Abstract
The present study investigated the anti-tumor activity of N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a potent and specific inhibitor of transient receptor potential cation channel subfamily M member 8 (TRPM8) in prostate cancer (PCa) DU145 cells. TRPM8 expression in DU145 and normal prostate PNT1A cells was detected by reverse transcription polymerase chain reaction and western blot analysis. The effect of BCTC on DU145 cells was analyzed by flow cytometry analysis, and MTT, scratch motility and Transwell invasion assays. The molecular mechanism through which BCTC acts was investigated by western blot analysis. TRPM8 expression was increased in DU145 cells compared with PNT1A cells at the mRNA and protein levels. The present study provided evidence that inhibition of TRPM8 by BCTC reduced the viability of DU145 cells, but not PNT1A cells. In addition, BCTC inhibited cell cycle progression, migration and invasion in DU145 cells. Cell cycle-associated proteins, including phosphorylated protein kinase B, cyclin D1, cyclin dependent kinase (CDK) 2 and CDK6 were downregulated by BCTC, while phosphorylated glycogen synthase kinase 3β was upregulated. However, investigations in the present study revealed that BCTC failed to trigger apoptosis in DU145 cells. In addition, in BCTC-treated DU145 cells, phosphorylated extracellular signal-regulated kinase 1/2 was downregulated substantially while phosphorylated p38 (p-p38) and phosphorylated c-Jun N-terminal kinases (p-JNK) were upregulated. The anti-proliferative activity of BCTC on DU145 cells was attenuated by p38 and JNK-specific inhibitors, suggesting that MAPK pathways are involved. Overall, the TRPM8 specific antagonist BCTC demonstrated excellent anti-tumor activity in PCa DU145 cells, and therefore has the potential to become a targeted therapeutic strategy against PCa.
Keywords: BCTC, inhibitor, prostate cancer, TRPM8, targeted therapy
Introduction
The literature reports that there are significant regional differences in the incidence and mortality rates of prostate cancer (PCa), particularly between Asian and European countries (1). Worldwide, ~900,000 men (33 per 100,000 men) were estimated to have been diagnosed with PCa in 2008, ~14% (122,000) of whom were diagnosed within the Asia-Pacific region (10 per 100,000 men), with three-quarters of these patients diagnosed in Japan (32%), China (28%) or Australia (15%) (2). In the early stages of PCa, the PCa cells depend on androgens for growth and survival, and androgen-ablative therapy is an effective therapeutic treatment (3,4). However, in later stages, PCa is more invasive and evolves to become androgen-independent, with a resistance to not only androgen-ablative therapy, but also to various other chemotherapy regimens (5–9). Currently, the treatment options for late-stage PCa remain relatively inefficient. Therefore, anti-tumor agents for PCa with high efficiency and safety are required.
Increasing evidence indicates that transient receptor potential (TRP) ion channels play important roles in human cancers (10,11). TRP melastatin 8 (TRPM8), a member of the TRP family, is a thermally regulated and nonselective Ca2+-permeable cation channel, and a novel specific marker of the prostate (12,13). TRPM8 is expressed abundantly in the prostate and its expression increases in PCa, suggesting that there is a huge potential for the successful treatment of PCa by specifically silencing or inhibiting TRPM8. The literature suggests that knockdown or blockade of TRPM8 has therapeutic potential in the treatment of several types of cancer, including prostate, pancreatic and oral squamous cancers (14–17). Zhang et al reported that knockdown of TRPM8 may lead to the suppression of proliferation in androgen-sensitive human prostate adenocarcinoma LNCaP cells (16). Valero et al demonstrated that inhibition of TRPM8 expression, by small interfering RNA, or function, by specific blockers such as AMTB and JNJ41876666, reduced the proliferation rate and proliferative fraction in PCa cells, but not in normal prostate cells (17). However, the current literature does not refer to the precise molecular mechanism underlying the action of TRPM8 gene silencing or its antagonists.
The aim of the present study was to identify whether N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a potent and specific antagonist of TRPM8 (18,19), exerts an anti-tumor effect on the androgen-independent PCa DU145 cells, and the mechanism of how the inhibition functions. The present study reports that BCTC exerts an anti-proliferative effect on DU145 cells and induces tumor suppression through G0/G1 cell cycle arrest, and inhibition of migration and invasion. This was demonstrated by cell cycle-associated molecules, consisting of phosphorylated protein kinase B (p-AKT), phosphorylated glycogen synthase kinase (p-GSK-3β), cyclin D1, cyclin dependent kinase (CDK) 2 and CDK6, and mobility-associated molecules, consisting of phosphorylated focal adhesion kinase (p-FAK) and matrix metalloproteinase (MMP) 2. These findings reveal that the blockade of TRPM8 by BCTC has the potential to become a targeted therapeutic strategy against PCa.
