Abstract
The objective of the present study was to evaluate the effects of novel porphyrin-based photosensitizer meso-5-[ρ-diethylene triamine pentaacetic acid- aminophenyl]-10,15,20-triphenyl-porphyrin (DTP)-mediated photodynamic therapy (PDT) on the HGC27 and SNU-1 human gastric cancer cell lines. The absorption spectrum of DTP was analyzed using a microplate spectrophotometer. The HGC27 or SNU-1 cells were incubated with DTP and exposed to illumination by a 650-nm laser. The experiments were divided into four groups: A blank control, cells treated with DTP without light, cells exposed to laser light without DTP and cells treated with a combination of DTP and light together. The phototoxicity of DTP was analyzed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay. Cell apoptosis was detected by flow cytometry and Hoechst 33342 staining. In addition, the intracellular distribution of DTP was investigated by laser scanning confocal microscopy. DTP-PDT demonstrated marked phototoxicity towards HGC27- and SNU-1 cells. The rate of cell death increased significantly in a DTP concentration-dependent and light dose-dependent manner, with maximum mortality rates of 74.14 and 67.76%, respectively. There were significant differences between the therapeutic and control groups (P<0.01). In addition, the growth of cells treated with DTP or laser light alone was not inhibited. Further evaluation revealed that, following DTP-PDT, HGC27 and SNU-1 cells demonstrated notable apoptotic changes, including condensed chromatin, fragmented nuclei and apoptotic bodies, and the percentage of apoptotic cells was significantly higher than that of the control groups (P<0.01). Furthermore, confocal laser scanning microscopy revealed that DTP localized to the lysosomes but not mitochondria in the two types of tumor cell. In conclusion, significant phototoxicity and reduced cytotoxicity in dark conditions make the novel photosensitizer DTP a promising potential PDT drug for future use in the treatment of human gastric cancer.
Keywords: gastric cancer, novel porphyrin-based photosensitizer, photodynamic therapy, apoptosis, subcellular localization
Introduction
Worldwide, gastric cancer is the fourth most common malignant disease and the second leading cause of cancer-associated mortality (1). According to the statistical data of the World Health Organization (2), there were 989,000 novel cases and 737,000 mortalities of gastric cancer in 2008, of which, the ratio of men to women was 2:1. Surgical excision, chemotherapy and radiotherapy are the basic treatment options for gastric cancer, among which, surgical excision is currently the first choice (3). However, the early diagnosis of gastric cancer is difficult and the majority of cases have progressed to a moderate or advanced stage prior to diagnostic confirmation, therefore, the postoperative 5-year survival rate of gastric cancer remains low at ~20–30%. Furthermore, elderly patients are frequently unable to tolerate surgery (4,5). In recent years, improvements to various resection methods, as well as chemotherapeutic and radiotherapeutic plans has had little effect (3). Surgical excision is incapable of removing all tumor cells, and therefore the risk of postoperative recurrence and metastasis remain (6). Furthermore, the major side effects of chemotherapy and radiotherapy, particularly for elderly patients, which include myelosuppression, immunosuppression and diarrhea, may outweigh the curative effects (7,8). Therefore, an effective therapy technique for the treatment of gastric cancer is urgently required.
Photodynamic therapy (PDT) is a non-invasive treatment method, used for a variety of malignant tumors (9,10). PDT has been known to be effective for the treatment of gastric cancer for a number of years (11,12). PDT typically involves the systemic administration of a photosensitizer (PS), followed by its activation by light at an appropriate wavelength, which results in the generation of reactive oxygen species (ROS) and eventually induces malignant cell death (13). PDT has numerous advantages over typical cancer treatment modalities, including surgery, chemotherapy and radiotherapy (14): i) PDT is relatively non-invasive, simply requiring illumination of the tumor site, and therefore inducing minimal injury to the adjacent normal tissues (15); ii) PDT does not induce systemic immunosuppressive effects that may be translated into clinical opportunistic infection (15); iii) PDT is able to be repeated without detrimental consequences to the patient (16); and iv) PDT is simple to conduct, and is particularly suitable for elderly patients who may be unable to endure surgery, chemotherapy or radiotherapy (16). In addition, since the laser light required for PDT may be delivered by an optical fiber, PDT is particularly useful for the treatment of cavity-tumor gastric cancer.
A novel porphyrin-based PS, named meso-5-[ρ-diethylene triamine pentaacetic acid (DTPA)-aminophenyl]-10,15,20-triphenyl-porphyrin (DTP), was recently developed by the present group (17). This compound is a porphyrin derivative and was designed to be a dissymmetrical DTPA-aminophenyl-based porphyrin. DTP is a chemically pure compound and is synthesized according to the patent specifications. The present study aimed to investigate the effects and mechanism of DTP-mediated PDT against human gastric cancer HGC27 and SNU-1 cells.
