Abstract
Polymorphisms in microRNA (miR) genes and their target sites are a distinct classification of variation in the human genome, which are rapidly being identified and investigated in human cancer. A polymorphism in the miR-196a-2 locus has demonstrated significant associations with various types of cancer, including lung, breast, esophageal and gastric tumors. However, miR-196a-2 has not been fully explored in ovarian cancer, which shares similar biological characteristics with other types of cancer. Therefore, the present study aimed to elucidate the association between a single nucleotide polymorphism (SNP) in the mature sequence of miR-196a-2 (rs11614913, T/C) and the clinical features of 479 Chinese patients with epithelial ovarian cancer (EOC). In addition, the biological significance of this polymorphism was investigated in the OVCAR3 ovarian cancer cell line. Risk association was evaluated in 479 cases of EOC patients and 431 controls. SNPs were analyzed by using polymerase chain reaction based restriction fragment length polymorphism assay. miR-196a expression was evaluated with reverse transcription polymerase chain reaction. The influence of miR-196a-2 rs11614913 T/C on EOC cell migration and invasion ability was further investigated in vitro. The results revealed significant differences in the homozygous CC genotype distribution in patients with EOC (n=479), compared with that of the control subjects (n=431; P=0.026). Analysis of the association between genotype and the risk of EOC revealed that individuals who carried the homozygous CC genotype were 1.34-fold more susceptible to EOC, compared with those carrying the wild-type TT and heterozygous CT genotypes [odds ratio, 1.34; 95% confidence interval, 1.04–2.17; P=0.023]. In addition, the role of this polymorphism in the production of mature miR-196a was investigated. Significantly enhanced production of mature miR-196a was revealed in the C-allelic compared with that of the T-allelic miR-196a-2 precursor (P<0.05). Further examination indicated that miR-196a significantly promoted cell migration and invasion ability in the human OVCAR3 ovarian cell line (P<0.05). In conclusion, the results indicated that the miR-196a-2 rs11614913 CC genotype may increase the risks of ovarian cancer by affecting the expression of mature miR-196a and enhancing cell migration/invasion. The current results provided evidence that the T>C polymorphism in the miR-196a-2 precursor may influence tumorigenesis and metastasis in EOC, and suggested that the functional SNP rs11614913 in the promoter region of pri-miR-196a-2 may be a potential indicator of EOC susceptibility in the population analyzed.
Keywords: rs11614913, epithelial ovarian cancer, polymorphism, microRNA-196a-2, reverse transcription-quantitative polymerase chain reaction
Introduction
Ovarian cancer only accounts for ~4% of cancer cases in women, but it is the eighth most common cause of cancer-associated mortality resulting from gynecological tumors worldwide (1,2). Epithelial ovarian cancer (EOC), which is categorized into serous, mucinous, endometrioid and non-clear cell types, accounts for 90% of all ovarian cancer (3,4). The earlier cancer is identified, the better the prognosis; however, ovarian cancer is difficult to detect early, as overt symptoms do not occur until later stages of the disease. Despite of advanced diagnostic technologies such as the combination of computed tomography/positron emission tomography scan and application of novel targeted drugs such as Bevacizumab and Olaparib, the 5-year survival rate ranges between ~30–50% (1,5). Furthermore, EOC is characterized by accelerated and aggressive growth, which may result in high recurrence rates. These features comprise the major challenges for the early diagnosis and effective treatment of patients with EOC (6). For a number of years, specific diagnostic biomarkers have been reported in various studies of EOC (7,8); Chong et al (9) conducted a microarray based microRNA expression study in primary and recurrent EOC patient tissues samples and identified 8 specific miRNAs whose expression was most significantly changed. In addition, Eitan et al (10) identified miR-200a, miR-34a, and miR-449b as the most downregulated miRNAs in advanced (stage III) ovarian tumors. Fan et al (11) reported the association of high levels of miR-196a expression and worse overall survival in ovarian cancer patients, especially in advanced stage tumors. Single nucleotide polymorphisms (SNPs) have also been examined as potential EOC risk indictors (12–14). However, the mechanism and genetic factors involved in the progression of this cancer remain to be elucidated.
MicroRNAs (miRNAs/miRs) are non-coding RNAs with complex biological functions that regulate the expression of numerous proteins through direct binding to the 3′-untranslated region of target genes (15,16). It has been well-documented that miRNAs are involved in a wide range of ovarian cancer tumorigenic processes, including malignant transformation, differentiation, proliferation and apoptosis (17–19).
Furthermore, SNPs located in miRNAs (miRSNPs) have begun to draw attention, due to their critical regulatory role in cancer progression. miRSNPs influence the transcription of the primary target gene, disturbing pri-/pre-miRNA processing, or affecting miRNA-mRNA interactions (20).
