Abstract
A growing body of evidence indicates that aberrant activation of epithelial-to-mesenchymal transition (EMT) plays a key role in tumor cell invasion and metastasis. Zinc finger E-box-binding homeobox factor 1 (ZEB1), as a crucial mediator of EMT, contributes to the malignant progression of various epithelial tumors. To determine whether ZEB1 is involved in the progression of ovarian cancer, we immunohistochemically evaluated the expression of ZEB1 in 238 cases of epithelial ovarian cancer (EOC) and analyzed its associations with clinicopathological parameters. Positive expression of ZEB1 was observed in 32.8% (78/238) of EOCs and it was found to be significantly associated with advanced tumor stage (P=0.001). The survival analysis indicated that the expression of ZEB1 was associated with a poor 5-year progression-free survival (PFS) (P=0.021). A similar tendency was also observed between the expression of ZEB1 and 5-year overall survival, although it did not reach statistical significance (P=0.118). Moreover, the multivariate analysis demonstrated that ZEB1 expression was an independent risk factor for 5-year PFS in ovarian cancer. Taken together, our data provide evidence that ZEB1 may play a crucial role in promoting aggressive ovarian carcinoma progression. Therefore, ZEB1 may serve as an effectively predictive marker and a potential target for therapeutic intervention in EOC.
Keywords: ovarian cancer, epithelial-to-mesenchymal transition, zinc finger E-box-binding homeobox 1, survival
Introduction
Although epithelial ovarian cancer (EOC) accounts for only a relatively small proportion of cancers among women, it is the leading cause of death from gynecological malignancies (1). Despite the advances in surgery and chemotherapy, the 5-year survival rate remains only ~30%, mainly due to the fact that these tumors are commonly diagnosed at an advanced stage (2). Thus, further investigation of the molecular mechanism underlying EOC metastasis is crucial.
Zinc finger E-box-binding homeobox 1 (ZEB1) is known to be an important regulator of epithelial-to-mesenchymal transition (EMT), which is required for cancer development and metastasis (3). ZEB1 promotes EMT by repressing genes, such as E-cadherin, which are involved in maintaining the epithelial phenotype, and activating those required for transformation to the mesenchymal phenotype (4,5). ZEB1 is a 190–210-kD protein, previously described as a transcriptional factor, repressor of cell adhesion molecules and cell polarity-associated genes (6). Aberrant expression of ZEB-1 in numerous cancers has been associated with aggressive disease, poor differentiation, rapid development of metastases and poor clinical outcome (7–11). Furthermore, high levels of ZEB1 promote the progression of gynecological cancer (12). However, the expression status of the ZEB1 protein in human ovarian carcinoma tissues and its role in clinical outcome requires further elucidation.
To investigate the expression pattern of ZEB1 in EOC tissues and evaluate its association with tumor progression and patient prognosis, we evaluated the expression of ZEB1 in 238 cases of ovarian cancer by immunohistochemistry (IHC) and analyzed the association between ZEB1 expression and clinicopathological parameters, including survival probability of EOC.
Patients and methods
Ethics statement
This study was approved by the Regional Committee for Medical Research Ethics South of Norway (S-06277a), The Social- and Health Directorate (06/3280) and The Data Inspectorate (06/5345).
Patients and materials
This study included 238 patients with EOC. All the patients underwent surgery at the Norwegian Radium Hospital, Oslo University Hospital (Oslo, Norway) between March, 1983 and May, 2001. Informed consent was obtained according to the institutional and national guidelines. The median age of the patients was 58 years (range, 19–89 years). The patients were followed up until January 1st, 2012. All the patients were clinically staged according to the International Federation of Gynecologists and Obstetricians (FIGO) staging system (13). The primary tumors were histologically graded as well-, moderately and poorly differentiated, according to the recommendations of the World Health Organisation (13). Disease progression was determined based on the definitions outlined by the Gynecologic Cancer Intergroup (14). Paraffin-embedded ovarian carcinoma tissues were obtained from the Department of Pathology, and 3-µm sections were cut and used for morphological examination and IHC.