Materials and methods
Preparation of DTP
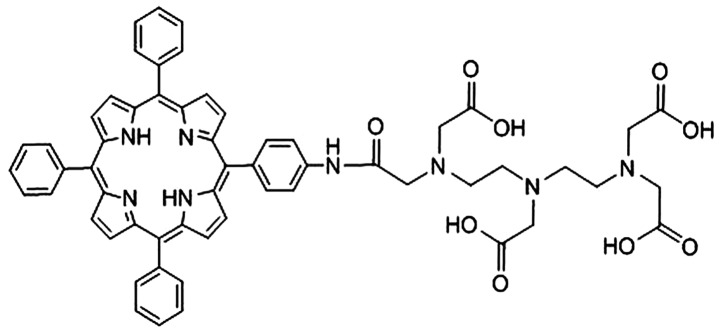
The synthetic process of DTP was obtained in detail according to the patent specifications (CN 101805362) (17). Initially, meso-5,10,15,20-tetraphenyl-porphyrin was synthesized in propionic acid solvent (Meryer Chemical Technology Co., Ltd., Shanghai, China), using the classical Adler method (18). Meso-5,10,15,20-tetraphenyl-porphyrin (0.01 mol) was added and stirred into 20 ml dichloromethane (Aladdin Industrial Inc., Shanghai, China) at −10°C until dissolved. Subsequently, 65% concentrated nitric acid (1 ml; Aladdin Industrial Inc.) was added to the solution. The nitrated product was reduced with sodium nitrite (Aladdin Industrial Inc.) in dichloromethane at room temperature, until a reduced mixture was gained. The meso-5-(ρ-aminophenyl)-10,15,20-triphenyl-porphyrin was obtained as the main product following separation of the mixture by silica gel chromatography. Next, 0.2 g meso-5-(ρ-aminophenyl)-10,15,20-triphenyl-porphyrin, 0.446 g diethylenetriaminepentaacetic acid dianhydride, 0.046 g dimethylaminopyridine (Aladdin Industrial Inc.) and 0.040 g triethylamine were dissolved in 10 ml dimethyl sulfoxide (DMSO; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) in a 25 ml flask. Following agitation for 12 h at room temperature, 5 ml H2O was added to the reaction solution and a precipitate was formed. The final DTP product was then obtained by crystallizing the precipitate of the mixed solution of DMSO and H2O. The chemical structure of DTP is shown in Fig. 1. DTP was dissolved in Roswell Park Memorial Institute (RPMI)-1640 medium and stored at 12.5×103 µM at 4°C, protected from visible light.
Figure 1.
Chemical structure of meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin.
Cells and culture conditions
The PDT effect of DTP was studied using the HGC27 and SNU-1 human gastric cancer cell lines, which were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The two types of cell were cultured in RPMI-1640 culture medium (Beijing Solarbio Science & Technology Co., Ltd.) with 10% Gibco fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a fully humidified atmosphere with 5% CO2.
Spectral analysis of DTP
DTP solution was prepared in phosphate-buffered saline (PBS; Beijing Solarbio Science & Technology Co., Ltd.) to produce a solution of 3.12 µM concentration. Subsequently, 100 µl DTP solution was added into a 96-well plate. The ultraviolet (UV)-visible absorption spectrum was recorded on a microplate spectrophotometer (Thermo 3001; Thermo Fisher Scientific, Waltham, MA, USA).
Photosensitization
HGC27 or SNU-1 cells were loaded into 96-well plates (1×104/well) and incubated in a humidified incubator containing 95% air and 5% CO2 until cell attachment to the substratum reached ~80% confluence. Subsequently, 100 µl of various concentrations of DTP were added to the wells and incubated for 24 h. Following incubation, the supernatant was replaced with fresh culture medium supplemented with 10% FBS and the cells were irradiated with 6 or 12 J/cm2 of laser light at a wavelength of 650 nm, followed by incubation for an additional 3 h at 37°C. The experiment was divided into groups, identical for HGC27 and SNU-1 cells, as follows: Control groups, including untreated cells, cells treated with PS alone and cells exposed to laser light alone; and treatment groups, including cells treated with DTP of various concentrations in combination with laser light energy. The treatment groups comprised: Group 1, 0.78 µM DTP + 6/12 J/cm2; group 2, 1.56 µM DTP + 6/12 J/cm2; group 3, 3.125 µM DTP + 6/12 J/cm2; group 4, 6.25 µM DTP + 6/12 J/cm2 and group 5 12.5 µM DTP + 6/12 J/cm2.
Colorimetric 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay
Cell viability was assessed using the MTT Cell Proliferation and Cytotoxicity Assay Kit’ (Amresco, Solon, OH, USA). Following photosensitization, 100 µg MTT in 20 µl PBS was added into each well (96-well plates) and the cells (1×104/well) were then incubated for a further 3 h. The reaction was stopped by the addition of 180 µl DMSO. The optical density (OD) of each sample was subsequently measured at a wavelength of 490 nm using a microplate spectrophotometer (Thermo 3001; Thermo Fisher Scientific). Cell survival rate (%) = (ODtreated/ODcontrol) × 100%.