A previous study located the common genetic variant rs11614913 in the 3p mature miRNA region of Homo sapiens (hsa)-miR-196a2, and revealed that it led to a variation from G:T to G:C in the stem region of the miR-196a-2 precursor (21). Previous studies have suggested that this SNP may affect the processing of pre-miRNA and is associated with risk in various types of cancer, including breast and colorectal cancer (22–24). However, the genetic factors associated with EOC susceptibility have not previously been identified. It was hypothesized that the miR-196a-2 genotype may be associated with susceptibility to EOC. The present study aimed to investigate this hypothesis in a population-based case-control study and determine the function of this variant in the OVCAR3 cell line in vivo.
Materials and methods
Study population
The study protocol was approved by the Institutional Review Board of Zhengzhou University (Zengzhou, China) and Henan Academy of Medical Science (Zengzhou, China). The present study included 479 women with EOC that underwent tumor resections in the First Affiliated Hospital of Zhengzhou University and Woman and Infants Hospital of Zhengzhou between 2006 and 2012. In addition, 431 healthy women living in the same area, without any history of hereditary or malignant disease were selected as a control group. All patients and controls were of the one ethnic group (Han Chinese). Healthy control individuals were recruited from a large pool of individuals seeing a physician for routine health checkups in the First Affiliated Hospital of Zhengzhou University and Woman and Infants Hospital of Zhengzhou. Both cases and controls were prospectively recruited and matched by age and ethnicity. Demographic and epidemiology information, including height and weight were collected for all subjects. Histopathological diagnoses and clinical stages were classified based on the criteria of the International Federation of Gynecology and Obstetrics (25). Genomic DNA was immediately extracted from 5 ml peripheral blood samples of all patients using a Wizard Genomic DNA Purification kit (Promega Corp., Madison, WI, USA) and stored at −80°C. Written informed consent was obtained from all participants or their families if direct consent could not be obtained.
Genotyping
Genotyping was performed using a polymerase chain reaction (PCR)-based restriction fragment length polymorphism assay. The following primers were used to amplify the rs11614913 T>C polymorphism site in the miR-196a-2 precursor: F 5′-CCCCTTCCCTTCTCCTCCAGATA-3′ and R 5′-CGAAAACCGACTGATGTAACTCCG-3′. All primers was synthesized by Shengong Company (Shanghai, China). PCR was performed with a total volume of 25 µl with 100 ng DNA template, 2.5 µl of 10X PCR buffer, 1 U of Taq DNA polymerase, 0.2 mM dNTPs (Invitrogen, Carslbad, CA, USA) and 0.5 µmol/l of each primer. The PCR conditions were 94°C for 5 min followed by 35 cycles of 30 sec at 94°C, 30 sec at 63°C, and 1 min at 72°C, and final elongation step at 72°C for 10 min. A total of 10 µl PCR product was then digested using 2 µl (10 U/µl) Fermentas MspI restriction enzyme (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) for 16 h at 37°C. The resulting fragments were separated by electrophoresis on a 3.0% agarose gel (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and visualized in three distinct patterns of restriction fragments. The CC genotype produced two fragments (125 and 24 bp), the TT homozygote produced one 149-bp fragment and the TC heterozygote produced three fragments (125, 149 and 24 bp). The experiment was performed in triplicate; 10% of the PCR products were randomly selected from each of the miRNA polymorphism patterns for repeat assay and validated by direct DNA sequencing using an ABI3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
Tissue samples
To detect the expression levels of miRNA-196a, ovarian cancer tissue samples were collected from 83 patients who were histopathologically diagnosed with EOC and had undergone resection for EOC between June and October 2010 at Henan Cancer Hospital (Zhengzhou, China). The recruitment process was the same as outlined above. No patients had received radiotherapy or chemotherapy prior to undergoing surgery.
Reverse transcription-quantitative PCR (RT-qPCR)
RNA was isolated from the ovarian cancer tissues using the Applied Biosystems mirVana miRNA Isolation kit (Thermo Fisher Scientific, Inc.). The quality of miRNA extracted was determined using a 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). Samples with a high RNA Integrate Number (RIN) of miRNA (>7.0) were included in subsequent investigations. RIN was calculated using Agilent 2100 Expert software (Agilent Biotechnology) (26). RNA was extracted from 83 tissue samples and nearly 40 were selected for RIN calculation in the present study.
