Abstract
Melittin, a significant constituent of Apis mellifera (honeybee) venom, is a water-soluble toxic peptide that has traditionally been used as an antitumor agent. However, the underlying mechanisms by which it inhibits tumor cell growth and angiogenesis remain to be elucidated. In the present study, screening for increased cathepsin S (Cat S) expression levels was performed in MHCC97-H cells and various other hepatocellular carcinoma cell lines by reverse transcription-polymerase chain reaction and western blot analysis. A pcDNA3.1-small hairpin RNA (shRNA)-Cat S vector was stably transfected into MHCC97-H cells (shRNA/MHCC97-H) in order to knockdown the expression of Cat S. The effects resulting from the inhibition of Cat S-induced proliferation, invasion and angiogenesis by melittin were examined using cell proliferation, cell viability, flat plate colony formation, migration, wound healing, Transwell migration and ELISA assays. In order to substantiate the evidence for melittin-mediated inhibition of Cat S-induced angiogenesis, Cat S RNA was transfected into primary human umbilical vein endothelial cells (Cat S-HUVECs) to induce overexpression of the Cat S gene. The effects of melittin on HUVECs were examined using Transwell migration and tube formation assays. The findings demonstrated that melittin was able to significantly suppress MHCC97-H cell (Mock/MHCC97-H) proliferation, invasion and angiogenesis, as well as capillary tube formation of Cat S-HUVECs, in a dose-dependent manner. However, proliferation, invasion and angiogenesis in shRNA/MHCC97-H and in native HUVECs (Mock-HUVECs) were unaffected. In addition, melittin specifically decreased the expression of phosphorylated (activated) Cat S, and components of the vascular endothelial growth factor (VEGF)-A/VEGF receptor 2 (VEGFR-2)/mitogen-activated protein kinase kinase 1 (MEK1)/extracellular signal-regulated kinase (ERK)1/2 signaling pathway in Mock/MHCC97-H cells. In conclusion, the inhibition of tumor cell growth and anti-angiogenic activity exerted by melittin may be associated with anti-Cat S actions, via the inhibition of VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling.
Keywords: melittin, cathepsin S, human hepatocellular carcinoma, invasion, angiogenesis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and the third leading cause of cancer-associated mortality globally (1,2). Despite significant improvements in surgical and drug treatments, the overall 5-year survival rate of HCC patients remains low (3–7); this is likely due to the high rate of recurrence and metastasis following curative resection and liver transplantation (8,9). It has been demonstrated that HCC metastasis is a multi-step process, involving invasion and degradation of extracellular matrix (ECM) proteins (10,11), translocation through the vasculature, subsequent migration to secondary sites and, finally, formation of metastatic nodules in future metastatic sites (12). Angiogenesis is reported to be a significant factor in the proliferation and migration of HCC (13). Although progress has been made in this field, more detailed research is required to investigate the mechanisms underlying HCC angiogenesis and metastasis.
Cathepsin S (Cat S), a member of the lysosomal cysteine cathepsin family, has been observed to play a significant role in cell proliferation, angiogenesis and metastasis (14). As a cysteine protease, Cat S is primarily localized in lysosomes that are able to retain proteolytic activity at a neutral pH (15). It has been demonstrated that Cat S is capable of being translocated from the lysosome to the cell surface and is ultimately released into the extracellular space (16,17). Furthermore, increased Cat S expression and activity has been associated with a number of malignancies, including HCC (18), pancreatic (14), breast (19) and prostate cancers (20). Angiogenesis has been identified to be associated with the growth and metastasis of human tumors (21). In addition, previous studies have indicated the significance of Cat S in tumor angiogenesis (17). Therefore, Cat S has become a compound of interest (18,22) and has been suggested as a potential therapeutic target for the suppression of tumor angiogenesis (18).
A previous study reported that vascular endothelial growth factor (VEGF) is essential for endothelial cells and plays a significant role in angiogenesis, tumor progression and vascular permeability (23). VEGF receptor 2 (VEGFR-2) is regarded as the most biologically important receptor for VEGF (24). VEGF has been observed to be frequently expressed in HCC (25), and is important in HCC proliferation and migration (26). Additional evidence has revealed that the VEGF-A/VEGFR-2/mitogen-activated protein kinase 1 (MEK1)/extracellular signal-regulated kinase (ERK)1/2 signaling pathway plays a central role in human cancer (27). However, the role of the VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway in HCC proliferation, invasion and angiogenesis is complex and remains to be elucidated.
Melittin is a water-soluble toxic peptide, produced by the honeybee, Apis mellifera. It is a small amphipathic peptide composed of 26 amino acids, with antitumor and antibacterial characteristics (28–33). Evidence has accumulated indicating that melittin is capable of causing growth arrest and exerting cytotoxic effects in HCC (34–36). It has been demonstrated that melittin is a significant factor in VEGF-A-induced angiogenesis via blocking of VEGFR-2 and the cyclooxygenase-2-mediated mitogen-activated protein kinase signaling pathway in endothelial cells (36). Melittin is thus considered to be an attractive anticancer therapeutic candidate (37). However, it is unclear at present whether melittin is able to regulate proliferation, invasion and angiogenesis via blocking of the VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway in MHCC97-H cells.
In the present study, interactions between melittin and Cat S-induced proliferation, invasion and angiogenesis in HCC, and the influence on the VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway were investigated. Initially, various hepatic cancer cell lines were screened for increased levels of Cat S expression. As MHCC97-H cells were identified to exhibit the highest expression levels of Cat S, the role of melittin and its effects on Cat S and the VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway were investigated in vitro in these cells.