Cell apoptosis
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) (Beyotime Institute of Biotechnology, Haimen, China) were used to detect cell apoptosis induced by DTP-PDT. HGC27 and SNU-1 cells were seeded in 6-well plates, followed by incubation with 6.25 µM DTP for 24 h. The cells were subsequently irradiated at 650 nm with 12 J/cm2 laser light. Three hours later, the irradiated cells were collected, rinsed with PBS and stained with Annexin V-FITC (200 µg/ml) for 10 min and 30 µg/ml PI in the dark at room temperature, successively. Immediately, the fluorescence was analyzed in 10,000 cells/sample using a FACScan flow cytometer (FCM; Beckman Coulter, Fullerton, CA, USA). The results were expressed as the percentage of cells exhibiting apoptosis relative to the total number of cells analyzed. Blank control and treatment with DTP or 6 J/cm2 laser light alone were included as control groups.
Morphological observations
HGC27 and SNU-1 cells were seeded in 24-well plates. Following attachment to the substratum to ~80% confluence, 6.25 µM DTP was added into each well for 24 h, followed by irradiation by a laser at 650 nm with 12 J/cm2. The cells were subsequently stained with Hoechst 33342 (Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at room temperature, washed twice with PBS and exposed to UV illumination using a fluorescent microscope (Leica DMIRE 2; Leica Microsystems GmbH, Wetzlar, Germany) to detect the differences in chromatin condensation and fragmentation between the control and treatment groups. The staining was evaluated by three investigators.
Intracellular distribution of PS by confocal laser scanning microscopy (CLSM)
HGC27 or SNU-1 cells were cultured on coverslips (Citoglas; Citotest Labware Manufacturing Co., Ltd., Jiangsu, China) placed in Petri dishes (Citoglas; Citotest Labware Manufacturing Co., Ltd.) with RPMI-1640 culture medium. Once the cells reached 85% confluence, 6.25 µM DTP in serum-free cell culture medium was added to the dishes and cells were incubated for 6 h, followed by incubation with: i) 150 nM MitoTracker Green FM for an additional 30 min or ii) 1.5 µM LysoSensor™ Green DND-189 (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at culture temperature. Following incubation, the staining solution was replaced with fresh culture media and the cells were observed by CLSM (TCS SP8; Leica Heidelberg GmbH, Heidelberg, Germany). The excitation wavelength used for DTP was 405 nm, and the fluorescence excitation and emission wavelengths for MitoTracker Green FM or LysoSensor Green DND-189 were 488 and 520 nm, respectively.
Statistical analysis
Statistical analysis was performed based on one way analysis of variance. All statistical analyses were performed using SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). The results were recorded as the mean ± standard error of the mean. P<0.05 was considered to indicate a statistically significant difference and all experiments were conducted at least 3 times.
Results
Spectral analysis of DTP
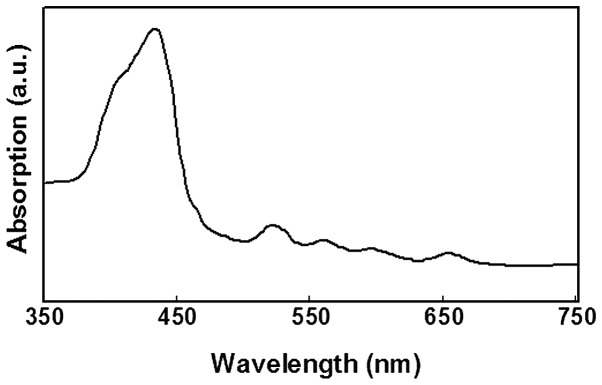
Initially, a spectral analysis of DTP was conducted (Fig. 2). The absorption spectrum indicated that DTP had significant absorption peaks at wavelengths of 432, 522, 560, 596 and 652 nm, respectively. Since the absorption was strongest at 432 nm, absorption at 405 nm was evaluated, as this was the closest wavelength which was able to be selected as the excitation laser channel during CLSM analysis. Furthermore, considering the penetration depth of light in PDT proportional to the wavelength, a long wavelength proximal to the red region of the spectrum should be used for laser illumination during treatment with PDT, and 650 nm is a suitable wavelength for the MTT and flow cytometry assays (19).
Figure 2.
Absorption spectrum of meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin.
DTP combined with laser light induces gastric cancer cell death
The photodynamic effect of DTP against human HGC27 and SNU-1 cells was evaluated by MTT assay. Table IA and B list the OD values of each group and Figs. 3 and 4 demonstrate the corresponding cell survival rates following PDT for HGC27 and SNU-1 cells, respectively.
Table I.