An RT-qPCR assay (Applied Biosystems TaqMan microRNA; Thermo Fisher Scientific, Inc.) was used to determine mature miRNA expression levels. The primers were as follows: Forward, 5′-GCTCTGGCTCCGTGTCTTCACTCCC-3′ and reverse, 5′-TGCCCCAGCACAGCCCCCGTCCCCC-3′. The following primers were used to amplify the β-actin gene, which was used as the internal control: Forward, 5′-AGAAAATCTGGCACCACACC-3′ and reverse, 5′-GGGGTGTTGAAGGTCTCAAA-3′. Small nuclear RNA U6 (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for normalization. The PCR was performed in a 20 µl reaction (2 µl of template cDNA, 1 µl of 10 µmol/l primers, 10 µl of 2X SYBR Green Master Mix, 4 µl of 25 mmol/l Mg2+ and 2 µl of ddH2O) was performed with the following cycling parameters: 3 min at 95°C followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec. All quantitative reactions were performed in an Applied Biosystems ABI 7000 Real-Time PCR System (Thermo Fisher Scientific, Inc.) in triplicate. Quantification cycle values were obtained from Applied Biosystems ABI PRISM 7000 SDS software, version 1.2.3 and used to calculate relative expression: Transcript levels were calculated according to the comparative cycle threshold (Cq). The 2−(∆∆Cq) method was applied to present final results, where ∆Cq for each sample was determined by subtracting the value for RNA U6 gene. Only triplicates with Cq values with a standard deviation <0.20 were acceptable.
Transient transfection and miRNA expression in an ovarian cancer cell line
To further explore the influence of this polymorphism on the production of mature miRNA, the commercial expression plasmid pCMV-MIR-196a-2 rs11614913-T/C (OriGene Technologies, Inc., Beijing, China) was transfected into OVCAR3 cells (American Type Culture Collection, Manassas, VA, USA), which exhibits wild-type and low expression of miR-196a. Briefly, 5×104 OVCAR3 cells/well were seeded on 24-well plates 24 h prior to transfection. A total of 1 µg of DNA vector diluted in 100 µl Opti-MEM I (ThermoFisher Scientific); 3 µl transfection agent MegaTran 1.0 (OriGene) was added to diluted DNA and incubate for 10 min at room temperature; 100 µl Megatran/DNA mixture was then added into each well and incubate 37°C for 48 h. An empty vector (pCMV-MIR) transfection was performed in parallel as a negative control. Transfection efficiency was confirmed by monitoring the GFP signal contained in the vector. The total RNA of cells that carried pCMV miR-196a-2 rs11614913 T or C was extracted and qPCR was used to detect miR-196a expression following 72 h of culture.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay
Cell proliferation was evaluated using an MTT cell viability assay. Cells (3×103/ml) were initially seeded in a 96-well plate and collected once growth in the log phase was achieved. A total of 10 µl MTT (5 mg/ml; Sigma-Aldrich) was added at 24 and 36 h following plasmid transfection, and cells were cultured for a further 4 h. Subsequently, cells were lysed with dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA). The ratio of optical density (OD) was measured at an absorbance of 490 nm using a Bio-Rad Laboratories 680 microplate reader, and the assay was repeated a minimum of three times.
Cell invasion and migration assay
Trypsinized cells of the various groups were collected from culture flasks and resuspended at a density of 2×105/ml in Dulbecco's modified Eagle's medium (Sigma-Aldrich). This cell suspension (200 µl) was added to the upper chamber of an 8-µm pore-size Transwell® insert (Corning Life Sciences, Carlsbad, CA, USA) in 6 wells. For the invasion assay, the Transwell was coated with 100 µl Matrigel (Corning Life Sciences). Subsequently, 600 µl RPMI-1640 culture solution (Sigma-Aldrich) containing 10% fetal bovine serum was added to the lower chamber of each well. Incubation at 37°C occurred for 24 h for migration and 48 h for invasion detection. Non-migratory cells on the upper surface of the membrane were removed, stained with 0.1% crystal violet and counted: the number of invading and migrated cells under the microscope at approximately 40X total magnification. Count cells in five randomly selected fields in triplicate.
Statistical analysis
The SPSS software package (version 11.5; SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Hardy-Weinberg equilibrium analysis was initially performed and then the χ2 test, was used to compare the distribution of SNPs between EOC patients and healthy controls. The odds ratio (OR) and 95% confidence interval (CI) were calculated using an unconditional logistic regression model. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The current results showed that 164 patients with EOC were stage I/II, while 315 were stage III/IV. The predominant tumor pathology type observed was serous-papillary (163 samples), followed by 121 endometrioid, 95 undifferentiated, 60 mucinous and 40 no clear cell-type samples (Table I). The 431 healthy control women exhibited a similar age distribution to that of the patients with EOC included in the current study.