IHC
Paraffin sections were immunostained by Dako Autostainer using Dako Envision™ FLEX+ system (K8012; Dako, Glostrup, Denmark) as described in our previous study (15). Briefly, the sections were deparaffinized, epitopes were unmasked in PT-link with low pH target retrieval solution (Dako) and then blocked with peroxidase blocking solution (Dako) for 5 min. The slides were incubated at room temperature with polyclonal rabbit anti-human ZEB1 antibody (cat. no. HPA027524; 1:500; Sigma-Aldrich, St. Louis, USA), followed by incubation with rabbit linker for 15 min and horseradish peroxidase for 30 min at room temperature. The slides were subsequently stained with 3,3′-diaminobenzidine tetrahydrochloride for 10 min and counter-stained with hematoxylin, dehydrated, and mounted in Richard-Allan Scientific Cytoseal XYL (Thermo Fisher Scientific, Waltham, MA, USA). A known ZEB1-positive human esophageal carcinoma slide was used as positive control. Serial negative controls were tested by the same concentration of normal rabbit serum as a substitute for the rabbit anti-human ZEB1 antibody.
IHC scoring method
The immunostaining of ZEB1 was evaluated by two pathologists (Z.S. and J.M.N.) from the Norwegian Radium Hospital. Only nuclear staining of ZEB1 was considered in this study. The case was classified as positive if immunostaining was observed in >10% of the tumor cells, as described in our previous study (16).
Statistical analysis
SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all the data analyses. Associations between categorical variables were analyzed by Chi-square tests (Pearson's and linear-by-linear, as appropriate). The Kaplan-Meier method was used for survival analysis and groups were compared with log-rank tests. For all the analyses, P<0.05 was considered as statistically significant.
Results
ZEB1 expression in tumor samples
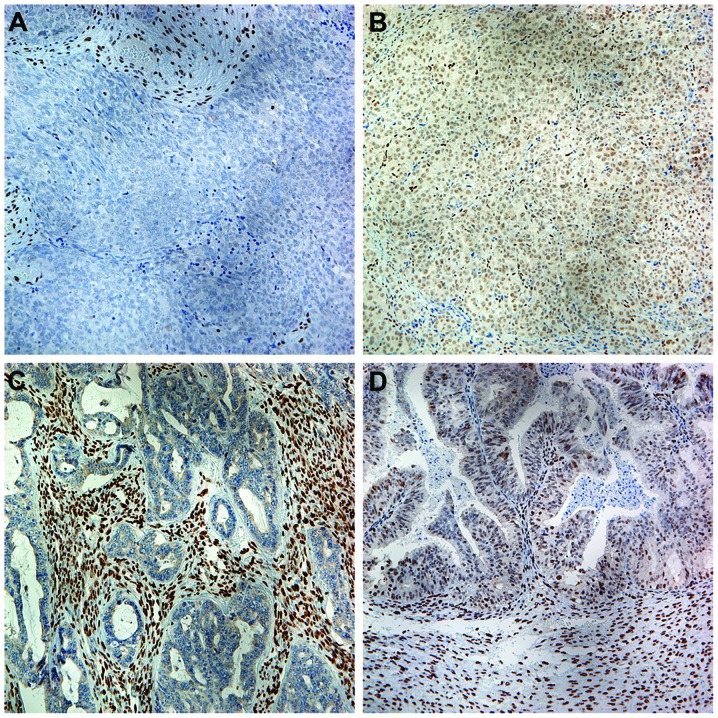
Variable nuclear immunoreactivity for ZEB1 was detected in ovarian carcinoma cells and the stromal cells in the ovarian primary tumor samples (Fig. 1). Of the 238 samples, 78 were positive for ZEB1 and the remaining 160 were negative (Table I). Compared with the tumor cells, ZEB1 expression in stromal cells was generally strong.
