Abstract
In 2013, a 76-year-old male with a cardiac pacemaker was diagnosed with adenosquamous carcinoma of the duodenum. Subsequently, a pancreatoduodenectomy and lymph node dissection were performed, and 12 cycles of adjuvant chemotherapy (modified FOLFOX6 regimen), which consisted of fluorouracil, leucovorin and oxaliplatin, were administered via a central venous catheter. At 5 months after the completion of adjuvant chemotherapy, the patient experienced the sudden onset of severe pain at the back right of the ear, edema of the right side of the face and right jugular vein dilatation. Computed tomography (CT) revealed filling defects in the superior vena cava (SVC) and right brachiocephalic vein, indicating catheter-induced venous thrombosis. Although the catheter was removed and anti-coagulation therapy, aspiration of the thrombosis and ballooning dilatation were performed immediately, the patient's symptoms were not ameliorated. Notably, histological examination following thrombus aspiration revealed metastatic cancer cells, and fluorodeoxyglucose-positron emission tomography/CT identified metabolically active nodules in the SVC at locations consistent with the initial duodenal tumors detected by CT and in the first thoracic vertebrae. The tumor thrombus rapidly increased in size and resulted in worsening dyspnea. Subsequently, radiotherapy was performed, followed by chemotherapy, which relieved the systemic symptoms and suppressed the tumor growth. Adenosquamous carcinoma of the duodenum is extremely rare, and to the best of our knowledge, intraluminal SVC metastasis as a result of adenosquamous carcinoma of the duodenum has not been reported previously. The placement of a cardiac pacemaker, central venous catheter and tumor cells possessing high metastatic potential are hypothesized to have contributed to this rare case of metastasis.
Keywords: intravascular metastasis, duodenal adenosquamous carcinoma, superior vena cava, E-cadherin, chemotherapy, radiotherapy
Introduction
Malignant neoplasms in the small intestine account for 1–2% of malignant neoplasms of the digestive organs (1). With regard to small intestinal neoplasms, carcinoid tumors are most common, accounting for 31% of all small intestinal neoplasms, followed by adenocarcinoma (30.1%), lymphoma (16.3%) and gastrointestinal stromal tumors (7.1%) (2). Duodenal adenosquamous carcinomas (DASC) are extremely rare, and to date, only a small number of cases have been published in the literature (3). Localized duodenal adenocarcinomas are treated with surgery, including pancreatoduodenectomy and regional lymph node dissection (4), and adjuvant chemotherapies are often administered according to the therapeutic strategy for colorectal cancer (5). In the case of DASCs, similar therapeutic strategies are employed.
Intraluminal metastasis of malignant tumors to the large vessels, such as the superior vena cava (SVC), is rarely observed (6). To the best of our knowledge, no cases of intraluminal metastasis to the SVC from DASCs have been reported. Risk factors for venous thrombosis include hypercoagulability due to underlying cancer, as well as the presence of a central venous catheter (7), and these clinical characteristics may also represent risk factors for intraluminal metastases of tumor cells. Obstruction of the SVC may result in a lethal SVC syndrome and thus, immediate treatment, as well as appropriate identification of the causes is required.
The current study presents an extremely rare case of SVC syndrome induced by the intraluminal metastasis of DASC.