Table I.
Baseline characteristics of the EOC cases and controls investigated.
| Characteristic | EOC patients, n(n=479) | Controls, n(n=431) |
|---|---|---|
| Age, years | ||
| <41 | 75 | 67 |
| 41–50 | 118 | 112 |
| 51–60 | 168 | 156 |
| >60 | 118 | 106 |
| Tumor histology | ||
| Serous | 163 | |
| Mucinous | 60 | |
| Endometrioid | 121 | |
| Undifferentiated | 95 | |
| No cell type | 40 | |
| Tumor stage | ||
| I/II | 164 | |
| III/IV | 315 |
EOC, epithelial ovarian cancer.
miR-196a-2 polymorphism CC genotype increases EOC risk
The observed genotype distribution of the miR-196a-2 polymorphism in the EOC and control groups conformed to the Hardy-Weinberg equilibrium (P=0.600 and P=0.243, respectively). The current results revealed a significantly higher frequency of CT and CC genotypes in the patients compared with that of the control group (51.6 vs. 47.1%, P=0.063 and 25.2 vs. 20.0%, P=0.018, respectively; Table II). Subsequent grouping of the TT and CT genotypes in the recessive genetic model revealed a significantly increased risk of ovarian cancer in CC genotype carriers when compared with that of the wild-type homozygous TT and heterozygous CT genotype carriers (OR, 1.36; 95% CI, 1.04–2.17; P=0.023).
Table II.
Association between the microRNA-196a-2 single nucleotide polymorphism and the risk of EOC.
| Genotype | EOC cases, n (%; n=479) | Control, n (%; n=431) | ORa (95% CI) | P-value |
|---|---|---|---|---|
| TT | 111 (23.2) | 142 (32.9) | 1 (Reference) | |
| CT | 247 (51.6) | 203 (47.1) | 1.53 (0.77–2.12) | 0.063 |
| CC | 121 (25.2) | 86 (20.0) | 1.78 (1.03–2.05) | 0.018 |
| TT/CTb | 358 (74.8) | 345 (80.0) | 1 (Reference) |
OR and P-values were calculated by multivariate unconditional logistic regression, adjusted for age.
Comparison of TT/CT vs. CC genotypes resulted in an OR (95% CI) of 1.36 (1.04–2.17) (P=0.023). EOC, epithelial ovarian cancer; OR, odds ratio; CI, confidence interval.
rs11614913-C enhances mature miR-196a expression
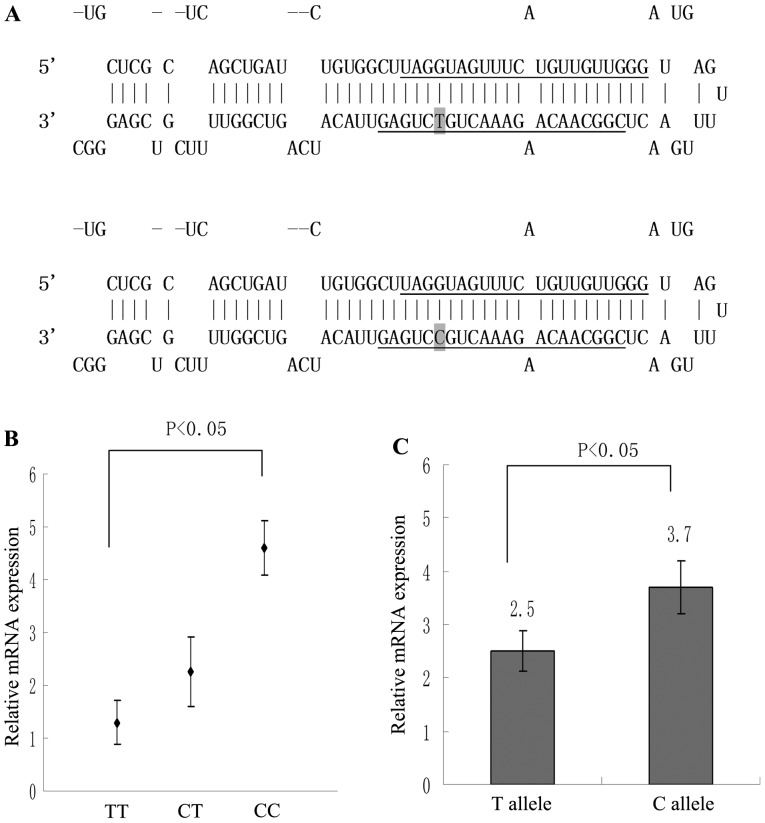
The effect of miR-196a rs11614913 on mature miR-196a expression was analyzed in 83 ovarian cancer tissue samples. Fig. 1A indicates the site-specific mutagenesis at rs11614913 from T to C in the plasmid used in the current study. Significantly upregulated expression of miR-196a was observed in CC genotype patients compared with that of the TT genotype (P<0.05; Fig. 1B). Furthermore, the CT genotype demonstrated increased expression of miR-196a compared with that of the TT genotype, however, no significant difference was observed (P>0.05; Fig. 1B). Following 72 h of culture, pCMV-MIR-miR-196a-2 rs11614913-C-transfected OVCAR3 cells expressed significantly increased expression levels of miR-196a when compared with those of the wild-type T allele group (P<0.01; Fig. 1C).