OD values of each group following treatment with DTP alone or in combination with laser light.
| A, HGC27 cells | ||||
|---|---|---|---|---|
| DTP + laser light, OD | ||||
| Group | DTP concentration, µM | DTP alone, OD | 6 J/cm2 | 12 J/cm2 |
| 1 | 0.78 | 0.8741±0.1365a | 0.7946±0.0895a | 0.7368±0.1305a |
| 2 | 1.56 | 0.8249±0.0549a | 0.6912±0.6521a | 0.5962±0.0895b |
| 3 | 3.125 | 0.8846±0.0745a | 0.4595±0.0352b | 0.3892±0.0421b |
| 4 | 6.25 | 0.8186±0.1026a | 0.3265±0.0451b | 0.2956±0.0512b |
| 5 | 12.5 | 0.8018±0.0982a | 0.2498±0.0213b | 0.2228±0.0231b |
| 6 | 0.0 | 0.8165±0.0951a | 0.8694±0.1543a | |
| 7 | Control | 0.8741±0.1116 | ||
| B, SNU-1 cells | ||||
| DTP + laser light, OD | ||||
| Group | DTP concentration, µM | DTP alone, OD | 6 J/cm2 | 12 J/cm2 |
| 1 | 0.78 | 0.8315±0.0865a | 0.7425±0.0745a | 0.6815±0.0351a |
| 2 | 1.56 | 0.8513±0.1065a | 0.6522±0.0534a | 0.6258±0.0541a |
| 3 | 3.125 | 0.7231±0.0256a | 0.5236±0.0982b | 0.4788±0.0255b |
| 4 | 6.25 | 0.7452±0.5614a | 0.4322±0.0415b | 0.3975±0.0413b |
| 5 | 12.5 | 0.7259±0.0635a | 0.2655±0.0265b | 0.2469±0.0215b |
| 6 | 0.0 | 0.7985±0.0261a | 0.7865±0.0452a | |
| 7 | Control | 0.7656±0.0915 | ||
P>0.05 vs. control;
P<0.01 vs. control. OD, optical density; DTP, meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin.
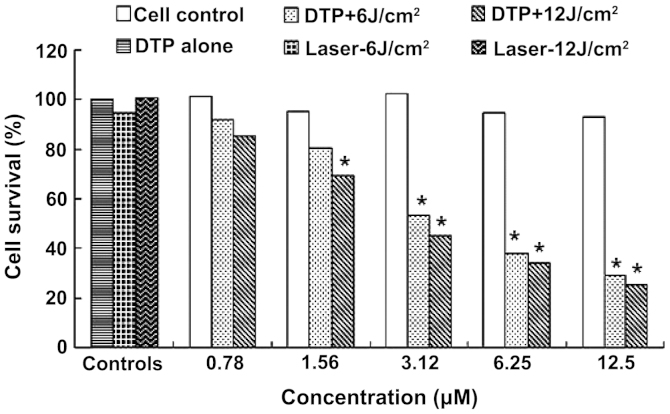
Figure 3.
Phototoxicity of DTP against HGC27 cells. DTP, meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin. *P<0.01 vs. control cells.
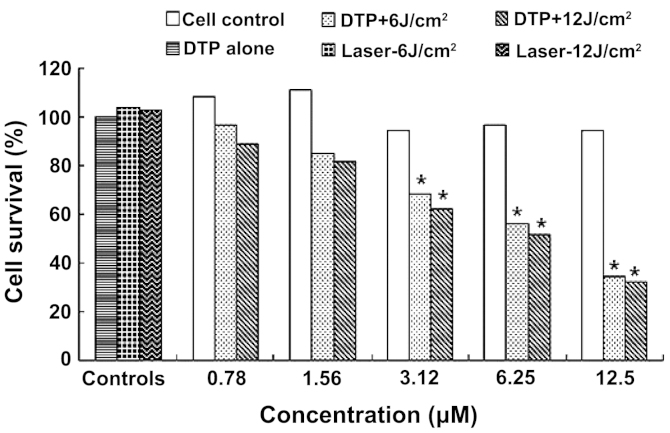
Figure 4.
Phototoxicity of DTP against SNU-1 cells. DTP, meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin. *P<0.01 vs. control cells.
Table IA and Fig. 3 exhibit the MTT assay results for DTP-PDT on HGC27 cells. The cell survival rate following DTP-PDT declined with increasing PS concentration and light dose (Fig. 3). Statistically significant differences were observed between treatment groups 3–5 and the cell control group (P<0.01) following exposure to 6 J/cm2 illumination, while following 12 J/cm2 treatment, a significant difference was identified between treatment groups 2–5 and the cell control group (P<0.01) (Table IA). Fig. 3 also demonstrated that 0.78 µM PS-mediated PDT only induced slight HGC27 cell death, with ~7.75 and 14.46% cell death at 6 and 12 J/cm2 illumination, respectively. However, treatment with 3.12 µM PS was able to induce 46.65 and 54.82% cell death. The highest concentration of DTP (12.5 µM) was found to be highly effective, inducing 70.99 and 74.14% mortality at light doses of 6 and 12 J/cm2, respectively. The IC50 of DTP was 4.75 and 2.98 µM at light doses of 6 and 12 J/cm2, respectively. DTP alone exerted no marked cytotoxicity on HGC27 cells at any concentration. In addition, laser light alone also did not exert any effects on the growth of HGC27 cells.