Case report
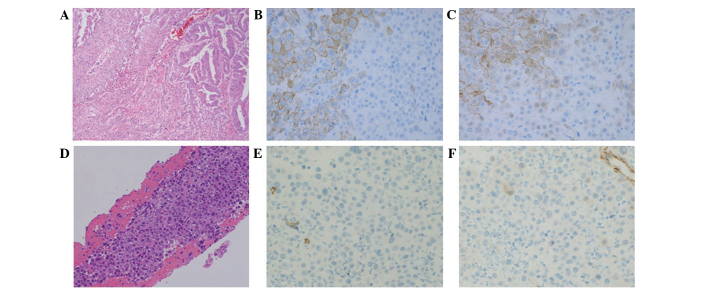
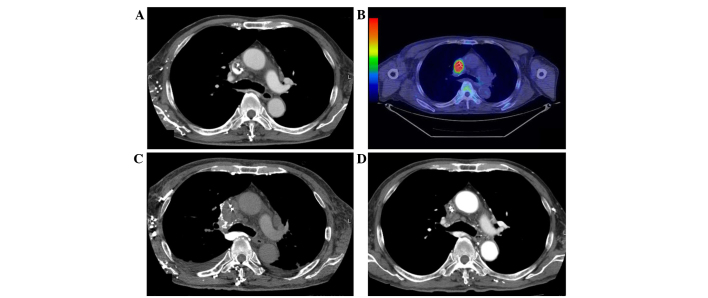
In January 2013, a 76-year-old male underwent placement of a cardiac pacemaker at Fukuoka Sanno Hospital (Fukuoka, Japan) for the management of sick sinus syndrome. In May 2013, the patient presented at Kyushu University Hospital (Fukuoka, Japan) with epigastric discomfort. Upper gastrointestinal endoscopy demonstrated an ulcerating protruding lesion at the descending section of the duodenum. Histological examination of a biopsy specimen obtained from the lesion showed clusters of polygonal atypical cells, with enlarged and pyknotic nuclei and keratinization. Immunohistochemically, the tumor cells were positive for keratin 903, AE1/AE3, p63 and cytokeratin 14, and strongly focally positive for involucrin, which indicated squamous cell carcinoma. Computed tomography (CT) and fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT revealed no metastasis. Therefore, a pancreatoduodenectomy and dissection of the second lymph node group, according to the General Rules for the Study of Pancreatic Cancer (8), were performed in June 2013. The diameter of the resected tumor was 5 cm, and the tumor was located separately from the duodenal papilla. Post-operative histopathological examination revealed adenosquamous carcinoma of the duodenum [pT4N1M0, stage IIIA, according to the TNM Classification of Malignant Tumours (9)] (Fig. 1A). The tumor was mainly composed of three types of tissue: i) Adenocarcinoma proliferating in a glandular pattern, ii) squamous cell carcinoma with apparent squamous differentiation and iii) poorly-differentiated carcinoma, which exhibited no features of adenocarcinoma or squamous cell carcinoma. Immunohistochemically, the adenocarcinoma and squamous cell carcinoma components were positive for membranous E-cadherin and β-catenin. By contrast, the poorly-differentiated carcinoma components were negative for E-cadherin and β-catenin (Fig. 1B and C). The patient underwent 12 cycles of adjuvant chemotherapy with a modified FOLFOX6 regimen, consisting of fluorouracil (400 mg/m2 bolus, followed by 2,400 mg/m2 46 h continuous infusion, day 1), leucovorin (200 mg, day 1) and oxaliplatin (85 mg/m2, day 1), which was repeated every 14 days and administered via a central venous catheter. No recurrent disease was observed for 5 months after the completion of treatment. However, in July 2014, the patient was admitted to Kyushu University Hospital following the sudden onset of severe pain at the back right of the ear, edema of the right side of the face and right jugular vein dilatation. Enhanced CT revealed filling defects extending from the SVC to the right brachiocephalic vein, indicating catheter-induced venous thrombosis (Fig. 2A). The central venous catheter was removed immediately, and anticoagulant therapy with heparin (17,500 units for 10 days) followed by warfarin (2 mg/day, continuously) was administered, however, the patient's symptoms were not ameliorated. Percutaneous transluminal aspiration of the thrombus and balloon dilatation of the vein using catheters were also performed, however, the symptoms showed no improvement. Pathological examination of the thrombus aspiration identified metastatic carcinoma, which exhibited similar features to that observed in the poorly-differentiated component of the primary tumor identified in the duodenum (Fig. 1D). Immunohistochemistry revealed that the tumor cells were negative for E-cadherin (Fig. 1E) and β-catenin (Fig. 1F). FDG-PET/CT revealed metabolically active nodules in the SVC at locations identical to those of the tumors identified on CT (Fig. 2B) and in the first thoracic vertebrae. The patient's symptoms rapidly worsened with respiratory discomfort and hypoxemia. Repeated enhanced CT scans performed at 3-week intervals showed rapid enlargement