Figure 1.
ZEB1 expression in ovarian carcinoma. (A) Poorly differentiated ovarian carcinoma exhibiting negative immunostaining for ZEB1, while most of the stromal cells surrounding the tumor cells exhibit strongly positive nuclear staining (magnification, ×200). (B) Another poorly differentiated ovarian carcinoma exhibiting positive nuclear immunostaining of both tumor and stromal cells (magnification, ×200). (C) The stromal cells in a well-differentiated ovarian carcinoma were all strongly positive, while the tumor cells were negative for ZEB1 (magnification, ×200). (D) Both the tumor and stromal cells in a well-differentiated ovarian carcinoma exhibited positive nuclear staining (magnification, ×200). ZEB1, zinc finger E-box-binding homeobox factor 1.
Table I.
Association of ZEB1 expression in ovarian carcinoma with clinicopathological characteristics.
| ZEB1 expression in tumor cells by IHC | ||||
|---|---|---|---|---|
| Characteristics | Total (n=238) | Negative, n (%) (n=160) | Positive, n (%) (n=78) | P-value |
| Age (years) | 0.423 | |||
| ≤39 | 16 | 9 (56.2) | 7 (43.8) | |
| 40–49 | 38 | 26 (68.4) | 12 (31.6) | |
| 50–59 | 61 | 40 (65.6) | 21 (34.4) | |
| 60–69 | 69 | 46 (66.7) | 23 (33.3) | |
| ≥70 | 39 | 28 (71.8) | 11 (28.2) | |
| Missing | 15 | |||
| Histological subtype | 0.278 | |||
| Serous carcinoma | 157 | 97 (61.8) | 60 (38.2) | |
| Mucinous carcinoma | 17 | 14 (82.4) | 3 (17.6) | |
| Endometrioid carcinoma | 19 | 16 (84.2) | 3 (15.8) | |
| Clear-cell carcinoma | 10 | 7 (70.0) | 3 (30.0) | |
| Mixed epithelial tumor | 11 | 9 (81.8) | 2 (18.2) | |
| Undifferentiated tumor | 5 | 3 (60.0) | 2 (40.0) | |
| Unclassified tumor and others | 19 | |||
| FIGO stage | 0.011 | |||
| I+II | 43 | 32 (74.4) | 11 (25.6) | |
| III | 113 | 81 (71.7) | 32 (28.3) | |
| IV | 76 | 41 (53.9) | 35 (46.1) | |
| Not staged or missing | 6 | |||
| Histological differentiation | 0.249 | |||
| High | 19 | 13 (68.4) | 6 (31.6) | |
| Moderate | 61 | 45 (73.8) | 16 (26.2) | |
| Poor | 126 | 79 (62.7) | 47 (37.3) | |
| Not graded or missing | 32 | |||
ZEB1, zinc finger E-box-binding homeobox factor 1; IHC, immunohistochemistry; FIGO, International Federation of Gynecology and Obstetrics.
ZEB1 expression and its clinicopathological associations
The association of ZEB1 expression with age, histological subtype, tumor differentiation grade and FIGO stage were investigated (Table I). ZEB1 expression in ovarian carcinoma cells was associated with advanced FIGO stage, but not with age, histological subtype or tumor differentiation.