Materials and methods
Cell lines, cell culture and reagents
Melittin (>90% pure) was obtained from Sigma-Aldrich (#M4171, St. Louis, MO, USA). A 5 µg/ml solution of melittin was prepared in sterile water, stored at −20°C and diluted to the required concentrations for the experiments performed. MHCC97-H, Bel-7402, LO2, HepG2, SMMC7721, Hep3B, HepG2, Huh7 cells and HUVECs were purchased from the Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Mouse anti-human antibodies against: Cat S (#sc-271619), anti-VEGF-A (#sc-53463) and anti-β-actin (#47778), were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit anti-human antibodies against: Phospho-VEGF receptor 2 (Tyr1175; #2478S), phospho-ERK1/2 (Thr202/Tyr204; #4370), ERK1/2 (#9194), phospho-MEK1 (Thr286; #9127S), MEK1(#12671), phospho-c-Raf (Ser259; # 9421S) and Raf (#9422S) were sourced from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-Ras was purchased from Epitomics (#1819-1; Burlingame, CA, USA). Matrigel (#356234) was obtained from BD Biosciences (San Jose, CA, USA). XTT stock solution (#M2128-1G) and Lipofectamine 2000 (#11668-027) was purchased from ThermoFisher Scientific, Inc. Hematoxylin solution (#KGA223) was purchased from Nanjing Sai hong rui Biological Technology Co., Ltd. (Nanjing, China). 24-well Transwell (#FK-cn018), BCA Protein Assay Kit (#23225) and chemiluminescence detection reagents (#32209) were purchased from ThermoFisher Scientific, Inc. Mitomycin C was purchased from Sigma-Aldrich. Highly specific quantitative sandwich ELISA kit for human VEGF was obtained from RayBiotech (#MAB293, Norcross, GA, USA). Endothelial cell medium and fetal bovine serum (FBS) were purchased from ScienCell (Carlsbad, CA, USA). All cells were grown at 37°C in a humidified atmosphere containing 5% CO2.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIzol® reagent (#15596-026; Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's protocols. Total RNA concentration and purity were determined by absorbance at 260 and 280 nm using a NanoVue Plus (#ND200: Gene Company Ltd.). Total RNA was reverse transcribed to complementary DNA (cDNA) using Superscript Reverse Transcriptase (Gibco; Thermo Fisher Scientific). cDNA was stored at −20°C until use. The following primers were used in the experiments: Cat S forward, 5′-ACGGCTTTCCAGTACATCATTGAT-3′, and reverse, 5′-CTTTGTAGGGATAGGAAGCGTCTG-3′; actin forward, 5′-CACCCAGCACAATGAAGATCAAGAT-3′, and reverse, 5′-CCAGTTTTTAAATCCTGAGTCAAGC-3′. RT-PCR was performed using the FastStart Universal SYBR® Green Master (#04913914001; Roche Ltd.). PCR experiments were performed in triplicate.
Cell transfection
In order to establish small hairpin RNA (shRNA)-Cat S-stably transfected cell lines, MHCC97-H cells at 70–80% confluence were transfected with 1 µg pcDNA3.1-shRNA-Cat S (shRNA/MHCC97-H) or pcDNA3.1 empty vectors (Mock/MHCC97-H: F 5′-UUCUCCGAACGUGUCACGU-3′ and R 5′-ACGUGACACGUUCGGAGAA-3′) utilizing Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific). The plasmid (pcDNA3.1-shRNA-Cat S) containing shRNA-Cat S Gene Operon was purchased from Caliper Life Sciences (PerkinElmer, Inc. Waltham, MA, USA). In order to obtain stable transformants, cells were selected with Geneticin (G418; 500 µg/ml; #108321; MP Biomedicals Ltd, Shanghai, China) following 24 h transfection. After a total of 3 weeks growth, the remainder of cells were plated with fresh Dulbecco's modified Eagle's medium (DMEM)/G418 (500 µg/ml) and 10% FBS in 96-well plates, until a single colony was formed. Subsequently, individual colonies were isolated from these cultures and expanded for in vitro study.
The pairs of Cat S RNA oligonucleotide sequences and the pair of control RNA oligonucleotide sequences were designed and synthesized by Shanghai GenePharma Co., Ltd (Shanghai, China). The primer sequences of Cat S utilized were as follows: Forward 5′-CGCAAATGGGCGGTAGGCGTG-3′, and reverse 5′-CAGCGGGGCTGCTAAAGCGCATGC-3′. HUVEC cells were transfected with the Cat S (Cat S-HUVECs) or with the vector alone (Mock-HUVECs). Cell lines were incubated in an atmosphere of 5% CO2 in air, at 37°C with DMEM containing 10% FBS. HUVEC cells were seeded into 6-well plates and grown to 50–60% confluence for 20–24 h prior to transfection. For transfection, Lipofectamine 2000 was mixed with 20 nM RNAs according to the manufacturer's protocols.
Cell proliferation assay
The quantity of viable cells in culture was determined by employing a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells (1×103) were seeded into a 96-well flat-bottomed titer plate and incubated for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, 10 µl MTT stock solution (5 mg/ml; Sigma-Aldrich) was added to each well, and additional incubation was performed for 4 h at 37°C. Adding 150 µl dimethyl sulfoxide (DMSO) to each well halted the reaction, and spectrophotometric absorbance was subsequently measured using a microplate reader (Model 3550; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm. The inhibition rate (%) was calculated as follows: (OD control group - OD experiment group)/(OD control group - OD blank group).
Cytotoxicity assay
A 2,3-bis-(2-methoxy-4-nitro-5- sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay was conducted to assess cytotoxicity. MHCC97-H cells were seeded into 96-well microplates at a density of 5×104 cells/well in 200 µl complete DMEM medium. Following 24 h of incubation at 37°C in a humidified incubator, cells were treated with various concentrations of melittin (0.5, 1, 2, 3, 4, 8, 16, 20 µg/ml). A total of 100 µl XTT (XTT II; Roche Diagnostics GmbH, Mannheim, Germany) reaction solution was added to each well. Cell viability was determined using the XTT assay with the Bio-Rad Model 3550 microplate reader, following 4 h of incubation.
Flat plate colony formation assay
MHCC97-H cells were collected and plated in 6 cm2 culture plates (1×103 cells/well) for colony formation. Cells were incubated at 37°C in a 5% CO2 atmosphere and treated with various concentrations of melittin (4 or 8 µg/ml) for 24 h. At the conclusion of treatment, cells were washed twice with phosphate-buffered saline (PBS) and subsequently incubated in 9 ml drug-free medium for 14 days, to allow colony formation of surviving cells. Following 14 days of incubation, colonies were washed with chilled PBS and fixed using 4% neutral-buffered formalin for 10 min, followed by staining with hematoxylin solution and counting of colony numbers (>50 cells was defined as a colony). The results are representative of three independent experiments.
Wound healing migration assay
MHCC97-H cells (8×105) were grown to 100% confluence in 6-well plates and subsequently incubated with 8 µg/ml Mitomycin C for 3 h, in order to inactivate cell proliferation. Confluent cells were subsequently scratched using a pipette with a 200-µl tip and incubated for 24 h in a CO2 incubator with a humidified atmosphere. Wounded cells were supplemented with a cell medium of 0.5% FBS and exposed to various concentrations (0, 4 or 8 µg/ml) of melittin. Images of MHCC97-H cells were captured following 24 h of incubation. The number of migrated cells in 6–8 randomly chosen fields for each well were counted under inverted microscope. Three independent experiments were performed.