Materials and methods
Cell lines and chemicals
The LNCaP cell line, which was derived from a metastatic site of the left supraclavicular lymph node, the DU145 cell line, which was derived from a metastatic site in the brain, and the human immortalized prostatic cell line PNT1A were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Gibco RPMI-1640 medium containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified atmosphere containing 5% CO2. BCTC and vehicle dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
MTT assay
The cell viability was assessed using standard MTT assay according to the manufacturer's instructions (Sigma-Aldrich). The protocol was performed as follows: DU145 or PNT1A cells (5×103 per well) were cultured in a 96-well plate (Corning Incorporated, Corning, NY, USA). The cells were treated with various concentrations of BCTC or vehicle (DMSO; maximum concentration ≤0.5%), with 10 wells per group for statistical analysis, following which the cells were cultured in drugs for 72 h, and 20 µl MTT solution (Sigma-Aldrich) was added subsequent to drawing off the medium. The mixture was incubated for an additional 4 h at 37°C. The supernatant was removed and 150 µl DMSO added per well. Using an ELISA kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA), the optical density was measured at 490 nm. All experiments were repeated in triplicate.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from the DU145, PNT1A or LNCaP cells using Invitrogen TRIzol reagent (Thermo Fisher Scientific, Inc.). For RT analysis, 1 µg total RNA was reverse transcribed, using the Moloney murine leukemia virus reverse transcription system (Thermo Fisher Scientific). RT-PCR was performed by adding 2 µl RT reaction mixture to make a final volume of 20 µl. PCR was performed as follows: Pre-heating to 94°C for 2 min; 35 cycles of 94°C for 30 sec, 50°C for 30 sec and 72°C for 60 sec; and 1 cycle of final extension at 72°C for 10 min. The PCR primers used were as follows: TRPM8 (first PCR) forward, 5′-TGTTTTGCCCAAGGAGGTGG-3′ and reverse, 5′-CAACCAGTTTCCAGACAAACG-3′; TRPM8 (second PCR) forward, 5′-ATGGGCAGCTGAAGCTTC-3′ and reverse, 5′-CTGCAGATTCCGGTACAC-3′; and β-actin forward, 5′-TTAGTTGCGTTACACCCTTTC-3′ and reverse, 5′-GTCACCTTCACCGTTCCAGTT-3′.
Western blot analysis
The cells were washed twice with ice-cold phosphate buffered saline (PBS) and solubilized in 1% Triton lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) on ice. The protein samples were resolved in 7.5, 10, 12.5 or 15% SDS–PAGE (Promoton Biotechnology, Shanghai, China) and analyzed with specific antibodies for western blot analysis, as follows: p-Akt (dilution, 1:1,000; catalog no. 4060), Akt (dilution, 1:1,000; catalog no. 9272), p-GSK-3β (dilution, 1:1,000; catalog no. 9323), GSK-3β (dilution, 1:1,000; catalog no. 12456), phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2; dilution, 1:2,000; catalog no. 4370P), ERK1/2 (dilution, 1:2,000, catalog no. 4695P), phosphorylated c-Jun N-terminal kinase (p-JNK; dilution, 1:1,000; catalog no. 4668P), JNK (dilution, 1:1,000; catalog no. 9258P), phosphorylated p38 (p-p38; dilution, 1:1,000; catalog no. 4511P), p38 (dilution, 1:1,000; catalog no. 9212P); p-FAK (dilution, 1:1,000; catalog no. 8556), FAK (dilution, 1:1,000; catalog no. 3285), cleaved caspase-3 (dilution, 1:500; catalog no. 8202) and MMP2 (dilution, 1:1,000; catalog no. 4022), all obtained from Cell Signaling Technologies (Danvers, MA, USA); CDK2/4/6 (dilution, 1:1,000; catalog no. MS-299-P0; Neomarkers, Union City, CA, USA); cyclin D1 (dilution, 1:1,000; catalog no. sc-735), cyclin B1 (dilution, 1:1,000; catalog no. sc-245) and GAPDH (dilution, 1:1,000; catalog no. sc-166574), all obtained from Santa Cruz Biotechnology (Dallas, TX, USA); B-cell lymphoma 2 (Bcl2; dilution, 1:1,000; catalog no. 12789-1-AP) and Bcl2-associated X protein (Bax; dilution, 1:1,000; catalog no. 50599-2-Ig), obtained from ProteinTech Group (Chicago, IL, USA); and TRPM8 (dilution, 1:500; catalog no. ab3243; Abcam, Cambridge, UK).