of the malignant thrombosis in the SVC extending to the right atrium (Fig. 2C), however, no pulmonary emboli were identified. Surgical resection of the tumor thrombus was attempted, however, surgery was terminated due to the patient's general condition and the identification of distant metastases. Immediate palliative radiotherapy (30 Gy in 10 fractions) was administered to the tumor thrombus, in addition to the continuation of anticoagulant therapy. The patient's systemic condition gradually improved following radiotherapy, with a decline in supplementary oxygen requirements. Subsequently, 5 cycles of chemotherapy with irinotecan (150 mg/m2, day 1, every 14 days) plus cetuximab (400 mg/m2, day 1 of cycle 1, followed by 250 mg/m2, every 7 days), which is a standard regimen for advanced colorectal cancer, was administered based on histological findings revealing that the primary tumor cells highly expressed the epidermal growth factor receptor and possessed a wild-type KRAS phenotype. A CT scan performed after two cycles of chemotherapy (November 2014) revealed a significant reduction of the tumor thrombus in the SVC (Fig. 2D). During treatment with irinotecan and cetuximab, the patient developed grade 3 pneumonia and grade 4 sepsis [according to the Common Terminology Criteria for Adverse Events (10)]. Thus, cetuximab single therapy was continuously performed for 11 cycles considering these adverse events. In March 2015, a CT scan revealed regrowth of the tumor thrombus in the SVC and FDG-PET/CT also revealed metabolically active nodules in the SVC. Palliative radiotherapy (40 Gy in 20 fractions) was administered to the tumor thrombus. The pacemaker leads were then removed, due to the possibility of the leads providing a nidus for tumor attachment and growth. Chemotherapy with paclitaxel single therapy (80 mg/m2 paclitaxel on days 1, 8 and 15, every 28 days) for 1 cycle. The physical condition of the patient had deteriorated after 1 cycle, and the patient succumbed 13 months after the diagnosis of intraluminal SVC metastasis.
Figure 1.
Histopathological examination of the tumors. (A) HE staining (magnification, ×100), and (B) E-cadherin and (C) β-catenin immunostaining (magnification, ×400) in the primary tumors. (D) HE staining (magnification, ×200), and (E) E-cadherin and (F) β-catenin immunostaining (magnification, ×400) in the intraluminal superior vena cava metastasis. HE, hematoxylin and eosin.
Figure 2.
CT and FDG-PET/CT of the chest. (A) CT scan and (B) FDG-PET/CT performed following the sudden onset of symptoms in July 2014. (C) CT scan performed 3 weeks after the appearance of symptoms. (D) CT scan following two cycles of chemotherapy. CT, computed tomography; FDG-PET, fluorodeoxyglucose-positron emission tomography.
Discussion
Advanced duodenal adenocarcinomas often metastasize to the lymph nodes, liver and lung, however, intraluminal metastasis to the large vessels, such as the SVC, are extremely rare (11). Furthermore, intraluminal metastasis to the SVC from DASCs has not been reported previously. To date, only a limited number of cases of malignant neoplasms of the skin (12,13), prostate (14), lung (15), thyroid (16), thymus (17) and colon (18) with intraluminal metastases to the SVC have been reported (Table I). We hypothesize that metastases to the large vessels are extremely rare due to the high volume and speed of blood flow, and the thick sub-endothelial layer, which prevent invasion by tumor cells. For the treatment of tumor thrombosis in the large vessels, various therapeutic strategies, including irradiation of the tumor and systemic chemotherapy, have been employed to control tumor growth. Notably, in the present case, treatment with 30 Gy of radiotherapy significantly improved the patient's systemic symptoms and suppressed the otherwise aggressive tumor growth.
Table I.