SNU-1 cells demonstrated similar MTT results to those observed in HGC27 cells (Table IB; Fig. 4). DTP exerted significant apoptosis induction in SNU-1 cells. A significant difference was observed between treatment groups 3–5 and the cell control group (P<0.01) for light doses of 6 and 12 J/cm2, while no difference was detected between treatment groups 1 and 2 and the cell control (P>0.05) (Table IB). The death of SNU-1 cells induced by DTP-PDT was proportional to PS concentration and light dose (Fig. 4). The lower concentrations of 0.78 and 1.56 µM resulted in the death of a small percentage cells at light doses of 6 and 12 J/cm2, respectively; however, 12.5 µM DTP induced 65.33 and 67.76% mortality and an IC50 of 4.75 and 6.38 µM, respectively. The data also revealed that DTP or laser treatment alone were nontoxic to SNU-1 cells.
DTP-PDT induces gastric cancer cell apoptosis
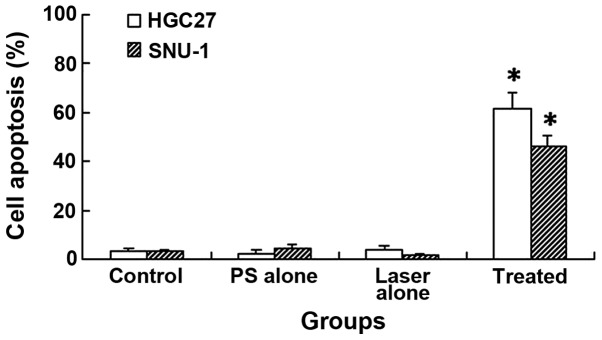
DTP-PDT induced significant cell apoptosis in HGC27 and SNU-1 cells, as determined by FCM analysis (Fig. 5). The apoptotic rates of HGC27 and SNU-1 cells were 61.5±6.56 and 45.9±4.62%, respectively. These values were significantly higher than those of the control groups (P<0.01), which demonstrated 3.1±1.25 and 2.9±0.85% apoptosis in HGC27 and SNU-1 cells, respectively. In the DTP alone group, only 2.5±0.98 and 4.3±1.25% cell apoptosis was detected, while 3.7±1.64 and 1.8±0.75% cell apoptosis were detected in the laser light alone group for HGC27 and SNU-1 cells, respectively. Therefore, no significant differences were detected, in comparison with the cell control (P>0.05).
Figure 5.
Cell apoptosis following meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin photodynamic therapy. *P<0.01 vs. control.
Morphological changes occur in gastric cancer cells following PDT
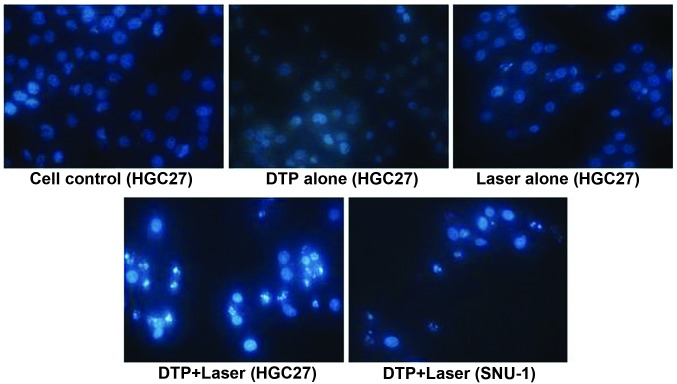
Morphological changes in the cellular nuclei of each group were observed using fluorescence microscopy (Fig. 6). A large quantity of apoptotic cells were detected following PS-PDT, demonstrating significant characteristics of apoptosis, including chromatin condensation, nucleus fragmentation and apoptotic bodies in HGC27 and SNU-1 cells. By contrast, in the control cell, DTP-alone or light-alone groups, the majority of the cells demonstrated intact, rounded/elliptical nuclei and demonstrated no morphological changes in either of the cell types.
Figure 6.
Morphological changes in the cells of each group. DTP, meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin.
DTP is localized to the lysosomes
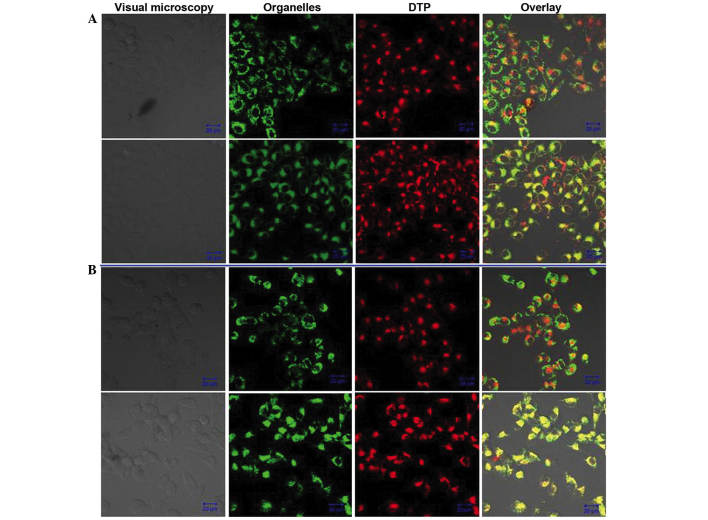
The distribution of DTP within HGC27 and SNU-1 cells was evaluated via fluorescent observation by CLSM (Fig. 7). Following the incubation of cells with DTP and MitoTracker Green Probe or LysoSensor Green Probe, the PS emitted red fluorescence that was excited by the 405 nm wavelength and the MitoTracker Green Probe and LysoSensor Green Probe emitted green fluorescence that was excited by the 488 nm wavelength in the same view. The lysosomal overlay images for HGC27 and SNU-1 cells revealed a large quantity of yellow-green fluorescent spots (Fig. 7, lower panels), indicating co-localization of DTP and the lysosomal probe. Conversely, no overlap was detectable between DTP and the mitochondrial probe for the two types of cell (Fig. 7, upper panels). The distribution of DTP in HGC27 and SNU-1 cells was analogous.