Flow cytometry analysis of cell cycle arrest and apoptosis
DU145 cells were synchronized by serum starvation in a medium containing 0.1% serum for 24 h and induced to re-enter the cell cycle by an exchange of 10% FBS with either vehicle (DMSO) or various concentrations of BCTC. Subsequent to 24 h of incubation, the cells were harvested and washed twice with PBS, then re-suspended with pre-cooled 75% ethanol overnight, at 4°C. The cells were washed again with PBS and centrifuged twice at 15,000 × g, treated with RNase A (100 mg/ml; BD Pharmingen, San Diego, CA, USA) and propidium iodide (PI; 50 mg/ml; BD Pharmingen) at room temperature for 30 min in darkness. Various phases of the cell cycle were analyzed by flow cytometry (Becton-Dickinson, San Jose, CA, USA). For analysis of apoptosis, following incubation with various concentrations (0, 10, 100 µM) of BCTC for 48 h, the cells were detached from monolayers with trypsin and incubated in a binding buffer containing FITC-conjugated Annexin V (BD Pharmingen) and PI at room temperature for 5 min in darkness, prior to analysis by flow cytometry.
Scratch motility and Transwell invasion assays
For the scratch motility assay, DU145 cells were plated on a six-well plate to form confluent monolayers in complete medium (RPMI-1640 and 10% FBS). A standard 200 µl pipette tip was used to scratch across the wells, which were then washed with PBS. The length of the scratch was monitored from either end when the scratch was created and 24 h later, which is shorter than the time required for DU145 cells to double in number. The migration rates of the DU145 cells to colonize the scratch were calculated as the proportion of the mean distance between the points of the scratch to the distance that remained cell-free subsequent to colonization. These were normalized against the control (DMSO). The Transwell invasion was performed using Matrigel-coated porous upper chamber inserts (Becton and Dickinson, San Jose, CA, USA), which were plated with 5×104 cells in 0.5% FBS medium, with 600 µl complete medium in the lower chamber. Cotton-tipped swabs were used to remove non-invasive cells in the interior of the inserts 48 h later. The inserts were incubated with 500 µl 0.5% crystal violet (Roche Biochemicals, Mannheim, Germany) for 20 min at room temperature. Subsequent to thorough washing in PBS, the inserts were observed using an Olympus IX70 inverted phase contrast microscope (Olympus Corporation, Tokyo, Japan).
Statistical Analysis
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for one-way analysis of variance tests for all the data. All data are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
TRPM8 expression is increased in DU145 cells compared with PNT1A cells
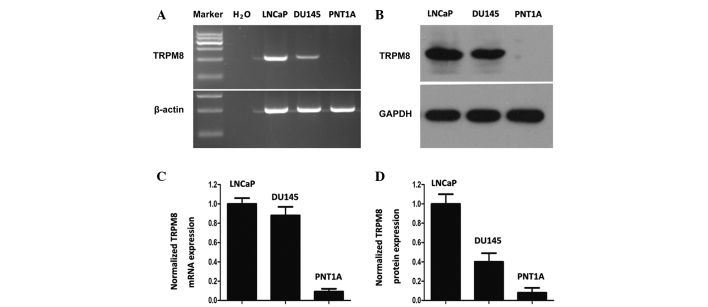
The expression of TRPM8 in DU145 cells was similar to that in LNCaP cells at the mRNA and protein levels (Fig. 1A and B). However, the PNT1A cells demonstrated little TRPM8 expression, as determined by grayscale analysis with Image J software (Fig. 1C and D). These results are mostly consistent with the results obtained by Valero et al (17). The differential expression profile of TRPM8 between tumor and non-tumor cells reveals the selective cytotoxic effect of chemical drugs towards tumor cells.
Figure 1.