Reported cases of intraluminal superior vena cava metastasis.
| First author (ref.) | Age, years/gender | Primary site | Histology | Advanced/relapse | Other lesions | Treatment |
|---|---|---|---|---|---|---|
| Blanco et al (12) | 72/M | Skin | Melanoma | Relapse | Left axillary mass Lung | CTx, RT, AC |
| Ghattas et al (13) | 42/F | Skin | Melanoma | Relapse | None | Surgery, CTx |
| Takeda et al (14) | 60/M | Prostate | Adenocarcinoma | Advanced | Lymph node | Endocrine therapy, AC |
| Wang et al (15) | 61/M | Lung | Adenocarcinoma | Advanced | Lymph node | RT, CTx |
| Murphy et al (16) | 75/F | Thyroid | Poorly-differentiated thyroid carcinoma | Relapse | Lung | RT |
| Matsuno et al (17) | 65/F | Thymus | Sarcomatoid | Advanced | None | Surgery, AC, carcinoma adjuvant CTx |
| Alzand et al (18) | 54/F | Colon | Adenocarcinoma | Relapse | None | Surgery |
AC, anticoagulant therapy; CTx, chemotherapy; RT, radiotherapy; F, female; M, male.
Intraluminal metastasis to the SVC in the present case may have been present at the time of the initial diagnosis, although it was not identified by radiological examination. Evident metastasis in the SVC appeared surrounding a lead of the cardiac pacemaker and surrounding the central vein catheter, suggesting that these leads and the catheter itself may have contributed to the onset of this extremely rare condition. Symptomatic upper extremity and central vein thrombosis attributed to pacemaker leads occurs in 1–3% of patients with permanent pacemakers (19). The pathogenesis of pacemaker lead-related thrombosis may involve local inflammation induced by foreign-body reactions (20) and endothelial cell injury as a result of lead-activated local hypercoagulability (19). Similarly, the pacemaker leads and/or the central venous catheter may provide a nidus for tumor attachment and growth.
The primary tumor in the present case was diagnosed as an adenosquamous carcinoma, which is rarely found among malignant tumors of the small intestine (3). It is not known whether adenosquamous carcinoma of the small intestine has a higher metastatic potential to distant organs than other histological types. With regard to colorectal tumors, significant differences in metastatic potential have been identified between adenocarcinomas and adenosquamous carcinomas (21). Therefore, in the present case, the histological characteristics of the tumor may correlate with the metastatic capacity. Notably, pathohistological examination of the SVC thrombus revealed similar features to those of the poorly-differentiated component of the primary tumor in the duodenum. Immunohistochemical examination of the poorly-differentiated carcinoma components of the primary tumor in the duodenum were negative for the expression of E-cadherin and β-catenin, which was consistent with that observed in the SVC thrombus. Adenocarcinomas of the small intestine, which possess specific markers of epithelial-mesenchymal transition, such as E-cadherin negativity, or vimentin and/or fibronectin positivity, are significantly associated with an undifferentiated histology and poor clinical outcomes (22). Loss of the E-cadherin protein was identified in 41.8% of small intestinal adenocarcinomas, and aberrant β-catenin protein expression was found in 40.7% of small intestinal adenocarcinomas (23). Furthermore, 24% of small intestinal adenocarcinomas exhibit the two phenotypes and were found to closely correlate with a poorly-differentiated histology (23). The decreased expression of E-cadherin may be associated with the loss of the intercellular junctional or cellular polarity of cancer cells and thus, theoretically, this may increase the metastatic potential of tumor cells (24–26). Additionally, the activation of β-catenin in tumor cells as a result of the loss of E-cadherin expression may induce the expression of metastasis-related genes, including Snail and Twist (27). The tumor phenotype (wild-type KRAS) in the present case may have induced an increased possibility of metastasis and also contributed to the intraluminal metastasis. A recent study showed that the KRAS mutation is associated with poor prognosis in colorectal cancer (28). However, whether KRAS mutation status correlates with poor prognosis and the metastatic capacity of duodenum carcinoma remains unclear.