Figure 7.
Intracellular distribution of DTP in (A) HGC27 and (B) SNU-1 cells (upper panels, mitochondria; lower panels, lysosomes). Green fluorescence is from the mitochondrial probe and red fluorescence is from the lysosomal probe. The overlay shows the green and red fluorescence together. Scale bar, 20 µm. DTP, meso-5-[ρ-diethylene triamine pentaacetic acid-aminophenyl]-10,15,20-triphenyl-porphyrin.
Discussion
PDT is a novel treatment for malignant tumors, which is derived from ‘photodynamic action’ (20). In 1900, Raab (21) discovered that paramecium, having absorbed acridines, were able to be killed by light illumination; however, light or acridines alone did not produce this effect. This phenomenon was subsequently known as ‘photodynamic action’. In 1960, Lipson et al (22) prepared the PS, hematoporphyrin derivative and evaluated its effects in the treatment of malignant tumors. Kato et al (23), in 1980, successfully introduced the application of an endoscope to deliver laser light during PDT treatment of lung cancer, which initiated the application of PDT in the therapy of cavity tumors. To date, numerous studies and practices have been focused on the PDT treatment of gastric cancer and have proven that this method is an effective technology for use in the treatment of gastric cancer (11,24).
PDT consists of a PS, laser light and oxygen, among which, PS is the most important component. The four main classes of PS are: Porphyrin derivatives, chlorins, phthalocyanines and porphycenes. These each possess distinct photochemical and photophysical properties, underlying their mechanisms of action and light activation (25). DTP, evaluated in the present study, belongs to the porphyrin derivatives family. The advantages of DTP when compared with other porphyrin derivatives were as follows: i) DTP is a pure compound with clear chemical and optical properties, and was able to be synthesized relatively easily, according to the patent specifications; ii) DTP exhibited improved chemical and physical stability; iii) DTP demonstrated little toxicity in dark conditions and is relatively safe for PDT treatment; iv) DTP has an absorption peak of 652 nm, which is optimal for PDT use; and v) the authors of the present study have applied for patent protection of the compound. These advantages make the novel photosensitizer DTP a promising potential PDT drug for use in the treatment of gastric cancer, and therefore prompted initiation of the present study.
Initially, the photodynamic effects of DTP on HGC27 and SNU-1 human gastric cancer cells were evaluated by MTT assay. The results revealed that DTP exerted marked phototoxicity on the two types of cell. In HGC27 cells, a concentration of 6.25 µM DTP-PDT resulted in 62.09 and 65.68% cell death at light doses of 6 and 12 J/cm2, respectively. Furthermore, treatment with 12.5 µM induced 70.99 and 74.14% mortality, respectively. Similar phototoxicity was observed in SNU-1 cells. A concentration of 12.5 µM DTP-PDT induced 65.33 and 67.74% cell death at light doses of 6 and 12 J/cm2, respectively, and the corresponding IC50 was 4.75 and 6.38 µM, respectively. However, in the absence of light stimulation, DTP did not exert any significant damage on the cells, even at the highest concentration of 12.5 µM. These data indicate that this PS is a safe and effective PDT drug for the treatment of gastric cancer cells, and the therapeutic effects were associated with PS concentration and light dose.
To further investigate the mechanism of DTP-mediated PDT on HGC27 and SNU-1 cells, flow cytometry and Hoechst 33342 staining were used to analyze cell apoptosis. Flow cytometric analysis revealed that DTP-PDT was able to induce marked apoptosis of HGC27 and SNU-1 cells, and that the apoptotic rate was 61.5±6.56 and 45.9±4.62%, respectively, significantly higher than that of the control group. Additionally, fluorescent microscope observation following Hoechst 33342 staining identified a large number of apoptotic cells in the DTP-PDT treatment group. By contrast, the majority of cells in the control groups remained rounded and intact. The above results supported the hypothesis that apoptosis is involved in the death process triggered by DTP-PDT in HGC27 and SNU-1 cells.