Expression of TRPM8 in tumor and non-tumor prostate cells. (A and B) Expression of TRPM8 in LNCaP, DU145 and PNT1A cells, detected by (A) RT-PCR and (B) western blot analysis. β-actin and GAPDH were used as positive controls in RT-PCR and western blot analysis, respectively. Water was used as a negative control in RT-PCR. (C and D) TRPM8 expression was quantified from (C) RT-PCR and (D) western blot analysis. LNCaP cells were used as positive controls for the expression of TRPM8. TRPM8, transient receptor potential melastatin 8; RT-PCR, reverse transcription-polymerase chain reaction.
BCTC arrests the growth of DU145 cells
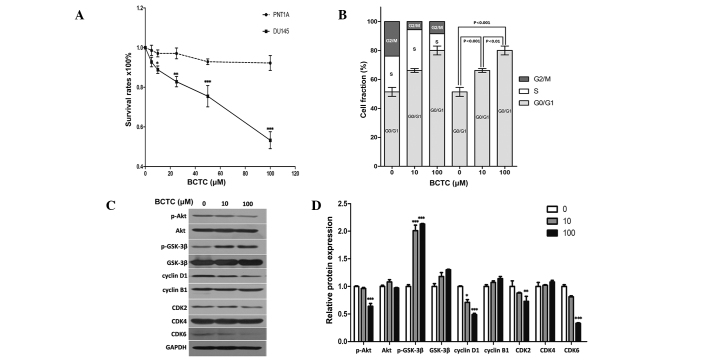
Since DU145 cells demonstrated considerable expression of TRPM8, the present study investigated whether the potent TRPM8 antagonist BCTC exerted an anti-proliferative effect in DU145 cells. The MTT assay established that BCTC reduced the growth of DU145 cells in a dose-dependent manner (Fig. 2A). Dose response data demonstrated a 12.03 and 50.69% growth inhibition at 10 µM and 100 µM, respectively, subsequent to a 72 h incubation. BCTC reduced proliferation rates in DU145 cells, but not in PNT1A cells, which indicated the potential for the highly selective anti-tumor activity of BCTC towards PCa cells, with minimal effects on normal prostate cells.
Figure 2.
BCTC suppressed the growth of DU145 cells and reduced G0/G1 phase cell cycle arrest. (A) BCTC reduced the growth of DU145 cells in a dose-dependent manner by standard MTT assay. Growth of DU145 cells was suppressed following incubation with 0, 20, 40, 60, 80 or 100 µM BCTC for 72 h, compared with PNT1A cells. MTT assays were conducted in triplicate. (B) BCTC induced G0/G1 phase arrest. DU145 cells were harvested 48 h subsequent to incubation with dimethyl sulfoxide, 10 µM BCTC or 100 µM BCTC, and the cell cycle distribution was examined. The G0/G1 phase rates of DU145 cells treated with various concentrations of BCTC were fractioned for statistical analysis. (C) Cell cycle-associated proteins, consisting of pAKT, AKT, pGSK-3β, GSK-3β, cyclin B1 and D1, and CDK2, 4 and 6 were detected by western blot analysis. Cells were harvested 48 h subsequent to the indicated drug incubation, and western blot analysis was performed. (D) Western blot analysis results were quantified and one-way analysis of variance was performed. *P<0.05; **P<0.01; ***P<0.001. Error bars indicate the mean ± standard deviation. BCTC, N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide; MTT assay, colorimetric assay; AKT, protein kinase B; GSK, glycogen synthase kinase; CDK, cyclin-dependent kinase; p-AKT, phosphorylated protein kinase B; p-GSK-3β, phosphorylated glycogen synthase kinase.
BCTC induces G0/G1 phase arrest rather than apoptosis in DU145 cells
Consistent with the results of the MTT assay, flow cytometric analysis of BCTC-treated DU145 cells indicated an increased proportion of cells in the G0/G1 phases of the cell cycle (Fig. 2B). Cell cycle-associated proteins, including Akt, GSK-3β, cyclin B1, cyclin D1 and CDK2/4/6, were detected by western blot analysis (Fig. 2C and D). p-Akt was downregulated by BCTC, while p-GSK-3β was upregulated, with each of their unphosphorylated forms unchanged. Cyclin D1, the most relevant protein in the cell cycle (20), was significantly downregulated, while cyclin-B1 remained unchanged. The expression levels of CDK2, 4 and 6 were also tested, and the expression of CDK2 and CDK6 was decreased by BCTC, but CDK4 levels remained unchanged. These results indicate that BCTC induced G0/G1 arrest by selectively modulating the expression levels of a subset of cell cycle regulator proteins.