ZEB1 expression and survival probability
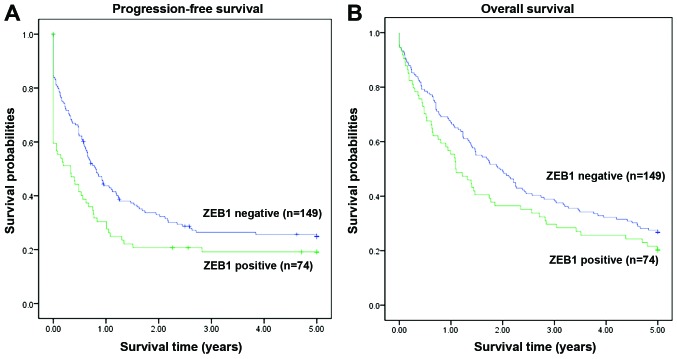
The survival analysis indicated that high expression of ZEB1 was associated with poor progression-free survival (PFS) (P=0.021, Fig. 2A). A similar tendency was also observed for the association of ZEB1 expression with 5-year overall survival (OS), although it did not reach statistical significance (P=0.118, Fig. 2B).
Figure 2.
Survival probabiliy in different zinc finger E-box-binding homeobox factor 1 (ZEB1) expression groups. (A) The group with positive ZEB1 expression in ovarian carcinoma cells had a poorer 5-year progression-free survival compared with the negative group (P=0.021, Kaplan-Meier method). (B) The ZEB1-negative group exhibited a trend for better 5-year-overall survival compared with the positive group, but the difference did not reach statistical significance (P=0.118, Kaplan-Meier method).
Multivariate analysis of 5-year PFS
The multivariate analysis revealed that the status of ZEB1 expression in cancer cells (P=0.021, Table II), tumor differentiation (P=0.020, Table II) and FIGO stage (P<0.001, Table II) were independent predictors of 5-year PFS in EOC.
Table II.
Multivariate analysis of 5-year progession-free survival in 238 confirmed ovarian carcinoma patients.
| Factors | HR | 95% CI | P-value |
|---|---|---|---|
| ZEB1 expressiona | 1.491 | 1.063–2.091 | 0.021 |
| Ageb | 1.104 | 0.961–1.269 | 0.163 |
| Differentiationc | 1.379 | 1.052–1.808 | 0.020 |
| FIGO staged | 1.689 | 1.334–2.140 | <0.001 |
| Histological subtypee | 0.905 | 0.811–1.267 | 0.905 |
Including negative and positive groups.
≤39 vs. 40–49 vs. 50–59 vs. 60–69 vs. ≥70 years.
High vs. moderate vs. poor differentiation.
Stage I vs. II vs. III vs. IV.
Serous vs. mucinous vs. endometrioid vs. clear-cell vs. mixed epithelial vs. undifferenciated. ZEB1, zinc finger E-box-binding homeobox factor 1; HR, hazard ratio; CI, confidence interval.
Discussion
Ovarian cancer has the highest mortality rate among all gynecological malignancies and EOC accounts for 90% of all ovarian cancers (2). Despite advances in surgery and chemotherapy, the 5-year survival rate remains ~30%, which is mainly attributed to these cancers being diagnosed at an advanced stage (2). The leading cause of relapse and death in patients with ovarian cancer is metastasis. Metastasis to the omentum, peritoneum, diaphragm and small bowel mesentery, have been confirmed as poor prognostic factors for EOC patients (17). Therefore, it is crucial to elucidate the mechanism underlying ovarian cancer metastasis.
The ZEB family of zinc finger transcription factors is necessary for embryonic development (12). Over the last few years, ZEB1 has emerged as an important regulator of EMT, required for cancer development and metastasis. A growing body of evidence indicates that ZEB1 overexpression may promote tumor progression (18–21). ZEB1 promotes EMT by repressing the genes contributing to the epithelial phenotype, while activating those associated with the mesenchymal phenotype (4,5). ZEB1 is expressed in estrogen-responsive tissues, such as breast, bone, uterus, endometrium, ovary, and the cardiovascular system, and high expression of this gene in normal ovarian and endometrial tissue is correlated with high estrogen levels. Measurements of ZEB1 mRNA in reproductive carcinomas have revealed high levels, yet independent of estrogen, in poorly differentiated endometrial and ovarian carcinomas (12). ZEB1 contributes to cell proliferation and migration and suppresses cell differentiation (4,5). Our study aimed to investigate the expression pattern of ZEB1 at the protein level in EOC tissues, evaluate its associations with tumor progression and patient prognosis, and evaluate ZEB1 as a prognostic marker and potential therapeutic target.