Transwell migration assay
Each well of the pre-chilled, 24-well Transwell (Corning Inc., Corning, NY, USA) plates was coated with 60 µl 1:8 diluted Matrigel (growth factor reduced) and incubated for 5 h. Cells (1×105/well) were seeded into the inner compartment of the invasion chamber with 200 µl 0.2% FBS/DMEM and various concentrations (4 or 8 µg/ml) of melittin. The inner chamber was placed into the outer chamber of the Transwell, which contained 500 µl 1% FBS/DMEM. Following incubation overnight, membranes were washed, and migrated cells were fixed using 4% paraformaldehyde with 0.5% crystal violet. Images were captured using an IX70 inverted microscope (Olympus Corporation, Tokyo, Japan) and the number of migrated cells was counted in 6–8 randomly selected fields. The number of invaded cells was quantified by manual counting relative to that of untreated controls.
ELISA assays for secretion of VEGF
MHCC97-H cells (5×105) were seeded into 6-well plates, incubated at 37°C in 5% CO2 atmosphere and treated with various concentrations of melittin. Cells were removed following 24 h of incubation, and culture medium was collected and used in order to determine the secretion of VEGF, using the VEGF ELISA kit in accordance with the manufacturer's protocol.
Tube formation assay
Pre-chilled 24-well plates were coated with 50 µl Matrigel (growth factor reduced) and incubated for 1 h at 37°C. The cells (1×105) were placed in 200 µl DMEM (supplemented with 0.1% FBS) with various concentrations of melittin (4 or 8 µg/ml). Following 8 h incubation, the endothelial cell tubular structure had formed. The tube area from 5 random fields per well were photographed using a high power Axiovert S100 light microscope (Carl Zeiss AG, Jena, Germany) at ×100 magnification. The lengths of tubes were measured using Image-Pro Plus software 4.5 (Media CyberMetics, Inc, Rockville, MD, USA). This experiment was repeated three times in triplicate. Data are expressed as the mean ± standard deviation.
Western blot analysis
Cells were harvested and washed twice with cold PBS and lysed in buffer (Beyotime Institute of Biotechnology, Shanghai, China). Samples were subsequently centrifuged for 30 min at 2,250 × g at 4°C, and supernatant was collected as total cell lysate. Protein concentration was measured using a bicinchoninic acid kit (Thermo Fisher Scientific). Approximately 40 µg cellular proteins were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Following blocking with 5% fat-free dry milk in 125 mM sodium chloride, 0.05% Tween-20 and 25 mM Tris base (1X TBS-T) for 1 h, cells were exposed overnight to primary antibodies (dilution, 1:1,000) against Cat S, phospho-VEGFR-2, VEGF-A, Ras, phospho-Raf, Raf, phospho-MEK1/2, MEK1/2, phospho-ERK1/2, ERK1/2 and β-actin. Following washing 3 times with 1X TBS-T for 10 min, the samples were incubated with secondary antibodies (dilution, 1:2,000) for 1 h. Membranes were subsequently incubated with enhanced chemiluminescence detection reagents (Thermo Fisher Scientific) according to the manufacturer's protocols. The antibody-specific proteins were visualized using an image analyzer (LAS-3000; Fujifilm, Tokyo, Japan) according to the manufacturer's protocol.
Statistical analysis
Data were expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). The significance of the difference between the experimental group and the control group was analyzed using a Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cat S is overexpressed in MHCC97-H cell lines
In order to elucidate the role of Cat S in HCC invasion and angiogenesis, Cat S messenger RNA (mRNA) and protein expression levels were measured in 7 HCC cell lines using RT-PCR and western blotting. It was revealed that Cat S mRNA and protein expression were highest in the MHCC97-H cell line, compared with the L02 normal liver cell line and other HCC cell lines (Bel-7402, HepG2, MHCC97-L, Hep3B, Huh7, and SMMC7721) investigated (Fig. 1A and B).
Figure 1.
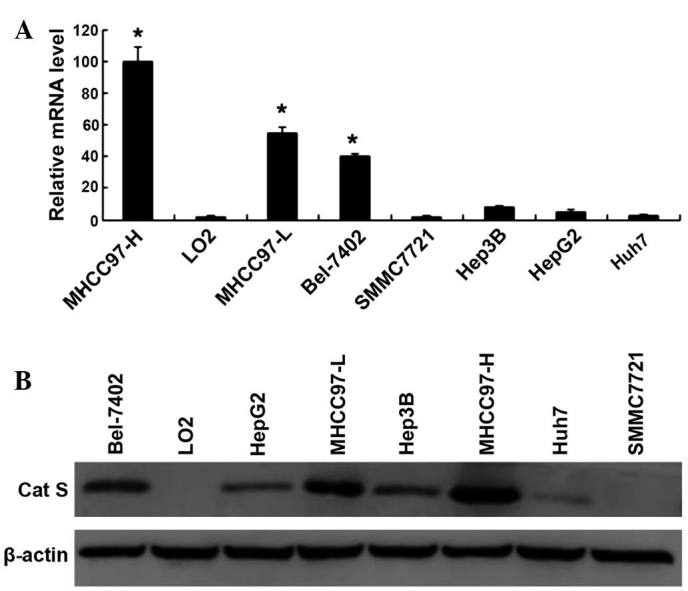
Expression of Cat S in HCC cell lines is positively correlated with metastatic capacity. (A) Results of reverse transcription-polymerase chain reaction assay of Cat S mRNA in 7 HCC cell lines (*P<0.05 compared with L02 normal liver cell line). (B) Expression of Cat S protein was determined by western blot analysis with 40 µg of protein from each sample. β-actin served as a loading control. HCC, hepatocellular carcinoma; Cat S, cathepsin S; mRNA, messenger RNA.
Although Cat S is considered to have a significant role in angiogenesis, invasion and metastasis in a number of tumor cell lines (38), it remains to be determined whether it exerts any effects in HCC cell lines. Therefore, the MHCC97-H cell line was chosen as a model cell for the present study.