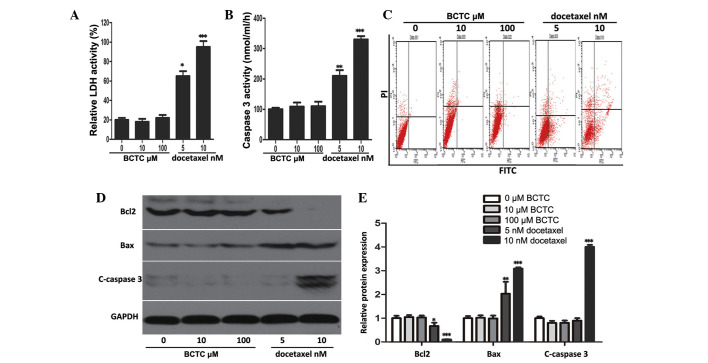
The ability of BCTC to trigger apoptosis was investigated through a series of experiments. It was found that BCTC did not elevate LDH or caspase-3 activity at concentrations of 5 and 10 nM, in comparison to docetaxel, a well-known and broad-spectrum chemotherapy agent (21), that acted as the positive control (Fig. 3A and B). Flow cytometric analysis revealed that BCTC did not increase the apoptotic rate of DU145 cells (docetaxel acted as the positive control and increased apoptosis) (Fig. 3C). Accordingly, to ascertain the mechanism of these results, western blot analysis was performed. The levels of proteins associated with apoptosis, consisting of Bcl2, Bax, and cleaved caspase-3, did not change in BCTC-treated DU145 cells, but in docetaxel-treated cells the levels of apoptosis-associated proteins changed (Fig. 3D and E). Overall, this data demonstrated that BCTC possesses the ability to induce cell cycle arrest, without triggering apoptosis.
Figure 3.
BCTC did not induce apoptosis in DU145 cells. (A and B) LDH release and caspase-3 activity were detected in BCTC-treated DU145 cells. The cells were treated with various concentrations of BCTC (0, 10 and 100 µM) or docetaxel (5 or 10 nM; positive control) for 72 h prior to performing LDH release and caspase-3 activity assays. LDH release and caspase-3 activity are expressed as a relative value to that of the untreated cells, which is set to 100%. (C) Cell apoptosis was analyzed by Annexin V-FITC assay. (D) Expression of apoptosis-associated proteins consisting of Bcl2, Bax, C-caspase-3, were investigated by western blot analysis. (E) Western blot analysis was quantified and one-way analysis of variance was performed. *P<0.05; **P<0.01; ***P<0.001. Error bars indicate the mean ± standard deviation. BCTC, N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide; LDH, lactate dehydrogenase; FITC, fluorescein isothiocyanate; Bcl2, B-cell lymphoma 2; c-caspase-3, cleaved-caspase-3; Bax, Bcl2-associated X protein.
BCTC inhibits the migration and invasion of DU145 cells
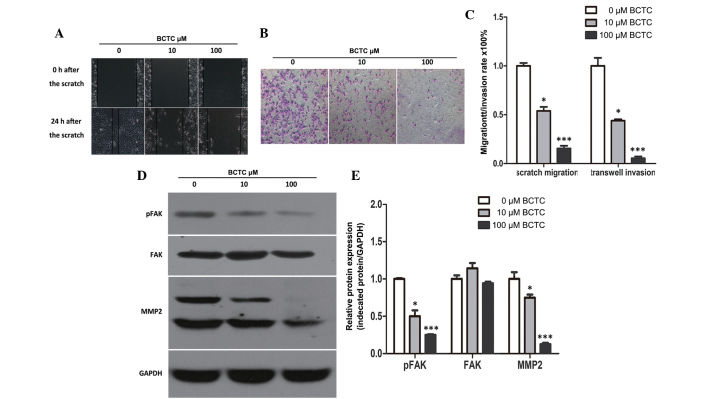
To investigate the potential effect of BCTC on the migration of DU145, a standard scratch motility assay was performed. Compared to the non-treated group, the migration of BCTC-treated DU145 cells was significantly reduced (Fig. 4A and C). Furthermore, BCTC reduced the invasion of DU145 cells in Matrigel-coated Transwell assays (Fig. 4B and C). Western blot analysis demonstrated the alteration of motility-associated protein levels in DU145 cells following incubation with BCTC. MMP2 and p-FAK were found to be downregulated by BCTC, which verified that BCTC inhibited the migration and invasion of DU145 cells (Fig. 4D and E).
