanithi S, Singh Dhull V, et al. Prostate cancer with lytic bone metastases: 18F-fluorodeoxyglucose positron emission tomography-computed tomography for diagnosis and monitoring response to medical castration therapy. Indian J Nucl Med. 2013;28:178–179. doi: 10.4103/0972-3919.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49:2031–2041. doi: 10.2967/jnumed.108.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadvar H. Molecular imaging of prostate cancer: PET radiotracers. AJR Am J Roentgenol. 2012;199:278–291. doi: 10.2214/AJR.12.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadvar H. Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317–323. doi: 10.1038/nrurol.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association of Urology. http://uroweb.org/wp-content/uploads/09-Prostate-Cancer_LR.pdf. [Jun 15;2015 ];Guidelines on Prostate Cancer. Accessed. [Google Scholar]
- 16.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American urological association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 17.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al., editors. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; [Dec 02;2013 ]. Accessed. [Google Scholar]
- 18.Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(Suppl 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 20.Smith TA. Mammalian hexokinases and their abnormal expression in cancer. Br J Biomed Sci. 2000;57:170–178. [PubMed] [Google Scholar]
- 21.Effert P, Beniers AJ, Tamimi Y, Handt S, Jakse G. Expression of glucose transporter 1 (GLUT-1) in cell lines and clinical specimen from human prostate adenocarcinoma. Anticancer Res. 2004;24:3057–3063. [PubMed] [Google Scholar]
- 22.Stewart GD, Gray K, Pennington CJ, et al. Analysis of hypoxia-associated gene expression in prostate cancer: Lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol Rep. 2008;20:1561–1567. [PubMed] [Google Scholar]
- 23.Kukuk D, Reischl G, Raguin O, et al. Assessment of PET tracer uptake in hormone-independent and hormone-dependent xenograft prostate cancer mouse models. J Nucl Med. 2011;52:1654–1663. doi: 10.2967/jnumed.110.086702. [DOI] [PubMed] [Google Scholar]
- 24.Jadvar H, Ye W, Groshen S, Conti PS. [F-18]-fl [F-18]-Fluorodeoxyglucose PET-CT of the normal prostate gland. Ann Nucl Med. 2008;22:787–793. doi: 10.1007/s12149-008-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiiba M, Ishihara K, Kimura G, et al. Evaluation of primary prostate cancer using 11C-methionine-PET/CT and 18F-FDG-PET/CT. Ann Nucl Med. 2012;26:138–145. doi: 10.1007/s12149-011-0551-6. [DOI] [PubMed] [Google Scholar]
- 26.Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, Shizukuishi K, Tateishi U, Kubota Y, Inoue T. The potential of FDG PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–27. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 27.Schöder H, Hermann K, Gönen M, Hricak H, Eberhard S, Scardino P, Scher HI, Larson SM. 2-[18F] fluoro-2-deoxyglucose positron emission tomography for detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11:4761–4769. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 28.Chang CH, Wu HC, Tsai JJ, et al. Detecting metastatic pelvic lymph nodes by 18F-2-deoxyglucose positron emission tomography in patients with prostate specific antigen relapse after treatment for localized prostate cancer. Urol Int. 2003;70:311–315. doi: 10.1159/000070141. [DOI] [PubMed] [Google Scholar]
- 29.Jadvar H, Desai B, Quinn D. Treatment response assessment of metastatic prostate cancer with FDG PET/CT. J Nucl Med. 2011;52(Suppl 1):431P. [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: Findings of the national oncologic PET registry. J Nucl Med. 2008;49:1928–1935. doi: 10.2967/jnumed.108.056713. [DOI] [PubMed] [Google Scholar]
- 33.Hwang I, Chong A, Jung SI, et al. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med. 2013;27:140–145. doi: 10.1007/s12149-012-0663-7. [DOI] [PubMed] [Google Scholar]
- 34.Minamimoto R, Senda M, Jinnouchi S, et al. The current status of an FDG-PET cancer screening program in Japan, based on a 4-year (2006–2009) nationwide survey. Ann Nucl Med. 2013;27:46–57. doi: 10.1007/s12149-012-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JL, Lin DW, Stanford JL. The effect of demographic and clinical factors on the relationship between BMI and PSA levels. Prostate. 2011;71:1631–1637. doi: 10.1002/pros.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koochekpour S, Majumdar S, Azabdaftari G, et al. Serum glutamate levels correlate with Gleason score and glutamate blockade decreases proliferation, migration and invasion and induces apoptosis in prostate cancer cells. Clin Cancer Res. 2012;18:5888–5901. doi: 10.1158/1078-0432.CCR-12-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartoletti R, Meliani E, Bongini A, Magno C, Cai T. Fluorodeoxyglucose positron emission tomography may aid the diagnosis of aggressive primary prostate cancer: A case series study. Oncol Lett. 2014;7:381–386. doi: 10.3892/ol.2013.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tombal B, Lecouvet F. Modern detection of prostate cancer's bone metastasis: Is the Bone Scan Era Over? Adv Urol. 2012;2012:893193. doi: 10.1155/2012/893193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulders PF, Schalken JA. Measuring therapeutic efficacy in the changing paradigm of castrate-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2009;12:241–246. doi: 10.1038/pcan.2009.25. [DOI] [PubMed] [Google Scholar]
- 40.Ryan CJ, Shah S, Efstathiou E, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabbani SA, Gladu J, Harakidas P, Jamison B, Goltzman D. Over-production of parathyroid hormone-related peptide results in increased osteolytic skeletal metastasis by prostate cancer cells in vivo. Int J Cancer. 1999;80:257–264. doi: 10.1002/(SICI)1097-0215(19990118)80:2<257::AID-IJC15>3.3.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Oyama N, Akino H, Suzuki Y, et al. Prognostic value of 2-deoxy-2-[F-18] fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99–104. doi: 10.1016/S1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 43.Meirelles GS, Schöder H, Ravizzini GC, et al. Prognostic value of baseline [18F] fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing prostate cancer. Clin Cancer Res. 2010;16:6093–6099. doi: 10.1158/1078-0432.CCR-10-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jadvar H, Xiankui L, Shahinian A, et al. Glucose metabolism of human prostate cancer mouse xenografts. Mol Imaging. 2005;4:91–97. doi: 10.1162/15353500200505118. [DOI] [PubMed] [Google Scholar]
- 45.Morris MJ, Akhurst T, Osman I, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–918. doi: 10.1016/S0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 46.Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, Pinski JK, Quinn DI. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Fakhri G, Surti S, Trott CM, Scheuermann J, Karp JS. Improvement in lesion detection with whole-body oncologic time-of-flight PET. J Nucl Med. 2011;52:347–353. doi: 10.2967/jnumed.110.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]