Overall, aggressive intraluminal SVC metastasis as a result of adenosquamous carcinoma of the duodenum may be associated with the placement of artificial devices in the large vessels, as well as the development of an aggressive metastatic phenotype in the tumor cells. The patient in the present case exhibited intraluminal superior vena cava metastasis from adenosquamous carcinoma of the duodenum. Radiotherapy, chemotherapy and anticoagulant therapy effectively suppressed tumor growth. Thus, the present study indicates that patients with advanced cancer harboring distant metastases to rare organ locations should undergo multidisciplinary therapy in accordance with the pathogenesis of the metastasis, with the type of therapy selected based on patient factors, as well as tumor factors.
References
- 1.Guo X, Mao Z, Su D, Jiang Z, Bai L. The clinical pathological features, diagnosis, treatment and prognosis of small intestine primary malignant tumors. Med Oncol. 2014;31:913. doi: 10.1007/s12032-014-0913-8. [DOI] [PubMed] [Google Scholar]
- 2.Hatzaras I, Palesty JA, Abir F, et al. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142:229–235. doi: 10.1001/archsurg.142.3.229. [DOI] [PubMed] [Google Scholar]
- 3.Wada T, Mizuno K, Itoh K, et al. Adenosquamous carcinoma of the jejunum. J Gatroenterol. 2003;38:786–790. doi: 10.1007/s00535-002-1147-7. [DOI] [PubMed] [Google Scholar]
- 4.Cecchini S, Correa-Gallego C, Desphande V, Ligorio M, Dursun A, Wargo J, Fernàndez-del Castillo C, Warshaw AL, Ferrone CR. Superior prognostic importance of perineural invasion vs. lymph node involvement after curative resection of duodenal adenocarcinoma. J Gastrointest Surg. 2012;16:113–120. doi: 10.1007/s11605-011-1704-6. [DOI] [PubMed] [Google Scholar]
- 5.Overman MJ, Kopetz S, Lin E, Abbruzzese JL, Wolff RA. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol. 2010;49:474–479. doi: 10.3109/02841860903490051. [DOI] [PubMed] [Google Scholar]
- 6.Otten TR, Stein PD, Patel KC, Mustafa S, Silbergleit A. Thromboembolic disease involving the superior vena cava and branchiocephalic veins. Chest. 2003;123:809–812. doi: 10.1378/chest.123.3.809. [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: Clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37–42. doi: 10.1097/01.md.0000198474.99876.f0. [DOI] [PubMed] [Google Scholar]
- 8.Japan Pancreas Society: General Rules for the Study of Pancreatic Cancer. 6th. Kanehara, Tokyo: 2009. [Google Scholar]
- 9.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 10.http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Nov 10;2015 ];National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Accessed. [Google Scholar]
- 11.Zaanan A, Costes L, Gauthier M, et al. Chemotherapy of advanced small-bowel adenocarcinoma: A multicenter AGEO study. Ann Oncol. 2010;21:1786–1798. doi: 10.1093/annonc/mdq038. [DOI] [PubMed] [Google Scholar]
- 12.Blanco P, Ly S, Beylot Barry M, Laurent F, Roques X, Doutre M, Beylot C. Surgical treatment of an endovascular metastatic melanoma of the superior vena cava. Dermatology. 1999;199:156–157. doi: 10.1159/000018225. [DOI] [PubMed] [Google Scholar]
- 13.Ghattas S, Howle J, Wang W, Kefford R, Gruenewald S. Intravascular metastatic melanoma: A difficult diagnosis. Australas J Dermatol. 2013;54:141–143. doi: 10.1111/ajd.12033. [DOI] [PubMed] [Google Scholar]