In this study, we observed that immunoreactive ZEB1 was variably detected in ovarian carcinoma cells and stromal cells. ZEB1 expression in the stromal cells was generally common and it was rather strong, which is seldom mentioned in other studies. Strong ZEB1 expression in stromal cells may be attributed to the fact that ZEB1 is able to activate genes required for the mesenchymal phenotype. However, the association of ZEB1 expression in the stromal cells with the clinicopathological parameters was not included in our present study.
IHC revealed that ZEB1 expression in ovarian carcinoma cells was significantly higher in FIGO stage IV cases (46.1%) (P=0.027, Table I). FIGO is the most popular clinically used staging system, and it is an important prognostic predictor in ovarian cancer. This supports the conclusion that ZEB1 may be associated with the development, as well as the invasion and metastasis of EOC. A study conducted by Yang et al (21) reported that overexpression of ZEB1 in esophageal squamous cell carcinoma was associated with tumor stage, lymph node metastasis, histological grade and depth of invasion. Furthermore, a study on gastric cancer conducted by Jia et al (19) demonstrated that overexpression of ZEB1 was associated with tumor differentiation, stage and depth of invasion. Theoretically, tumor differentiation is associated with the invasive and metastatic ability of tumors. Unlike the abovementioned studies on other carcinomas, the present investigation found no significant correlation between the expression of ZEB1 and tumor differentiation in EOC (P=0.249). Additionally, the expression of ZEB1 in gastric cancer tissue was independent of the patients' age (P>0.05); however, ZEB1 expression in tumor cells tended to be negative in mucinous and endometrioid carcinoma, although no significant difference was found in the present study.
The survival analysis indicated that high expression of ZEB1 was associated with poor 5-year PFS. A similar tendency was also observed for the association between high expression of ZEB1 and 5-year OS, although it did not reach statistical significance. In light of these findings, we suggest that the expression of ZEB1 in EOC is associated with an unfavorable prognosis. In addition to its effect on EMT, ZEB1 expression was also reported to be significantly associated with poor response to chemotherapy at diagnosis (22). Moreover, the multivariate analysis in our study demonstrated that the status of ZEB1 expression was an independent predictor of 5-year PFS in EOC, together with histological grade and FIGO stage.
Taken together, our data support the evidence suggesting that ZEB1 may play a crucial role in promoting aggressive EOC behavior and progression. Therefore, ZEB1 may serve as a predictive marker and a potential target for therapeutic intervention in EOC.
Acknowledgements
We would like to thank the Inger and John Fredriksen Foundation, the Radium Hospital Research Foundation and The Norwegian Cancer Society for the financial support. We would also like to thank Ellen Hellesylt, Mette Synnøve Førsund, Mai Nguyen and Don Trinh for technical support with IHC.