Figure 1.
miR-196a-2 stem loop and influence of rs11614913 on the production of mature miR-196a. (A) Schematic diagram of the hairpin loop structure of the T and C allelic miR-196a-2 precursor. The sequences for mature miR-196a-2 5p and 3p are underlined. The polymorphism site is indicated by grey highlighting. The T/C polymorphism is located in the stem region opposite to the mature miR-196a-2 sequence and results in a change from a G:U to a G:C pair in the stem structure of the precursor. (B) Correlation between miR-196a expression and various genotypes of rs11614913 in epithelial ovarian cancer tissue samples. (C) Expression of miR-196a in mature cells transfected with various rs11614913 plasmids. The result was reproducible in three independent experiments. miR, microRNA; mRNA, messenger RNA. Data are presented as the mean ± SEM; error bars represent SEM.
miR-196a SNP promotes cell proliferation, migration and invasion
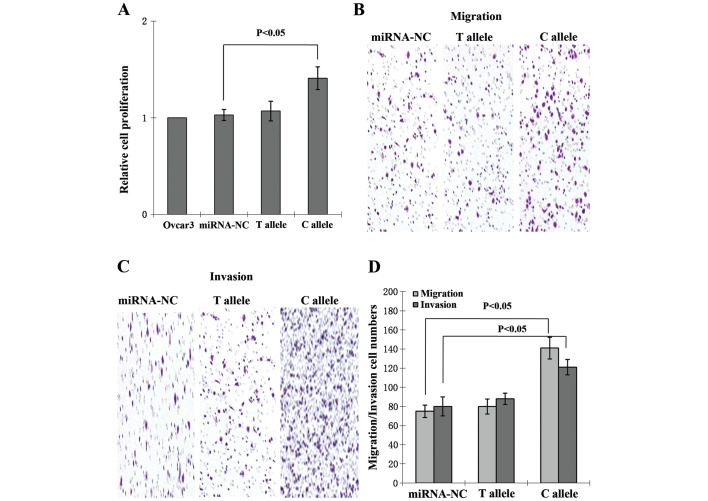
Following transfection, no significant differences in cell viability were observed between the control and T allele groups. Proliferation in the C allele group was significantly increased compared with that of the control group (P<0.05; Fig. 2A). The migration capacity of C allele-transfected cells was significantly enhanced compared with that of the non-transfected and T allele-transfected groups (Fig. 2B). The number of cells migrating to Matrigel was counted, and compared with non-transfected and T allele-transfected cells, invasion was increased in C allele-transfected cells (Fig. 2C). The increases in migration and invasion rates were significant (P<0.05; Fig. 2D).
Figure 2.
Promotion of cell proliferation and migration/invasion ability by miR-196a-2 rs11614913. (A) Cell viability was significantly increased in C allele cells, as demonstrated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test. (B) Crystal violet staining of EOC cells that crossed the polycarbonate membrane of the Transwell® chamber, revealing the migration of cells (magnification, ×200). (C) Crystal violet staining of EOC cells that crossed the Matrigel®-coated polycarbonate membrane of the Transwell chamber, demonstrating the invasion of cells (magnification, ×200). (D) Migration and invasion ability was significantly enhanced in C allele plasmid-transfected cells. miRNA-NC, microRNA negative control; EOC, epithelial ovarian cancer. Data are presented as the mean ± SEM; error bars represent SEM.
Discussion
It is well established that miRNAs participate in the initial and developmental stages of numerous types of cancer, as oncogenes or tumor suppressors (27–29). However, the correlation between miRNA coding region variants and cancer risk, as well as prognosis, has not been fully elucidated (30,31). The present study revealed the distribution frequencies of miRNA miR-196a-2 rs11614913 in a Han Chinese population with EOC. An association between the CC genotype of miR-196a-2 rs11614913 and a 1.34-fold increase in ovarian cancer risk was observed. In addition, it was demonstrated that the miR-196a-2 rs11614913 C allele induced mature miR-196a expression in vivo in tissue and in vitro in cells. Finally, mechanism data suggested that the alternation of mature miR-196a-2 expression may result in abnormal cell viability and migration/invasion capacity, which may eventually be responsible for increasing susceptibility to ovarian cancer. To the best of our knowledge, the present study is the first combined clinical and cell functional study of the association between rs11614913 SNP and susceptibility to ovarian cancer.