- 14.Takeda T, Saitoh M, Takeda S. Superior vena cava syndrome caused by an intravascular thrombosis due to underlying prostate carcinoma. Intern Med. 2008;47:2007–2009. doi: 10.2169/internalmedicine.47.1428. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Liang J, Wang W, Ouyang H, Wang L. Malignant thrombosis of the superior vena cava caused by non-small-cell lung cancer treated with radiation and erlotinib: A case with complete and prolonged response over 3 years. Onco Targets Ther. 2013;6:749–753. doi: 10.2147/OTT.S45660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy C, Schwalb H, Berlangieri S, Eek R. Intraluminal superior vena cava metastasis in a patient with poorly differentiated thyroid carcinoma. J Clin Oncol. 2014 Apr 21; doi: 10.1200/JCO.2013.51.8753. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Matsuno Y, Takama N, Yasuhara K, Koyano T, Obayashi T, Sasaki T, Kanesawa N, Kurabayashi M. Long-survival case of thymic carcinoma with superior vena cava tumor thrombus. Ann Thorac Surg. 2012;94:1729–1731. doi: 10.1016/j.athoracsur.2012.02.081. [DOI] [PubMed] [Google Scholar]
- 18.Alzand BS, Geyik Z, Dannert R, Cheriex EC. Superior vena cava syndrome as a complication of colon carcinoma. Int J Cardiol. 2009;132:e45–e47. doi: 10.1016/j.ijcard.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 19.Spittell PC, Hayes DL. Venous complications after insertion of a transvenous pacemaker. Mayo Clin Proc. 1992;67:258–265. doi: 10.1016/S0025-6196(12)60103-7. [DOI] [PubMed] [Google Scholar]
- 20.Krug H, Zerbe F. Major venous thrombosis. A complication of transvenous pacemaker electrodes. Br Heart J. 1980;44:158–161. doi: 10.1136/hrt.44.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cagir B, Nagy MW, Topham A, Rakinic J, Fry RD. Adenosquamous carcinoma of the colon, rectum, and anus: Epidemiology, distribution, and survival characteristics. Dis Colon Rectum. 1999;42:258–263. doi: 10.1007/BF02237138. [DOI] [PubMed] [Google Scholar]
- 22.Kim A, Bae YK, Gu MJ, Kim JY, Jang KY, Bae HI, Lee HJ, Hong SM. Epithelial-mesenchymal transition phenotype is associated with patient survival in small intestinal adenocarcinoma. Pathology. 2013;45:567–573. doi: 10.1097/PAT.0b013e3283650bab. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Lee OJ, Jang KT, Bae YK, Chung JY, Eom DW, Kim JM, Yu E, Hong SM. Combined loss of E-cadherin and aberrant β-catenin protein expression correlates with a poor prognosis for small intestinal adenocarcinomas. Am J Clin Pathol. 2013;139:167–176. doi: 10.1309/AJCPS54RTFCTHGWX. [DOI] [PubMed] [Google Scholar]
- 24.Kase S, Sugio K, Ymazaki K, Okamoto T, Yano T, Sugimachi K. Expression of E-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–4796. [PubMed] [Google Scholar]
- 25.Liu S, Liao G, Ding J, Ye K, Zhang Y, Zeng L, Chen S. Dysregulated expression of snail and E-cadherin correlates with gastrointestinal stromal tumor metastasis. Eur J Cancer Prev. 2014;23:329–335. doi: 10.1097/CEJ.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 26.Fernebro E, Bendahl PO, Dictor M, Persson A, Fernö M, Nilbert M. Immunohistochemical patterns in rectal cancer: Application of tissue microarray with prognostic correlations. Int J Cancer. 2004;111:921–928. doi: 10.1002/ijc.20229. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Sorbye H, Dragomir A, Sundström M, Pfeiffer P, Thunberg U, Bergfors M, Aasebø K, Eide GE, Ponten F, Qvortrup C, Glimelius B. High BRAF mutation frequency and marked survival differences in subgroups according to KRAS/BRAF mutation status and tumor tissue availability in a prospective population-based metastatic colorectal cancer cohort. PLoS One. 2015;29:e0131046. doi: 10.1371/journal.pone.0131046. [DOI] [PMC free article] [PubMed] [Google Scholar]