Figure 4.
BCTC inhibited migration and invasion in DU145 cells. (A) BCTC inhibited cell migration in DU145 cells (magnification, ×400). The migration rate was expressed as a percentage of DU145 cells treated with a vehicle (dimethyl sulfoxide). Migration was suppressed in BCTC-treated DU145 cells. (B) BCTC inhibited cell invasion. Following pre-treatment for 48 h with 10 or 100 µM of BCTC, the number of invaded cells was significantly reduced compared to the untreated cells (magnification, ×100). (C) Quantitative data from (A) scratch and (B) Transwell invasion assays. (D) BCTC suppressed the expression of pFAK and MMP2, as assessed by western blot analysis. (E) Western blot analysis was quantified and one-way analysis of variance was performed. Error bars indicate the mean ± standard deviation. *P<0.05; ***P<0.001. BCTC, N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide; FAK, focal adhesion kinase; MMP2, matrix metalloproteinase-2; p-FAK, phosphorylated FAK.
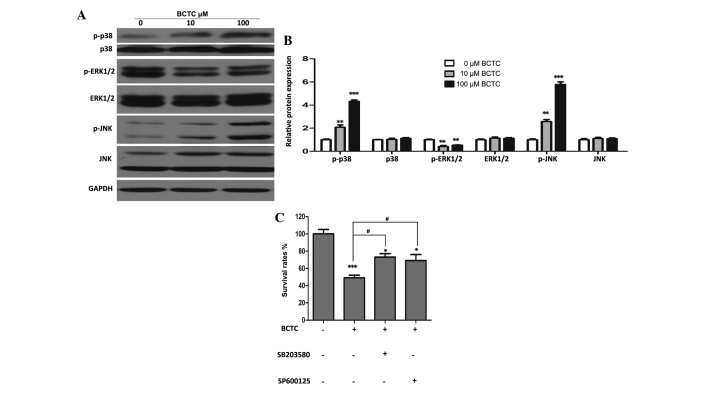
MAPK signal pathways may partially participate in the anti-tumor activity of BCTC
Western blot analysis revealed that p-ERK1/2 was downregulated substantially BCTC-treated DU145 cells, while p-p38 and p-JNK increased in comparison to the non-treated group, with all of their unphosphorylated forms unchanged (Fig. 5A and B). In addition, specific inhibitors of p38 and JNK (SB203580 and SP600125, respectively) attenuated the inhibition of proliferation of DU145 cells by BCTC (Fig. 5C), suggesting that MAPK pathways partially participate in the anti-tumor activity of BCTC.
Figure 5.
Mitogen-activated protein kinase signal pathways may partially be involved in the anti-tumor activity of BCTC towards DU145 cells. (A) DU145 cells were treated with various concentrations of BCTC for 48 h. Western blot analysis was performed to investigate the expression of p-p38, p38, p-ERK1/2, ERK1/2, p-JNK and JNK. (B) Western blot analysis was quantified and a one-way analysis of variance was performed. (C) DU145 cells were pre-treated with a p38 inhibitor (SB203580; 20µM) and a JNK inhibitor (SP600125; 10 µM) for 4 h, and then treated with 100 µM BCTC. After 48 h treatment, cell viability was determined by a MTT assay. Experiments were performed in triplicate. Western blot analysis was quantified and one-way analysis of variance was performed. Error bars indicate the mean ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001 vs. control; #P<0.05 vs. BCTC group. BCTC, N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide; p-ERK, phosphoryalted extracellular signal-regulated kinase; p-JNK, phosphorylated c-Jun N-terminal kinase; p-p38, phosphorylated p38.