The intracellular localization of a PS is crucial for photodynamic damage. It is the ROS generated by PS that induce cell death during PDT. Since ROS have a short half-life and are only able to act close to the site of generation (26), photodynamic damage is closely associated with the generation of ROS within cells, and therefore to the precise intracellular localization of PS. PSs are able to localize various organelles within cells, including the mitochondria, lysosomes, endoplasmic reticulum, Golgi apparatus and plasma membranes (27). Generally, PS distribution in mitochondria commonly activates cell apoptosis through various pathways, while PS distribution in lysosomes frequently leads to cell necrosis (28). The present study showed that DTP localized within the lysosomes of HGC27 and SNU-1 cells, but induced apoptosis as the major death mode. This phenomenon may be due to the indirect mitochondrial injury induced by direct lysosome damage by DTP-PDT. It has been reported that the permeability of the mitochondrial membrane was increased by PDT-mediated lysosomal damage, which indirectly results in apoptosis of tumor cells (29). Photodynamically-produced ROS by lysosomal PS, induced rapid destruction of lysosomes, resulting in the release of cathepsins, inducing the following spatiotemporal sequence of cellular events: Bid/Bax activation, cytochrome c release from the mitochondria to cytosol, activation of downstream caspases and subsequent apoptisis (30,31). Nagata et al (32) revealed that the PS ATX-S10 (Na) had a primary site of accumulation in lysosomes, however cells underwent apoptosis following illumination, leading to 70% cell death. The results of these studies were in agreement with those of the present study.
In conclusion, the results of the present study indicate that the novel porphyrin-based PS, DTP, exhibited significant PDT effects on HGC27 and SNU-1 human gastric cancer cells in vitro, and that DTP is safe for use in the PDT process. This compound localized in the lysosomes of HGC27 and SNU-1 cells, but indirectly induced the mitochondrial apoptotic pathway. Further investigations, including animal experiments and additional molecular mechanism of action investigations should be conducted for this compound in order to verify its potential clinical application for the treatment of human gastric cancer.
References
- 1.Wong JE, Ito Y, Correa P, Peeters KC, van de Velde CJ, Sasako M, Macdonald J. Therapeutic strategies in gastric cancer. J Clin Oncol. 2003;21(23 Suppl):267s–269s. doi: 10.1200/JCO.2003.08.536. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Gu JR, Cao XT. Conceptual consideration of cancer, challenges and opportunities for cancer biotherapy. Zhong Guo Zhong Liu Sheng Wu Zhi Liao Za Zhi Bian Ji Bu. 2008;15:2–7. (In Chinese) [Google Scholar]
- 4.Liu X, Ru J, Zhang J, Zhu LH, Liu M, Li X, Tang H. miR-23a targets interferon regulatory factor 1 and modulates cellular proliferation and paclitaxel-induced apoptosis in gastric adenocarcinoma cells. PLoS One. 2013;8:e64707. doi: 10.1371/journal.pone.0064707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Li H, Liu Z, Xu C, Chen Y, Zhang J, Cui B, Chen X, An G, She X, Liu H, et al. Overexpression of S100A4 is closely associated with the progression and prognosis of gastric cancer in young patients. Oncol Lett. 2013;5:1485–1490. doi: 10.3892/ol.2013.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang ZQ, Yao HL, Wen Y, Miao XY, Xiong L. Advances in photodynamic therapy for gastric cancer. Ji Guang Sheng Wu Xue Bao Bian Ji Bu. 2012;21:289–293. (In Chinese) [Google Scholar]
- 7.Pan XR, Chen XX. Recent development on adverse reactions induced by cancer chemotherapy treated with traditional Chinese medicine. Yi Yao Yu Bao Jian Bian Ji Bu. 2010;16:2358–2360. (In Chinese) [Google Scholar]
- 8.Ma XQ, He TQ, Zheng Tao, Xu BX, Xiao CR, Zheng SX, Lin XR, Gao PY, Shen G, Bao YH, et al. Clinical study on protective effect of diltiazem against radiation injuries on cancer. Zhong Guo Yao Xue Za Zhi Bian Ji Bu. 2004;39:231–232. (In Chinese) [Google Scholar]
- 9.Vrouenraets MB, Visser GWM, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003;23:505–522. [PubMed] [Google Scholar]
- 10.Nowak-Stepniowska A, Pergoł P, Padzik-Graczyk A. Photodynamic method of cancer diagnosis and therapy - mechanisms and applications. Postepy Biochem. 2013;59:53–63. (In Polish) [PubMed] [Google Scholar]
- 11.Karanov SI. Long-term results after photodynamic therapy (PDT) of T1N0M0 gastrointestinal (GI) tumors. Hepatogastroenterology. 2002;49:1579–1581. [PubMed] [Google Scholar]