Melittin prevents MHCC97-H cell invasion, metastasis and angiogenesis
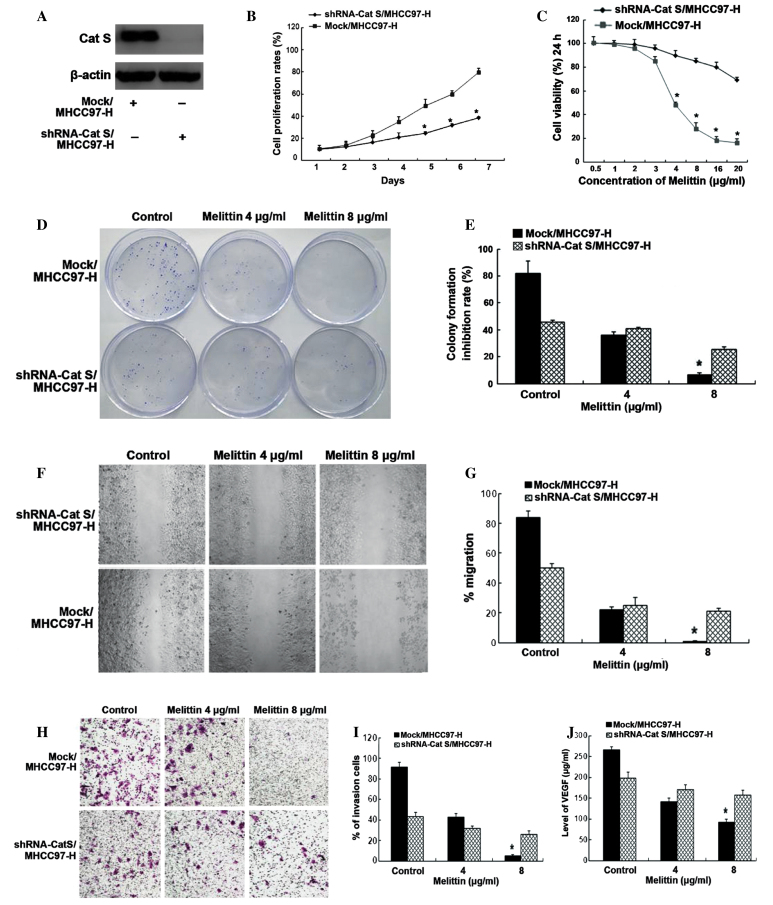
It has previously been demonstrated qualitatively that Cat S was aberrantly overexpressed in human tumor tissues of HCC patients (25). It is plausible that the activity of Cat S contributes to the invasion and metastasis of HCC cells. In order to investigate this hypothesis, the association between cell motility and Cat S expression was investigated in MHCC97-H cells, which were stably transfected with pcDNA3.1-shRNA-Cat S (shRNA-Cat S/MHCC97-H) or pcDNA3.1 empty vectors (Mock/MHCC97-H). Downregulation of Cat S in MHCC97-H cells was confirmed using western blotting. As shown in Fig. 2A, the levels of Cat S expression were significantly inhibited following transfection with pcDNA3.1-shRNA-Cat S, compared with MHCC97-H cells transfected with empty vectors.
Figure 2.
Melittin prevents stably transfected MHCC97-H cell growth, invasion and angiogenesis. (A) Expression of Cat S was analyzed by western blotting. Levels of β-actin protein were used to monitor for equal loading. (B) Growth curves of stably transfected MHCC97-H cells assessed by MTT assay. Data are represented as the mean ± standard deviation of 3 independent experiments (*P<0.05 compared with DMSO treated Mock/MHCC97-H cells). (C) Inhibitory effect of melittin on stably transfected MHCC97-H cells detected by XTT assay. Data are represented as the mean ± standard deviation of ≥3 measurements (*P<0.05 compared with DMSO treated Mock/MHCC97-H cell). (D) Effect of melittin on colony formation of stably transfected MHCC97-H cells. (E) Quantification of colony formation assay. Data are represented as the mean ± standard deviation of 3 independent experiments (*P<0.05 compared with DMSO treated Mock/MHCC97-H cell). (F) Effect of melittin on migration of stably transfected MHCC97-H cells was evaluated by wound-healing assay. (G) Quantification of wound-healing assay. Magnification, ×100. Data are represented as the mean ± standard deviation of 3 independent experiments. (H) Effect of melittin on invasion of stably transfected MHCC97-H cells was evaluated by Transwell chamber assay. Magnification, ×100. (I) Cell number counts of Transwell chamber assay. Data are represented as the mean ± standard deviation of 3 independent experiments (*P<0.05 compared with DMSO treated Mock/MHCC97-H cell). (J) Enzyme-linked immunosorbent assay for melittin inhibition of Cat S-associated angiogenesis. Data are represented as the mean ± standard deviation of 3 independent experiments (*P<0.05 compared with shRNA-Cat S/MHCC97-H cells). Cat S, cathepsin S; shRNA, small hairpin RNA.
Cat S has been demonstrated to be a significant factor in the degradation of ECM elements, and is closely associated with regulation of tumor cell invasion and metastasis (35). It is possible that Cat S additionally regulates the metastasis of HCC cells (39). Therefore, in the present study, an MTT cell assay was performed to determine whether shRNA-Cat S affects the proliferation of MHCC97-H cells. As shown in (Fig. 2B), shRNA-Cat S significantly reduced the growth of shRNA-Cat S/MHCC97-H cells, compared with that of Mock/MHCC97-H cells.
A previous study demonstrated that melittin was able to significantly inhibit the growth of the SMMC-7721 HCC cells (40). It is possible that melittin affects MHCC97-H cell invasion and angiogenesis via inhibition of Cat S activity (22,35). In order to test this hypothesis, the effects of melittin treatment on the viability of MHCC97-H cells were investigated in the present study using a XTT assay. As demonstrated in Fig. 2C, the present study determined the IC50 of melittin in shRNA-Cat S/MHCC97-H cells to be 9.94 µg/ml; in Mock/MHCC97-H cells, the IC50 was 4.03 µg/ml. The results indicated that the viability of melittin-treated Mock/MHCC97-H cells was significantly reduced compared with that of melittin-treated shRNA-Cat S/MHCC97-H cells. These results support the hypothesis that melittin is capable of inhibiting MHCC97-H cell viability, and that this is correlated with increased expression levels of the Cat S oncogene.
The present study subsequently investigated whether melittin was able to inhibit long-term clonogenic survival of MHCC97-H cells, using a colony formation assay. As demonstrated in Fig. 2D and E, in the control (0.1% DMSO treatment) group, shRNA-Cat S/MHCC97-H cells (45.5±1.7%) exhibited significantly reduced colony formation compared with Mock/MHCC97-H cells (82.1±9.3%). Notably, treatment with melittin was able to markedly reduce the number of colonies formed in Mock/MHCC97-H cells relative to the control treatment group (4 µg/ml and 8 µg/ml of melittin treated Mock/MHCC97-H cells was 36.1±8.7% and 6.5±8.9%, respectively P<0.05), however, it did not markedly reduce the colony growth rates in shRNA-Cat S/MHCC97-H cells (4 µg/ml and 8 µg/ml of melittin treated shRNA-Cat S/MHCC97-H cell was 40.7±1.2% and 26.4±1.6%, respectively), indicating that melittin specifically inhibits Cat S-induced colony formation.