Discussion
TRPM8 is a receptor-activated non-selective cation channel that is highly expressed in PCa cells, and in previous years has emerged as a promising prognostic marker and putative therapeutic target in PCa (11,16,17,22). Notably, TRPM8 induces tumor suppression when it is inhibited and also when activated. Since TRPM8 plays a key role in Ca2+ homeostasis of prostate epithelial cells, either over-expression or repression of TRPM8 will lead to a loss of Ca2+ homeostasis, and also to the degradation of cell viability (16,22,23). Previous studies have provided clear evidence that over-expression of TRPM8 in the androgen-independent PC3 cell line derived from metastatic sites in bone or the activation of TRPM8 using the classical TRPM8 agonist menthol in DU145 cells suppressed PCa cell viability (24,25). By contrast, Zhang et al and Valero et al demonstrated the anti-tumor effect of the knockdown or blockade of TRPM8 in PCa cells (16,17). In particular, Valero et al provided evidence that knockdown and antagonists of TRPM8, including BCTC, possessed the ability to inhibit the proliferation, cell cycle progression and migration of PCa cells (17). However, this study presented only the anti-tumor phenomena and the precise molecular mechanism was not discerned, which consequently was a major aim of the present study. To further explore the feasibility of targeting TRPM8, the present study chose the potent and specific antagonist BCTC to determine the precise mechanism of repressing PCa cells by inhibiting TRPM8.
In the majority of the literature associated with TRPM8, the dose of BCTC is usually <10 µM; however, the present study used 10 µM and 100 µM. In addition, in the literature, BCTC was mainly used to inhibit the internal Ca2+ flux of cells induced by exogenous TRPM8 agonists or cold temperature, and not to disturb the normal function of TRPM8 at physiological conditions (18,26,27). The techniques performed most often were Ca2+ imaging and patch clamp technique. However, these detect the immediate changes of Ca2+ flux or membrane potential. A higher dose of BCTC would be required if continuing changes of Ca2+ flux or membrane potential are expected to affect the normal function of cells. The present study revealed that BCTC did not clearly affect the proliferation of PNT1A cells, even at the dose of 100 µM, thereby demonstrating the safety and selectivity of this drug.
The present study demonstrated that BCTC induced evident G0/G1 cell cycle arrest in DU145 cells, through selectively modulating the expression of a subset of cell cycle regulators, including cyclin D1, CDK2 and CDK6. However, BCTC did not induce apoptosis of DU145 cells, which was verified by a series of experiments, including LDH and caspase-3 activity testing, flow cytometric analysis and determining the expression of apoptosis-associated proteins by western blot analysis.
In addition to cell cycle deregulation, metastasis is another important manifestation of cancer (25). FAK is a non-receptor protein tyrosine kinase that regulates adhesion-dependent cell signaling and is required for the invasion and metastasis of cancer cells (28,29). The MMP family plays a central role in cell migration, and the literature has indicated that stromal connective tissue and basement membranes are mostly degraded by the MMP family of proteins, which comprise >20 zinc-dependent endopeptidases (30). The present study indicated that blockade of TRPM8 by BCTC, through the downregulation of MMP2 and p-FAK, reduced the motility of DU145 cells.
MAPK family members are known to control cell cycle progression at various stages in a cell type- and context-specific manner (31). The present study revealed that p-ERK1/2 was substantially downregulated, while p-p38 and p-JNK were upregulated in BCTC-treated DU145 cells. Furthermore, specific inhibitors of p38 and JNK attenuated the inhibition of proliferation by BCTC, which suggests that MAPK pathways are partially involved in the anti-tumor activity of BCTC towards DU145 cells.
In summary, the present study demonstrated that blockade of TRPM8 by BCTC reduced proliferation, cell cycle progression, migration and invasion of DU145 cells, which is partially attributed to the alteration of MAPK signal pathways. These findings indicate that the TRPM8 inhibitor BCTC is a potential therapeutic strategy against PCa and provide novel insight into the understanding of PCa biology.
Acknowledgements
This study was supported by the Natural Science Foundation of China (grant nos.81172734 and 81202027) and the Specialized Research Fund for the Doctoral Program of Higher Education (grant no. 20120141120052).