It is widely accepted that gene variants located in pri- and pre-miRNA regions may influence the biological functions of miRNAs and eventually lead to alterations in disease incidence (32). Considering this, various studies have investigated the application of pri- and pre-miRNA polymorphisms as predictors of cancer risk (29,31,33). Notably, miRNAs are frequently located in cancer-associated genomic regions (34) and may regulate almost all cancer-associated genes (35). The SNPs in miRNAs may perturb multiple miRNA-mediated gene regulations and make it a novel cancer diagnostic and prognostic biomarker. One example is the G to C variant (rs2910164) of the miR-146a precursor, which induces a change from a G:U pair to a C:U mismatch in the stem region. Evidence indicates that this variant may facilitate early diagnosis in patients with ovarian cancer (36). Another study demonstrated that carriers of the variant homozygote CC of miR-196a-2 were more likely to develop gastric cancer compared with wild-type homozygote TT and heterozygote CT carriers (adjusted OR, 1.57; 95% CI, 1.03–2.39; P=0.038) (37). In the same study, it was also indicated this C allele was significantly associated with lymph node metastasis of gastric cancer (adjusted OR, 2.25; 95% CI, 1.21–4.18; P=0.011) (37).
Hu et al (30) previously reported significantly higher expression of miR-196a in non-small cell lung tumor samples with CC genotypes compared with that of CT and TT individuals. However, no observed differential expression was found in pri- and pre-miRNA sequences based on genotype in subsequent studies (21,38). Hoffman et al (33) demonstrated upregulation of mature miR-196a expression in breast cancer cells transfected with pre-miR-196a-C compared with pre-miR-196a-T-transfected empty vector control; however, marked differential expression of the precursor miRNA was not observed in this study. Similarly, the present study revealed upregulation of miR-196a in patients with ovarian cancer who carried the C allele and in pre-miR-196a-C-transfected cells. Similarly, the present study identified that CC genotype carriers were more susceptible to ovarian cancer compared with TT and CT genotype carriers. Together, these results suggest that the rs11614913 polymorphism may affect the processing of the pre-miRNA to its mature form.
To clarify the impact of miR-196a on tumor cells, a series of functional experiments were performed in the present study. It was observed that elevated miR-196a expression from C allele-transfected cells promoted cell proliferation, migration and invasion capacity in vitro. A previous study, conducted in colorectal cancer, demonstrated a positive correlation between enhanced migration, invasion ability and increased expression levels of miR-196a (39). Results derived from studies of other types of cancer, including lung (38), colorectal (40) and early breast (22) cancer, also demonstrated similar results, suggesting that the miR-196a-2 genotype may result in altered processing of the pre-miRNA. However, other studies have reported the absence of an association between miR-196a-2 rs11614913 and the risk for various types of cancer or disease (41–43). These contradictory results may be a result of population sample selection bias or differences in human genotype distribution. A large population-based gene variant correlation study should be conducted to overcome this limitation.
Despite the merits of the current study, including it being the first report of miRNA SNPs and EOC risk, as well as having a relatively large study population and a high statistical power, certain limitations should be taken into consideration. All the patients were clinically followed up for 12–24 months. The short follow-up period for certain patients, particularly those of stage I, mean that analysis of the association between this SNP and EOC prognosis could not be performed. In addition, the direct target genes of hsa-miR-196a-2 miRNA were not identified in the current study; this will require further exploration in future in vitro and in vivo investigations.
In conclusion, the present study suggested a potential role for miR-196a-2 rs11614913 in predicting EOC risk, and indicated the critical use of miRNAs as diagnostic and prognostic biomarkers in cancer.
Acknowledgements
The present study was supported by a grant from the National Natural and Science Foundation of China (grant no. 81202070).