- 12.Ell C, Gossner L, May A, Schneider HT, Hahn EG, Stolte M, Sroka R. Photodynamic ablation of early cancers of the stomach by means of mTHPC and laser irradiation: Preliminary clinical experience. Gut. 1998;43:345–349. doi: 10.1136/gut.43.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm S, Mvondo D, Grune T, Breusing N. The outcome of 5-ALA-mediated photodynamic treatment in melanoma cells is influenced by vitamin C and heme oxygenase-1. Biofactors. 2011;37:17–24. doi: 10.1002/biof.129. [DOI] [PubMed] [Google Scholar]
- 14.Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ. Photodynamic therapy in oncology. Expert Opin Pharmacother. 2001;2:917–927. doi: 10.1517/14656566.2.6.917. [DOI] [PubMed] [Google Scholar]
- 15.Selbo PK, Høgset A, Prasmickaite L, Berg K. Photochemical internalisation: A novel drug delivery system. Tumour Biol. 2002;23:103–112. doi: 10.1159/000059713. [DOI] [PubMed] [Google Scholar]
- 16.Rockson SG, Lorenz DP, Cheong WF, Woodburn KW. Photoangioplasty. An emerging clinical cardiovascular role for photodynamic therapy. Circulation. 2000;102:591–596. doi: 10.1161/01.CIR.102.5.591. [DOI] [PubMed] [Google Scholar]
- 17.Liu TJ, Wang YM, Wu L, Lv F. [Diethylenetriaminepentaacetaacetato-N,N',N”]gadolinium-modified porphyrins, and preparation method and application thereof. 0086. CN 101805362 Filed. 2010 Mar 30; issued August 18, 2010. [Google Scholar]
- 18.Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J, Korsakoff L. A simplified synthesis for meso-tetraphenylporphine. J Org Chem. 1967;32:467–477. doi: 10.1021/jo01288a053. [DOI] [Google Scholar]
- 19.Yan YJ, Zheng MZ, Chen ZL, Yu XH, Yang XX, Ding ZL, Xu L. Studies on preparation and photodynamic mechanism of chlorin P6-13,15-N-(cyclohexyl)cycloimide (Chlorin-H) and its antitumor effect for photodynamic therapy in vitro and in vivo. Bioorg Med Chem. 2010;18:6282–6291. doi: 10.1016/j.bmc.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Juarranz A, Jaén P, Sanz-Rodríguez F, Cuevas J, González S. Photodynamic therapy of cancer. Basic principles and applications. Clin Transl Oncol. 2008;10:148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 21.Raab O. The effect of fluorescent substances on infusoria. Z Biol. 1900;39:524–526. [Google Scholar]
- 22.Lipson RL, Baldes EJ, Olsen AM. Hematoporphyrin derivative: A new aid for endoscopic detection of malignant disease. J Thorac Cardiovasc Surg. 1961;42:623–629. [PubMed] [Google Scholar]
- 23.Kato H, Konaka C, Saito M, Nishimiya K, Kawate N, Aizawa K, Hayata Y. Laser photodynamic therapy with hematoporphyrin derivative in lung cancer. Nihon Geka Gakkai Zasshi. 1985;86:1059–1063. (In Japanese) [PubMed] [Google Scholar]
- 24.Nakamura H, Yanai H, Nishikawa J, Okamoto T, Hirano A, Higaki M, Omori K, Yoshida T, Okita K. Experience with photodynamic therapy (endoscopic laser therapy) for the treatment of early gastric cancer. Hepatogastroenterology. 2001;48:1599–1603. [PubMed] [Google Scholar]
- 25.De Rosa FS, Bentley MVLB. Photodynamic therapy of skin cancers: Sensitizers, clinical studies and future directives. Pharm Res. 2000;17:1447–1455. doi: 10.1023/A:1007612905378. [DOI] [PubMed] [Google Scholar]
- 26.Waidelich R. Laser-induced lithotripsy and photodynamic therapy in urology - A short introduction to current laser applications. Med Laser Appl. 2010;25:14–19. doi: 10.1016/j.mla.2009.11.003. [DOI] [Google Scholar]
- 27.Pazos M, Nader HB. Effect of photodynamic therapy on the extracellular matrix and associated components. Braz J Med Biol Res. 2007;40:1025–1035. doi: 10.1590/S0100-879X2006005000142. [DOI] [PubMed] [Google Scholar]
- 28.Ali SM, Olivo M. Bio-distribution and subcellular localization of Hypericin and its role in PDT induced apoptosis in cancer cells. Int J Oncol. 2002;21:531–540. [PubMed] [Google Scholar]
- 29.Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis. 2010;15:1050–1071. doi: 10.1007/s10495-010-0479-7. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Zhang Z, Xing D. Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage. Free Radic Biol Med. 2011;51:53–68. doi: 10.1016/j.freeradbiomed.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Chiu SM, Xue LY, Lam M, Rodriguez ME, Zhang P, Kenney ME, Nieminen AL, Oleinick NL. A requirement for bid for induction of apoptosis by photodynamic therapy with a lysosome- but not a mitochondrion-targeted photosensitizer. Photochem Photobiol. 2010;86:1161–1173. doi: 10.1111/j.1751-1097.2010.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata S, Obana A, Gohto Y, Nakajima S. Necrotic and apoptotic cell death of human malignant melanoma cells following photodynamic therapy using an amphiphilic photosensitizer, ATX-S10(Na) Lasers Surg Med. 2003;33:64–70. doi: 10.1002/lsm.10190. [DOI] [PubMed] [Google Scholar]