Abstract
Worldwide, colon cancer is the third most common cancer in terms of incidence, following lung and breast cancer. Resistance to psoralidin frequently occurs following its use as an anticancer treatment. However, the mechanisms underlying the effects of psoralidin on colon cancer, remain to be elucidated. Hence, the present study investigated the anticancer effects and potential mechanism of action of psoralidin on SW480 human colon cancer cells. In the present study, an MTT assay was performed to measure the viability of SW480 cells. Additionally, an Annexin V-fluorescein isothiocyanate/propidium iodide apoptosis detection kit, DAPI staining assay and caspase-3 colorimetric assay kits were used to analyze the cellular apoptosis of SW480 cells. The nuclear factor-κB (NF-κB) p65 activity and B-cell lymphoma-2 (Bcl-2)/Bcl-2-associated X protein (Bax) protein expression of SW480 cells was detected using NF-κB colorimetric assay kits and western blot analysis, respectively. Bcl-2 inhibitor ABT-737 was added to SW480 cells and the subsequent effects and mechanism of action of psoralidin on SW480 colon cancer cells was studied. In the present study, psoralidin reduced SW480 cell viability and enhanced the cellular apoptosis of SW480 cells in a dose-dependent manner. Caspase-3 activity of SW480 cells was increased following treatment with psoralidin. Additionally, psoralidin was able to reduce the NF-κB p65 activity of SW480 cells. Furthermore, psoralidin was able to reduce Bcl-2 protein expression and increase Bax protein expression in SW480 cells. Notably, Bcl-2 inhibitor was observed to enhance the effects of psoralidin on SW480 cells. The results of the present study suggest that psoralidin may be a candidate drug for the treatment of colon cancer by inhibition of the NF-κB and Bcl-2/Bax signaling pathways.
Keywords: nuclear factor-κB, B-cell lymphoma-2/B-cell lymphoma-2-associated X protein, colon cancer, psoralidin
Introduction
Colon cancer is a type of malignancy with high levels of incidence; worldwide, it is the third most commonly occurring malignancy in males, and the second most common in females (1). According to estimates from the International Agency for Research on Cancer, ~1.2 million novel cases of colon cancer were diagnosed in 2008, of which ~8% led to mortality (2). Colon cancer is more common in developed countries and regions, however due to economic development and the acceleration of urbanization, the diet and lifestyle of the population in developing countries have been altered (3). In recent years, the incidence and mortality of colon cancer has demonstrated an increasing trend in developing countries, which is higher than the world average (4).
Nuclear factor-κB (NF-κB) is a ubiquitous transcription factor that is able to mediate inflammation and the immune response (5). Previous studies have revealed that NF-κB is associated with the occurrence and development of tumors, the inhibition of apoptosis and the induction of drug resistance (6,7). Identifying a natural inhibitor of NF-κB activity, and blocking the NF-κB signaling pathway which inhibits apoptosis, may be able to provide promising drug candidates for the treatment of malignant tumors (8). Yu et al (9) demonstrated that berberine was able to enhance the chemosensitivity of colon cancer cells to irinotecan via the suppression of NF-κB. Tanwar et al (10) concluded that etoricoxib reduced colon cancer development by inhibition of NF-κB.
The levels and interaction of B-cell lymphoma-2 (Bcl-2) family gene products are important for the regulation of apoptosis, during which the ratio of Bcl-2/Bcl-2-associated X protein (Bax) is critical (11). Investigating the expression of Bcl-2/Bax may be significant in the improvement of the study, diagnosis, treatment efficacy and prognosis assessment of tumors (12,13). Ko et al (14) demonstrated that soy soluble polysaccharide induced Bcl-2/Bax-mediated apoptosis of HCT-116 human colon cancer cells. Mao et al (15) reported that gastrin accelerated the action of the cell apoptosis regulation complex Bcl-2/Bax in large intestine carcinoma. Zhao et al (16) revealed that β-sitosterol inhibited cell growth and induced apoptosis of human stomach cancer cells via a reduction of the Bcl-2/Bax ratio.
Current research has revealed that psoralidin contains a number of compounds, including coumarin, flavonoids and monoterpene phenols, which possess immunomodulatory, anti-inflammatory, antioxidant and anti-tumor effects (17). Furthermore, Hao et al (18) reported that psoralidin inhibited the proliferation of A549 human lung cancer cells through the generation of reactive oxygen species (ROS). Additionally, psoralidin has been observed to inhibit cell proliferation and induce apoptosis of androgen-independent prostate cancer cells through phosphatidylinositol 3-kinase-mediated Akt signaling (17). However, to the best of our knowledge, the mechanisms underlying the anticancer effects of psoralidin on colon cancer cells have not previously been studied. Therefore, in the present study, the mechanism of action of psoralidin was investigated in human colon cancer cells.