References
- 1.Dutta DK, Dutta I. Origin of ovarian cancer: Molecular profiling. J Obstet Gynaecol India. 2013;63:152–157. doi: 10.1007/s13224-013-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 4.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P, Sun Y, Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng G, Wang X, Cao X, Shen L, Zhu J. ZEB1 expression in endometrial biopsy predicts lymph node metastases in patient with endometrial cancer. Dis Markers. 2014;2014:680361. doi: 10.1155/2014/680361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Wang J, Chu M, Zhang K, Yang R, Gao WQ. Zeb1 promotes androgen independence of prostate cancer via induction of stem cell-like properties. Exp Biol Med (Maywood) 2014;239:813–822. doi: 10.1177/1535370214538727. [DOI] [PubMed] [Google Scholar]
- 9.Quan Y, Jin R, Huang A, Zhao H, Feng B, Zang L, Zheng M. Downregulation of GRHL2 inhibits the proliferation of colorectal cancer cells by targeting ZEB1. Cancer Biol Ther. 2014;15:878–887. doi: 10.4161/cbt.28877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayan AE. Tumour-promoting role of EMT-inducing transcription factor ZEB1 in mantle cell lymphoma. Cell Death Differ. 2014;21:194–195. doi: 10.1038/cdd.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellner U, Brabletz T, Keck T. ZEB1 in pancreatic cancer. Cancers (Basel) 2010;2:1617–1628. doi: 10.3390/cancers2031617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurt EM, Saykally JN, Anose BM, Kalli KR, Sanders MM. Expression of the ZEB1 (deltaEF1) transcription factor in human: Additional insights. Mol Cell Biochem. 2008;318:89–99. doi: 10.1007/s11010-008-9860-z. [DOI] [PubMed] [Google Scholar]
- 13.Cho KR, Shih IeM. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zivanovic O, Sima CS, Iasonos A, Hoskins WJ, Pingle PR, Leitao MM, Jr, Sonoda Y, Abu-Rustum NR, Barakat RR, Chi DS. The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by the initial tumor burden in the upper abdomen cephalad to the greater omentum. Gynecol Oncol. 2010;116:351–357. doi: 10.1016/j.ygyno.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang R, Ma Y, Holm R, Trope CG, Nesland JM, Suo Z. Sex hormone-binding globulin (SHBG) expression in ovarian carcinomas and its clinicopathological associations. PloS one. 2013;8:e83238. doi: 10.1371/journal.pone.0083238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang R, Wu D, Yuan Y, Li X, Holm R, Trope CG, Nesland JM, Suo Z. CD117 expression in fibroblasts-like stromal cells indicates unfavorable clinical outcomes in ovarian carcinoma patients. PloS One. 2014;9:e112209. doi: 10.1371/journal.pone.0112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehouli J, Senyuva F, Fotopoulou C, Neumann U, Denkert C, Werner L, Gülten OO. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol. 2009;99:424–427. doi: 10.1002/jso.21288. [DOI] [PubMed] [Google Scholar]
- 18.Harada K, Miyake H, Kusuda Y, Fujisawa M. Expression of epithelial-mesenchymal transition markers in renal cell carcinoma: Impact on prognostic outcomes in patients undergoing radical nephrectomy. BJU Int. 2012;110:E1131–E1137. doi: 10.1111/j.1464-410X.2012.11297.x. [DOI] [PubMed] [Google Scholar]
- 19.Jia B, Liu H, Kong Q, Li B. Overexpression of ZEB1 associated with metastasis and invasion in patients with gastric carcinoma. Mol Cell Biochem. 2012;366:223–229. doi: 10.1007/s11010-012-1299-6. [DOI] [PubMed] [Google Scholar]
- 20.Miyahara S, Hamasaki M, Hamatake D, Yamashita S, Shiraishi T, Iwasaki A, Nabeshima K. Clinicopathological analysis of pleomorphic carcinoma of the lung: Diffuse ZEB1 expression predicts poor survival. Lung Cancer. 2015;87:39–44. doi: 10.1016/j.lungcan.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Wang Q, Dai W, Zhang J, Chen X. Overexpression of zinc finger E-box binding homeobox factor 1 promotes tumor invasiveness and confers unfavorable prognosis in esophageal squamous cell carcinoma. Tumour Biol. 2014;35:11977–11984. doi: 10.1007/s13277-014-2494-8. [DOI] [PubMed] [Google Scholar]
- 22.Davidson B, Holth A, Hellesylt E, Tan TZ, Huang RY, Tropé C, Nesland JM, Thiery JP. The clinical role of epithelial-mesenchymal transition and stem cell markers in advanced-stage ovarian serous carcinoma effusions. Hum Pathol. 2015;46:1–8. doi: 10.1016/j.humpath.2014.10.004. [DOI] [PubMed] [Google Scholar]