Subsequently, the present study investigated the effects of melittin on MHCC97-H cell migration using Transwell chamber and wound-healing assays. In the wound healing assay (Fig. 2F and G), shRNA-Cat S/MHCC97-H cells (44.5±3.2%) demonstrated significantly reduced migration compared with Mock/MHCC97-H cells (84.3±4.1%) when treated with 0.1% DMSO (control). When cells were treated with melittin, migration of Mock/MHCC97-H cells reduced significantly compared with DMSO-treated Mock/MHCC97-H cells (4 µg/ml and 8 µg/ml of melittin treated Mock/MHCC97-H cells was 22.5±2.1% and 1.5±1.9%, respectively P<0.05); however, no such reduction in migration was observed in shRNA-Cat S/MHCC97-H cells (4 µg/ml and 8 µg/ml of melittin treated shRNA-Cat S/MHCC97-H cells was 27.2±5.9% and 21.5±2.3%). Similarly, when treated with 0.1% DMSO (control), the number of shRNA-Cat S/MHCC97-H cells invading through the Matrigel (43.4±3.9%) was significantly lower compared with that of Mock/MHCC97-H cells (91.3±4.2%). However, when exposed to melittin, the number of Mock/MHCC97-H cells invading across the membrane (4 µg/ml and 8 µg/ml of melittin treated Mock/MHCC97-H cell was 42.9±3.9% and 5.2±7.4% respectively; P<0.05) was significantly reduced compared with that of shRNA-Cat S/MHCC97-H cells (4 µg/ml and 8 µg/ml of melittin treated shRNA-Cat S/MHCC97-H cell was 31.7±2.1% and 25.8±3.5 %respectively; Fig. 2H and I). These results suggest that melittin was able to effectively suppress Cat S-induced migration and invasion.
ELISA was used to detect and quantify VEGF in the melittin-treated cell culture supernatant. It was revealed that treatment with melittin markedly suppressed secretion of VEGF from Mock/MHCC97-H cells into the cell culture supernatant: The VEGF level for DMSO treated cells, 4 µg/ml and 8 µg/ml of melittin treated Mock/MHCC97-H cells was 265.5±8.1, 140.9±9.4 and 91.7±7.9, respectively P<0.05; compared with shRNA-Cat S/MHCC97-H cells. The VEGF level for DMSO, 4 µg/ml and 8 µg/ml of melittin treated shRNA-Cat S/MHCC97-H cells was 198.2±14.5, 170.1±11.8 and 157.5±12.1, respectively (Fig. 2J). The results indicate that Cat S may play a significant role as a target for angiogenesis inhibition by melittin.
Melittin inhibits Cat S-induced invasion and angiogenesis of HUVECs
Considering that melittin treatment was able to significantly inhibit Mock/MHCC97-H cell migration, invasion and angiogenesis, the present study additionally investigated the effects of melittin inhibition on Cat S-induced angiogenesis in HUVECs.
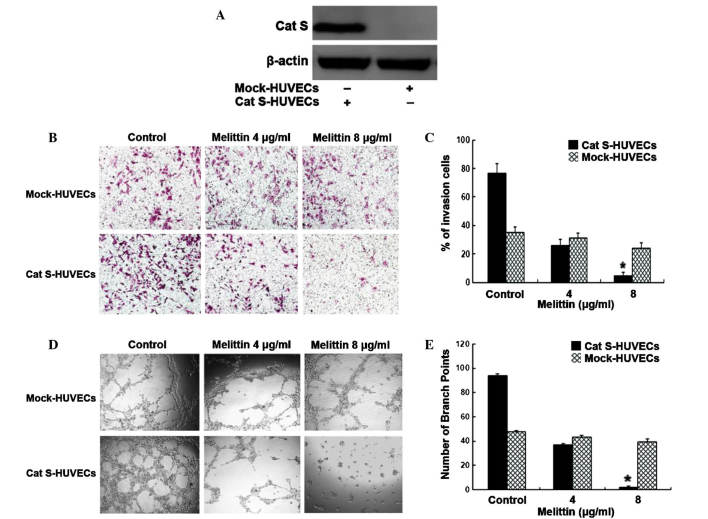
In order to confirm the inhibitory effects of melittin on Cat S-induced invasion and angiogenesis in HUVECs, HUVECs were transfected with Cat S RNA (Cat S-HUVECs) to induce overexpression of Cat S, or with the empty vector (Mock-HUVECs). As demonstrated in Fig. 3A, an increase in the level of Cat S protein was observed in Cat S-HUVECs, whilst no such increase was observed in Mock-HUVECs.
Figure 3.
Melittin suppresses Cat S-induced invasion and angiogenesis of HUVECs. (A) Western blotting detected the expression of Cat S in transient transfection of HUVECs. β-actin served as a loading control. (B) Effect of melittin on invasion of transfected HUVECs was evaluated by Transwell chamber assay. Magnification, ×100. (C) Counted cell numbers from Transwell assay. Data are represented as the mean ± standard deviation of 3 independent experiments (*P<0.05 compared with control). (D) Effect of melittin on tube-formation in transfected HUVECs. Magnification, ×100. (E) Lengths of tubes were measured using Image-Pro Plus software. Data are expressed as the mean ± standard deviation. *P<0.05 compared with the control group. Cat S, cathepsin S; HUVECs, human umbilical vein endothelial cells.
Subsequently, the effects of melittin on HUVEC motility were investigated by Transwell assay. As shown in Fig. 3B and C, Cat S-HUVECs in the control group (76.4±4.1%) demonstrated an increase in the percentage of invading cells compared with Mock-HUVECs (35.2±3.5%). In the melittin-treated groups, the percentage of invading Cat S-HUVECs (4 µg/ml and 8 µg/ml of melittin treated Cat S-HUVECs was 25.6±4.5 and 4.6±2.4%, respectively, P<0.05) was significantly reduced relative to the control group (76.4±4.1%), whilst the migration of Mock-HUVECs was not: The migration rate for DMSO, 4 µg/ml and 8 µg/ml of melittin treated Mock-HUVECs was 35.2±3.5, 31.2±3.8 and 24.1±3.6%, respectively.