References
- 1.Akaza H. Asian trends in primary androgen depletion therapy on prostate cancer. Cancer Biol Med. 2013;10:187–191. doi: 10.7497/j.issn.2095-3941.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal SA, Sandler HM. Treatment strategies for high-risk locally advanced prostate cancer. Nat Rev Urol. 2010;7:31–38. doi: 10.1038/nrurol.2009.237. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: New agents for an established target. Lancet Oncol. 2009;10:981–991. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–1721. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 6.Taplin ME. Drug insight: Role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–244. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan GF, Amenta PS, Villanueva JD, Alvarez CJ, Yang JM, Hait WN. The expression of drug resistance gene products during the progression of human prostate cancer. Clin Cancer Res. 1998;4:1393–1403. [PubMed] [Google Scholar]
- 8.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 9.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, Scher HI. Prostate cancer cell cycle regulators: Response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–1876. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 10.Wissenbach U, Niemeyer BA, Flockerzi V. TRP channels as potential drug targets. Biol Cell. 2004;96:47–54. doi: 10.1016/j.biolcel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937–946. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- 13.Henshall SM, Afar DE, Hiller J, Horvath LG, Quinn DI, Rasiah KK, Gish K, Willhite D, Kench JG, Gardiner-Garden M, et al. Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 2003;63:4196–4203. [PubMed] [Google Scholar]
- 14.Okamoto Y, Ohkubo T, Ikebe T, Yamazaki J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int J Oncol. 2012;40:1431–1440. doi: 10.3892/ijo.2012.1340. [DOI] [PubMed] [Google Scholar]
- 15.Yee NS, Brown RD, Lee MS, Zhou W, Jensen C, Gerke H, Yee RK. TRPM8 ion channel is aberrantly expressed and required for preventing replicative senescence in pancreatic adenocarcinoma: Potential role of TRPM8 as a biomarker and target. Cancer Biol Ther. 2012;13:592–599. doi: 10.4161/cbt.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 17.Valero ML, de Queiroz Mello F, Stühmer W, Viana F, Pardo LA. TRPM8 ion channels differentially modulate proliferation and cell cycle distribution of normal and cancer prostate cells. PloS One. 2012;7:e51825. doi: 10.1371/journal.pone.0051825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madrid R, Donovan-Rodríguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci. 2006;26:12512–12525. doi: 10.1523/JNEUROSCI.3752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mergler S, Mertens C, Valtink M, Reinach PS, Székely VC, Slavi N, Garreis F, Abdelmessih S, Türker E, Fels G, Pleyer U. Functional significance of thermosensitive transient receptor potential melastatin channel 8 (TRPM8) expression in immortalized human corneal endothelial cells. Exp Eye Res. 2013;116:337–439. doi: 10.1016/j.exer.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Alao JP. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trudeau ME. Docetaxel: a review of its pharmacology and clinical activity. Can J Oncol. 1996;6:443–457. [PubMed] [Google Scholar]
- 22.Kulkarni P. TRPM8 and prostate cancer: To overexpress or repress, that is the question-comment on “Effects of TRPM8 on proliferation and motility of prostate cancer PC-3 cells” by Yang ZH et al in Asian Journal of Andrology. Asian J Androl. 2009;11:150–151. doi: 10.1038/aja.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flourakis M, Prevarskaya N. Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta. 2009;1793:1105–1109. doi: 10.1016/j.bbamcr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Yang ZH, Wang XH, Wang HP, Hu LQ. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J Androl. 2009;11:157–165. doi: 10.1038/aja.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang X, Yang Z, Zhu G, Chen D, Meng Z. Menthol inhibits the proliferation and motility of prostate cancer DU145 cells. Pathol Oncol Res. 2012;18:903–910. doi: 10.1007/s12253-012-9520-1. [DOI] [PubMed] [Google Scholar]
- 26.Mälkiä A, Madrid R, Meseguer V, de la Peña E, Valero M, Belmonte C, Viana F. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol. 2007;581:155–174. doi: 10.1113/jphysiol.2006.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valero M, Morenilla-Palao C, Belmonte C, Viana F. Pharmacological and functional properties of TRPM8 channels in prostate tumor cells. Pflugers Arch. 2011;461:99–114. doi: 10.1007/s00424-010-0895-0. [DOI] [PubMed] [Google Scholar]
- 28.Parsons JT, Slack-Davis J, Tilghman R, Roberts WG. Focal adhesion kinase: Targeting adhesion signaling pathways for therapeutic intervention. Clin Cancer Res. 2008;14:627–632. doi: 10.1158/1078-0432.CCR-07-2220. [DOI] [PubMed] [Google Scholar]
- 29.Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway corassociate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- 30.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacCorkle RA, Tan TH. Mitogen-activated protein kinases in cell-cycle control. Cell Biochem Biophys. 2005;43:451–461. doi: 10.1385/CBB:43:3:451. [DOI] [PubMed] [Google Scholar]