References
- 1.Ovarian Cancer 2014 Report. www.wcrf.org/sites/default/files/Ovarian-Cancer-2014-Report.pdf. [Jul 21;2014 ];World Cancer Research Fund/American Institute for Cancer Research, Food, Nutrition, Physical Activity, and the Prevention of Cancer. Accessed. [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Lyon, France: International Agency for Research on Cancer; [Jul 21;2014 ]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Accessed. [Google Scholar]
- 3.Sung PL, Chang YH, Chao KC, Chuang CM. Task Force on Systematic Review and Meta-analysis of Ovarian Cancer: Global distribution pattern of histological subtypes of epithelial ovarian cancer: A database analysis and systematic review. Gynecol Oncol. 2014;133:147–154. doi: 10.1016/j.ygyno.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Guo Z, Wu C, Li Y, Kang S. A polymorphism at the miR-502 binding site in the 3′ untranslated region of the SET8 gene is associated with the risk of epithelial ovarian cancer. Cancer Genet. 2012;205:373–376. doi: 10.1016/j.cancergen.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Suh DH, Lee KH, Kim K, Kang S, Kim JW. Major clinical research advances in gynecologic cancer in 2014. Gynecol Oncol. 2015;26:156–167. doi: 10.3802/jgo.2015.26.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 7.Munksgaard PS, Blaakaer J. The association between endometriosis and gynecological cancers and breast cancer: A review of epidemiological data. Gynecol Oncol. 2011;123:157–163. doi: 10.1016/j.ygyno.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Gram IT, Lukanova A, Brill I. Cigarette smoking and risk of histological subtypes of epithelial ovarian cancer in the EPIC cohort study. Int J Cancer. 2012;130:2204e2210. doi: 10.1002/ijc.26235. [DOI] [PubMed] [Google Scholar]
- 9.Chong GO, Jeon HS, Han HS, Son JW, Lee YH, Hong DG, Lee YS, Cho YL. Differential MicroRNA Expression Profiles in Primary and Recurrent Epithelial Ovarian Cancer. Anticancer Res. 2015;35:2611–2617. [PubMed] [Google Scholar]
- 10.Eitan R, Kushnir M, Lithwick-Yanai G, David MB, Hoshen M, Glezerman M, Hod M, Sabah G, Rosenwald S, Levavi H. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol. 2009;114:253–259. doi: 10.1016/j.ygyno.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Fan Y, Fan J, Huang L, Ye M, Huang Z, Wang Y, Li Q, Huang J. Increased expression of microRNA-196a predicts poor prognosis in human ovarian carcinoma. Int J Clin Exp Pathol. 2015;8:4132–4137. [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan C, Liu X, Yan S, Wang C, Kong B. Analyzing association of the XRCC3 gene polymorphism with ovarian cancer risk. BioMed Res Int. 2014;2014:648137. doi: 10.1155/2014/648137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Padilla I, Amir E, Marsh S, Liu G, Mackay H. Genetic polymorphisms as predictive and prognostic biomarkers in gynecological cancers: A systematic review. Gynecol Oncol. 2012;124:354–365. doi: 10.1016/j.ygyno.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Vella N, Aiello M, Russo AE, Scalisi A, Spandidos DA, Toffoli G, Sorio R, Libra M, Stivala F. ‘Genetic profiling’ and ovarian cancer therapy (Review) Mol Med Rep. 2011;4:771–777. doi: 10.3892/mmr.2011.512. [DOI] [PubMed] [Google Scholar]
- 15.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 17.Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579:5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 18.Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Yu CY, Wang JL, Guan J, Chen HY, Fang JY. MicroRNA sequence polymorphisms and the risk of different types of cancer. Sci Rep. 2014;4:3648. doi: 10.1038/srep03648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 21.Christensen BC, Avissar-Whiting M, Ouellet LG, Butler RA, Nelson HH, McClean MD, Marsit CJ, Kelsey KT. Mature microRNA sequence polymorphism in MIR196A2 is associated with risk and prognosis of head and neck cancer. Clin Cancer Res. 2010;16:3713–3720. doi: 10.1158/1078-0432.CCR-10-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Seo JW, Chae YS, Kim JG, Kang BW, Kim WW, Jung JH, Park HY, Jeong JY, Park JY. Genetic polymorphism of miR-196a as a prognostic biomarker for early breast cancer. Anticancer Res. 2014;34:2943–2949. [PubMed] [Google Scholar]