In order to determine whether melittin was capable of direct anti-angiogenic effects in Cat S-HUVECs, an endothelial cell tube formation assay was performed. In the control group (0.1% DMSO), Cat S-HUVECs formed a robust and more complete tube-like network (94.3±2.1 branch points) compared with Mock-HUVECs (67.3±2.1 branch points; Fig. 3D and E). As predicted, there was a significantly reduced number and length of tube structures in Cat S-HUVECs with increased concentrations of melittin (4 µg/ml and 8 µg/ml of melittin treated Cat S-HUVECs was 37.1±1.4 and 1.82±1.2 branch points, respectively; P<0.05). However, no such reduction was observed in Mock-HUVECs (4 µg/ml and 8 µg/ml of melittin treated Mock-HUVECs was 45.5±1.7 and 39.1±2.5 branch points, respectively).
Melittin inhibits Cat S expression in HCC cell lines
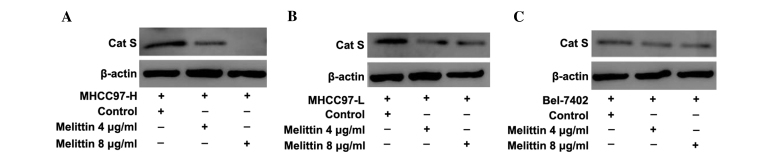
In order to understand the effects of melittin on Cat S activity and expression, additional HCC cell lines with relatively high levels of Cat S expression were selected (Fig. 1A and B), including MHCC97-H, MHCC97-L and Bel-7402 cells, for additional measurements. Cells were treated with 0.1% DMSO (control) or melittin (4 or 8 µg/ml), for 24 h. Following incubation, total cell lysates were prepared and the level of Cat S proteins was determined using western blot analysis. The results revealed that melittin inhibited Cat S expression in MHCC97-H cells more markedly than in MHCC97-L and Bel-7402 cells (Fig. 4), thereby further supporting the hypothesis that melittin is a Cat S inhibitor.
Figure 4.
Effects of melittin on inhibition of the expression of metastasis-associated Cat S protein in MHCC97-H, MHCC97-L and Bel-7402 cell lines. (A) Melittin markedly inhibited the expression of metastasis-associated Cat S protein in MHCC97-H cells compared with (B) MHCC97-L and (C) Bel-7402 cells. Blots represent 3 independent experiments with qualitatively similar results. The expression of β-actin protein served as a loading control. Cat S, cathepsin S.
Melittin inhibits the activation of Cat S-mediated signaling pathways in stably transfected MHCC97-H cells
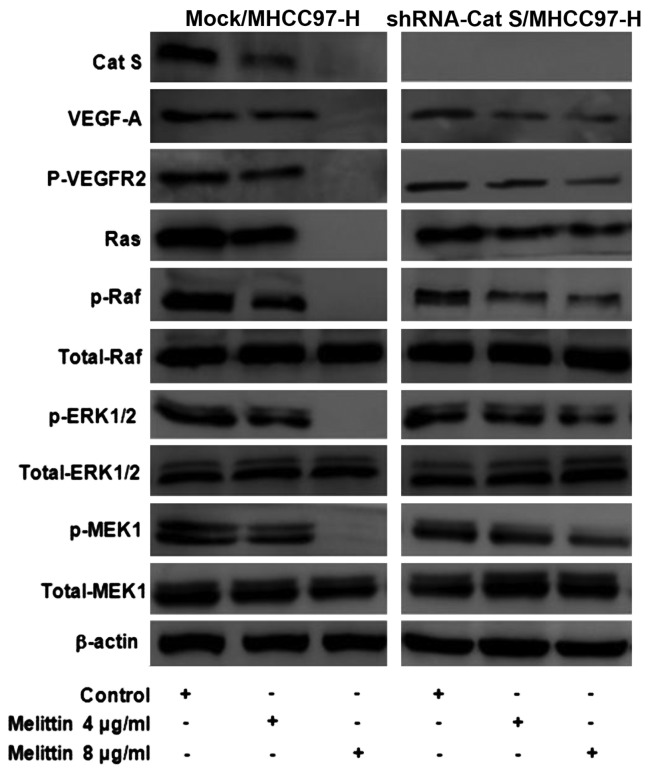
In order to clarify whether melittin was capable of inhibiting Cat S-induced migration, invasion and angiogenesis via blocking of the VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway in MHCC97-H cells, Cat S gene expression was silenced in MHCC97-H cells and the cells were treated with various concentrations of melittin. Protein levels from cells exposed to various concentrations of melittin treatment were measured using western blotting, and probed with specific antibodies targeting certain proteins. As demonstrated in Fig. 5, when treated with 0.1% DMSO (control), the levels of phosphorylation/activation of Cat S, VEGF-A, phosphorylated (p)-VEGFR-2, Ras, p-Raf, p-MEK1 and p-ERK1/2 were significantly increased in Mock/MHCC97-H cells compared with shRNA-Cat S/MHCC97-H cells. Furthermore, the expression of the above proteins was significantly downregulated in a dose-dependent manner in the Mock/MHCC97-H cells as a result of melittin treatment. By contrast, this inhibitory effect was less marked in shRNA-Cat S/MHCC97-H cells. Therefore, it appears that melittin inhibited the VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway in Mock/MHCC97-H cells. The results of the present study suggested that Cat S may be involved in MHCC97-H cell invasion, and that Cat S expression may be markedly suppressed by melittin treatment in vitro.
Figure 5.
Effects of melittin on the phosphorylation/activation of VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway. Melittin specifically decreased the expression of phosphorylated/activated Cat S, VEGF-A, p-VEGFR-2, Ras, p-Raf, p-MEK1 and p-ERK1/2 in Mock/MHCC97-H cells, however, did not affect shRNA-Cat S/MHCC97-H cells. Results are from a representative experiment performed with qualitatively similar results. β-actin served as an internal control in each sample. VEGFR, vascular endothelial growth factor receptor; MEK, mitogen-activated protein kinase; ERK, extracellular regulated mitogen-activated protein kinase; p, phosphorylated, shRNA, small hairpin RNA.
Discussion
Previous studies have implicated Cat S in the processes of invasion and metastasis in several types of malignancy (14,41). In addition, the overexpression and secretion of Cat S by tumor cells has been observed to positively increase tumor invasiveness (42,43). Therefore, inhibition of Cat S-induced invasion and angiogenesis may be a potential therapeutic strategy for the treatment of cancer (38). Although progress has been made in this field, the function of Cat S in HCC progression and metastasis has not been fully elucidated. In the present study, it was identified that Cat S protein and mRNA were expressed at increased levels in MHCC97-H cells, which demonstrate high metastatic potential. In order to confirm the effects of Cat S on the invasion and angiogenesis of HCC cells, the present study utilized shRNA knockdown and overexpression of Cat S; this demonstrated that Cat S was able to promote MHCC97-H cell and HUVEC migration and angiogenesis, respectively.
A number of studies have reported that certain natural products may possess significant potential as anti-angiogenic agents for the control of cancer development and metastasis (44). It was proposed by Gajski et al (37) that melittin may be a potential candidate for a cancer treatment strategy utilizing natural products. However, the mechanisms underlying the inhibitory action of melittin on Cat S-induced invasion and angiogenesis in MHCC97-H cells remain to be elucidated.
In the present study, the effects of melittin on Cat S-induced invasion and angiogenesis in stably transfected MHCC97-H cells were investigated using cell proliferation, cell viability, flat plate colony formation, wound healing, migration, Transwell migration and ELISA assays. It was revealed that melittin was able to significantly inhibit Mock/MHCC97-H cell invasion, migration and angiogenesis in a dose-dependent manner; however, it did not affect shRNA-Cat S/MHCC97-H cells. Similarly, melittin treatment was able to significantly inhibit invasion and tube formation of Cat S-HUVECs in a concentration-dependent manner. It thus appears that melittin exerted a direct inhibitory effect on Cat S-induced invasion and angiogenesis in vitro.
Angiogenesis is considered to be crucial in the growth, invasion and metastatic spreading of cancer (45), and inhibition of angiogenesis provides a potential strategy for the modulation of cellular growth and prevention of malignancies (46). The VEGF-A/VEGFR-2/Ras/Raf/MEK1/ERK1/2 signaling pathway has been identified as a potentially significant target for anti-angiogenic tumor therapy (47,48). It is well known that the biological functions of VEGF in angiogenesis, including microvascular permeability, invasion, migration and survival, are primarily mediated through VEGFR-2 (49). Melittin has been demonstrated to play an important role in the inhibition of angiogenesis and metastasis, through decreasing VEGF and VEGFR-2, and blocking the VEGFR-2/Ras/Raf signaling pathway via interfering with the activation of MEK1/ERK1/2 (50). In the present study, it was identified that melittin specifically abrogated phosphorylation/activation of Cat S, VEGF-A, p-VEGFR-2, Ras, p-Raf, p-MEK1 and p-ERK 1/2 in Mock/MHCC97-H cells; however, it exerted little effect on the shRNA-Cat S/MHCC97-H cells. This finding demonstrated that melittin was able to significantly inhibit the activation of VEGF-A/VEGFR-2/MEK1/ERK1/2 protein kinases in a concentration-dependent manner, in Mock/MHCC97-H cells in vitro, suggesting that modulation of Cat S expression may underlie the anticancer effects of melittin.
In conclusion, the present study demonstrated that Cat S may be a significant regulator of growth and angiogenesis in MHCC97-H cells, and that melittin is capable of inhibiting Cat S-induced invasion and angiogenesis via blocking of the VEGF-A/VEGFR-2/MEK1/ERK1/2 signaling pathway, in a dose-dependent manner. Furthermore, the present study provides additional evidence for the anti-angiogenic role of melittin. Melittin may be considered as a selective Cat S inhibitor, which possesses potential therapeutic value for the treatment of HCC cases exhibiting increased levels of Cat S expression.
Acknowledgements
The present study was supported by the Guangxi Natural Science Foundation (grant no. 2011GXNSFA018284), the Department of Health Care of Guangxi (grant no. GZKZ10-114), and the National Natural Science Foundation of China (grant no. 81360372).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Lau WY, Lai EC. Hepatocellular carcinoma: Current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–257. [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Tateishi R, Yasunaga H, Horiguchi H, Yoshida H, Matsuda S, Koike K. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: A national survey of 54,145 patients. J Gastroenterol. 2012;47:1125–1133. doi: 10.1007/s00535-012-0569-0. [DOI] [PubMed] [Google Scholar]
- 7.Xu ZY, Zheng Z. An optimized 3-step strategy for preventing and treating post-operation relapse and metastasis of malignant tumors with traditional Chinese medicine. Zhong Xi Yi Jie He Xue Bao. 2007;5:5–10. doi: 10.3736/jcim20070102. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 8.Lu X, Zhao H, Yang H, Mao Y, Sang X, Miao R, Xu Y, Du S, Xu H, Chi T, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009;100:488–493. doi: 10.1002/jso.21354. [DOI] [PubMed] [Google Scholar]
- 9.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 10.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: Current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spano D, Zollo M. Tumor microenvironment: A main actor in the metastasis process. Clin Exp Metastasis. 2012;29:381–395. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Wang JN, Tang JM, Kong X, Yang JY, Zheng F, Guo LY, Huang YZ, Zhang L, Tian L, et al. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Mol Biol Rep. 2012;39:5085–5093. doi: 10.1007/s11033-011-1304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brömme D, Bonneau PR, Lachance P, Wiederanders B, Kirschke H, Peters C, Thomas DY, Storer AC, Vernet T. Functional expression of human cathepsin S in Saccharomyces cerevisiae. Purification and characterization of the recombinant enzyme. J Biol Chem. 1993;268:4832–4838. [PubMed] [Google Scholar]
- 16.Levicar N, Strojnik T, Kos J, Dewey RA, Pilkington GJ, Lah TT. Lysosomal enzymes, cathepsins in brain tumour invasion. J Neurooncol. 2002;58:21–32. doi: 10.1023/A:1015892911420. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, Kalluri R, Shi GP. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281:6020–6029. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Li D, Ke Z, Liu R, Maubach G, Zhuo L. Cathepsin S is aberrantly overexpressed in human hepatocellular carcinoma. Mol Med Rep. 2009;2:713–718. doi: 10.3892/mmr_00000161. [DOI] [PubMed] [Google Scholar]