- 23.Hoffman AE, Zheng T, Yi C, Leaderer D, Weidhaas J, Slack F, Zhang Y, Paranjape T, Zhu Y. microRNA miR-196a-2 and breast cancer: A genetic and epigenetic association study and functional analysis. Cancer Res. 2009;69:5970–5977. doi: 10.1158/0008-5472.CAN-09-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang N, Li Y, Zhu LJ, Zhou RM, Jin W, Guo XQ, Wang CM, Chen ZF, Liu W. A functional polymorphism rs11614913 in microRNA-196a2 is associated with an increased risk of colorectal cancer although not with tumor stage and grade. Biomed Rep. 2013;1:737–742. doi: 10.3892/br.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prat J. FIGO Committee on Gynecologic Oncology: Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen PS, Su JL, Hung MC. Dysregulation of microRNAs in cancer. J Biomed Sci. 2012;19:90. doi: 10.1186/1423-0127-19-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbri M, Calore F, Paone A, Galli R, Calin GA. Epigenetic regulation of miRNAs in cancer. Adv Exp Med Biol. 2013;754:137–148. doi: 10.1007/978-1-4419-9967-2_6. [DOI] [PubMed] [Google Scholar]
- 29.Salzman DW, Weidhaas JB. SNPing cancer in the bud: microRNA and microRNA-target site polymorphisms as diagnostic and prognostic biomarkers in cancer. Pharmacol Ther. 2013;137:55–63. doi: 10.1016/j.pharmthera.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang D, Meyer L, Chang DW, Lin J, Pu X, Ye Y, Gu J, Wu X, Lu K. Genetic variants in MicroRNA biosynthesis pathways and binding sites modify ovarian cancer risk, survival, and treatment response. Cancer Res. 2010;70:9765–9776. doi: 10.1158/0008-5472.CAN-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Q, Dong Q, He C, Liu W, Sun L, Liu J, Xing C, Li X, Wang B, Yuan Y. A new polymorphism biomarker rs629367 associated with increased risk and poor survival of gastric cancer in Chinese by up-regulated miRNA-let-7a expression. PLoS One. 2014;9:e95249. doi: 10.1371/journal.pone.0095249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slaby O, Bienertova-Vasku J, Svoboda M, Vyzula R. Genetic polymorphisms and microRNAs: New direction in molecular epidemiology of solid cancer. J Cell Mol Med. 2012;16:8–21. doi: 10.1111/j.1582-4934.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava K, Srivastava A. Comprehensive review of genetic association studies and meta-analyses on miRNA polymorphisms and cancer risk. PLoS One. 2012;7:e50966. doi: 10.1371/journal.pone.0050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 35.Nicoloso MS, Sun H, Spizzo R, Kim H, Wickramasinghe P, Shimizu M, Wojcik SE, Ferdin J, Kunej T, Xiao L, et al. Single-nucleotide polymorphisms inside microRNA target sites influence tumor susceptibility. Cancer Res. 2010;70:2789–2798. doi: 10.1158/0008-5472.CAN-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen J, Ambrosone CB, DiCioccio RA, Odunsi K, Lele SB, Zhao H. A functional polymorphism in the miR-146a gene and age of familial breast/ovarian cancer diagnosis. Carcinogenesis. 2008;29:1963–1966. doi: 10.1093/carcin/bgn172. [DOI] [PubMed] [Google Scholar]
- 37.Peng S, Kuang Z, Sheng C, Zhang Y, Xu H, Cheng Q. Association of microRNA-196a-2 gene polymorphism with gastric cancer risk in a Chinese population. Dig Dis Sci. 2010;55:2288–2293. doi: 10.1007/s10620-009-1007-x. [DOI] [PubMed] [Google Scholar]
- 38.Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L, Zeng Y, Miao R, Jin G, Ma H, et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schimanski CC, Frerichs K, Rahman F, Berger M, Lang H, Galle PR, Moehler M, Gockel I. High miR196a levels promote the oncogenic phenotype of colorectal cancer cells. World J Gastroenterol. 2009;15:2089–2096. doi: 10.3748/wjg.15.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan JF, Chen LH, Chen ZX, Yuan YW, Xie GZ, Sun AM, Liu Y. A functional variant in microRNA196a2 is associated with susceptibility of colorectal cancer in a Chinese population. Arch Med Res. 2011;42:144148. doi: 10.1016/j.arcmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Sun LY, Chen LL, Zheng HQ, Zhang QF. A variant in microRNA196a2 is not associated with susceptibility to and progression of colorectal cancer in Chinese. Intern Med J. 2012;42:e115–e119. doi: 10.1111/j.1445-5994.2011.02434.x. [DOI] [PubMed] [Google Scholar]
- 42.Pu JY, Dong W, Zhang L, Liang WB, Yang Y, Lv ML. No association between single nucleotide polymorphisms in pre-mirnas and the risk of gastric cancer in Chinese population. Iran J Basic Med Sci. 2014;17:128–133. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu R, Liu X, He Z, Li Q. miR-146a and miR-196a2 polymorphisms in patients with ischemic stroke in the northern Chinese Han population. Neurochem Res. 2014;39:1709–1716. doi: 10.1007/s11064-014-1364-5. [DOI] [PubMed] [Google Scholar]