- 19.Sevenich L, Schurigt U, Sachse K, Gajda M, Werner F, Müller S, Vasiljeva O, Schwinde A, Klemm N, Deussing J, et al. Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc Natl Acad Sci USA. 2010;107:2497–2502. doi: 10.1073/pnas.0907240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández PL, Farré X, Nadal A, Fernández E, Peiró N, Sloane BF, Shi GP, Chapman HA, Campo E, Cardesa A. Expression of cathepsins B and S in the progression of prostate carcinoma. Int J Cancer. 2001;95:51–55. doi: 10.1002/1097-0215(20010120)95:1<51::AID-IJC1009>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Semin Oncol. 2002;29(6 Suppl 16):10–14. doi: 10.1016/S0093-7754(02)70064-X. [DOI] [PubMed] [Google Scholar]
- 22.Fan Q, Wang X, Zhang H, Li C, Fan J, Xu J. Silencing cathepsin S gene expression inhibits growth, invasion and angiogenesis of human hepatocellular carcinoma in vitro. Biochem Biophys Res Commun. 2012;425:703–710. doi: 10.1016/j.bbrc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 25.Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma: A review of literature. Cancer. 2009;115:4895–4906. doi: 10.1002/cncr.24537. [DOI] [PubMed] [Google Scholar]
- 26.Yen CJ, Lin YJ, Yen CS, Tsai HW, Tsai TF, Chang KY, Huang WC, Lin PW, Chiang CW, Chang TT. Hepatitis B virus X protein upregulates mTOR signaling through IKKbeta to increase cell proliferation and VEGF production in hepatocellular carcinoma. PLoS One. 2012;7:e41931. doi: 10.1371/journal.pone.0041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldner MJ, Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:5–13. doi: 10.1517/14728222.2011.641951. [DOI] [PubMed] [Google Scholar]
- 28.Boman HG, Wade D, Boman IA, Wåhlin B, Merrifield RB. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989;259:103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 29.Jang HS, Kim SK, Han JB, Ahn HJ, Bae H, Min BI. Effects of bee venom on the pro-inflammatory responses in RAW264.7 macrophage cell line. J Ethnopharmacol. 2005;99:157–160. doi: 10.1016/j.jep.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258:72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Lee JD, Kim SY, Kim TW, Lee SH, Yang HI, Lee DI, Lee YH. Anti-inflammatory effect of bee venom on type II collagen-induced arthritis. Am J Chin Med. 2004;32:361–367. doi: 10.1142/S0192415X04002016. [DOI] [PubMed] [Google Scholar]
- 32.Park HJ, Lee HJ, Choi MS, Son DJ, Song HS, Song MJ, Lee JM, Han SB, Kim Y, Hong JT. JNK pathway is involved in the inhibition of inflammatory target gene expression and NF-kappaB activation by melittin. J Inflamm (Lond) 2008;5:7. doi: 10.1186/1476-9255-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu ST, Cheng HH, Huang CJ, Chang HC, Chi CC, Su HH, Hsu SS, Wang JL, Chen IS, Liu SI, et al. Phospholipase A2-independent Ca2+ entry and subsequent apoptosis induced by melittin in human MG63 osteosarcoma cells. Life Sci. 2007;80:364–369. doi: 10.1016/j.lfs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Li B, Gu W, Zhang C, Huang XQ, Han KQ, Ling CQ. Growth arrest and apoptosis of the human hepatocellular carcinoma cell line BEL-7402 induced by melittin. Onkologie. 2006;29:367–371. doi: 10.1159/000094711. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Yu M, He Y, Xiao L, Wang F, Song C, Sun S, Ling C, Xu Z. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology. 2008;47:1964–1973. doi: 10.1002/hep.22240. [DOI] [PubMed] [Google Scholar]
- 36.Huh JE, Kang JW, Nam D, Baek YH, Choi DY, Park DS, Lee JD. Melittin suppresses VEGF-A-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J Nat Prod. 2012;75:1922–1929. doi: 10.1021/np300446c. [DOI] [PubMed] [Google Scholar]
- 37.Gajski G, Garaj-Vrhovac V. Melittin: A lytic peptide with anticancer properties. Environ Toxicol Pharmacol. 2013;36:697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, Pan JH, Lu ML, Cheng XW, Iguchi A, et al. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003;92:493–500. doi: 10.1161/01.RES.0000060485.20318.96. [DOI] [PubMed] [Google Scholar]
- 39.Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, Tong M, Lo J, Ng IO. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:179–191. doi: 10.1002/hep.27070. [DOI] [PubMed] [Google Scholar]
- 40.Hu H, Chen D, Li Y, Zhang X. Effect of polypeptides in bee venom on growth inhibition and apoptosis induction of the human hepatoma cell line SMMC-7721 in-vitro and Balb/c nude mice in-vivo. J Pharm Pharmacol. 2006;58:83–89. doi: 10.1211/jpp.58.1.0010. [DOI] [PubMed] [Google Scholar]
- 41.Yusuf N, Irby C, Katiyar SK, Elmets CA. Photoprotective effects of green tea polyphenols. Photodermatol Photoimmunol Photomed. 2007;23:48–56. doi: 10.1111/j.1600-0781.2007.00262.x. [DOI] [PubMed] [Google Scholar]
- 42.Flannery T, Gibson D, Mirakhur M, McQuaid S, Greenan C, Trimble A, Walker B, McCormick D, Johnston PG. The clinical significance of cathepsin S expression in human astrocytomas. Am J Pathol. 2003;163:175–182. doi: 10.1016/S0002-9440(10)63641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohamed MM, Sloane BF. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 44.Quesada AR, Muñoz-Chápuli R, Medina MA. Anti-angiogenic drugs: From bench to clinical trials. Med Res Rev. 2006;26:483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Song CC, Lu X, Cheng BB, Du J, Li B, Ling CQ. Effects of melittin on growth and angiogenesis of human hepatocellular carcinoma BEL-7402 cell xenografts in nude mice. Ai Zheng. 2007;26:1315–1322. (In Chinese) [PubMed] [Google Scholar]
- 47.Huh JE, Lee EO, Kim MS, Kang KS, Kim CH, Cha BC, Surh YJ, Kim SH. Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis. 2005;26:1436–1445. doi: 10.1093/carcin/bgi097. [DOI] [PubMed] [Google Scholar]
- 48.Toomey DP, Murphy JF, Conlon KC. COX-2, VEGF and tumour angiogenesis. Surgeon. 2009;7:174–180. doi: 10.1016/S1479-666X(09)80042-5. [DOI] [PubMed] [Google Scholar]
- 49.Lu N, Gao Y, Ling Y, Chen Y, Yang Y, Gu HY, Qi Q, Liu W, Wang XT, You QD, Guo QL. Wogonin suppresses tumor growth in vivo and VEGF-induced angiogenesis through inhibiting tyrosine phosphorylation of VEGFR2. Life Sci. 2008;82:956–963. doi: 10.1016/j.lfs.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Huh JE, Baek YH, Lee MH, Choi DY, Park DS, Lee JD. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett. 2010;292:98–110. doi: 10.1016/j.canlet.2009.11.013. [DOI] [PubMed] [Google Scholar]