Materials and methods
Reagents and chemicals
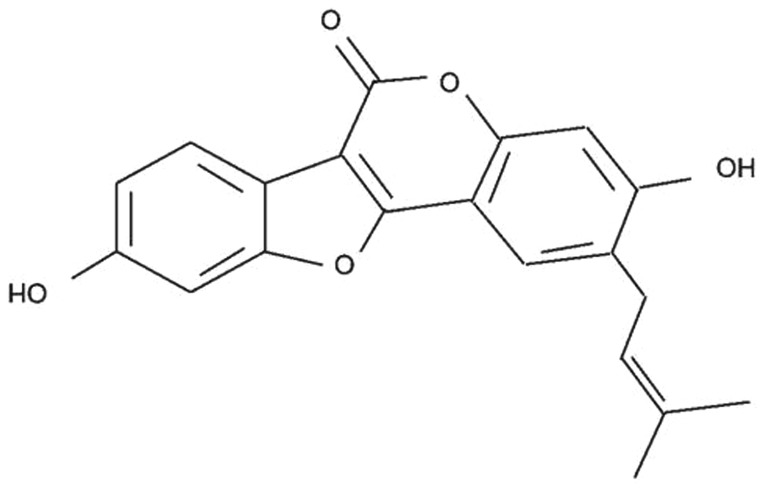
The chemical structure of psoralidin (with purity ≥98%) is presented in Fig. 1. Psoralidin and DAPI reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 medium was obtained from KeyGen Biotechnology Co., Ltd. (Nanjing, China). Fetal bovine serum (FBS) was obtained from HyClone (GE Healthcare Life Sciences, Logan, UT, USA). MTT was purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit, caspase-3 colorimetric assay and NF-κB ELISA assay kits were purchased from Beyotime Institute of Biotechnology (Nanjing, China). ABT-737 was purchased from EMD Millipore (#HY-50907; Billerica, MA, USA).
Figure 1.
Chemical structure of psoralidin.
Cell line and culture
The SW480 human colon cancer cell line was obtained from the Department of Oncology (Central Hospital of Jingzhou, Jingzhou, China). Cells were grown in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was replaced every 2–3 days with fresh complete medium.
MTT assay
SW480 cells (2.0×104 cells/well) were cultured with psoralidin (0, 5, 10 and 20 µM) at 37°C in a humidified atmosphere containing 5% CO2 for 0, 24, 48 and 72 h in 96-well plates. SW480 cells were washed twice with phosphate-buffered saline (PBS; Sangon Biotech Co., Ltd.), prior to the addition of 10 µl MTT to each well. SW480 cells were incubated at 37°C for 4 h. Subsequently, the culture medium was removed and 150 µl dimethyl sulfoxide (Invitrogen Life Technologies) was added to each well. SW480 cells were incubated for 20 min at room temperature with agitation. Cell viability of SW480 cells was determined by the MTT assay as described previously (19). Briefly, absorbance was measured at a wavelength of 570 nm using a microplate reader (2104-0010; PerkinElmer, Inc., Waltham, MA, USA). Cell viability was calculated as follows: Cell viability (%) = (mean absorbance of psoralidin treated groups/mean absorance of 0 µM psoralidin group) × 100.
Flow cytometric analysis of cellular apoptosis
SW480 cells (2×106 cells/well) were cultured with psoralidin (0, 5, 10 and 20 µM) (17) at 37°C in a humidified atmosphere containing 5% CO2 for 48 h in 6-well plates. SW480 cells were harvested and washed twice with cold PBS. Subsequently, SW480 cells (1×106 cells/ml) were resuspended in Annexin-V binding buffer. Annexin V-FITC (10 µl) was added and cells were incubated for 30 min in the dark at 4°C. Subsequently, 5 µl PI was added to the cells and incubated for 10 min in the dark at room temperature. Cellular apoptosis of SW480 cells was immediately detected using flow cytometry (EPICS® ALTRA™; Beckman Coulter, Inc., Brea, CA, USA).
DAPI staining assay
SW480 cells (2×106 cells/well) were cultured with psoralidin (0, 5, 10 and 20 µM) at 37°C in a humidified atmosphere containing 5% CO2 for 0, 24, 48 and 72 h in 6-well plates. SW480 cells were washed twice with PBS. Subsequently, 0.5 ml 4% paraformaldehyde (Beijing DingGuo ChangSheng Biotechnology Co., Ltd., Beijing, China) was added to each well and fixed for 30 min at 4°C. SW480 cells were washed twice with PBS, prior to the addition of sodium citrate (0.1%) containing 0.1% Triton X-100 and incubated for 5 min at 4°C. Subsequently, DAPI was added to each well and incubated for 10–15 min at 4°C in the dark. Apoptotic cells were excited by ultraviolet light. SW480 cells were observed and images were captured under a fluorescent microscope (Zeiss Axio Observer A1; Ziess AG, Oberkochen, Germany) at 340 nm.
Detection of caspase-3 activity
SW480 cells (2×106 cells/well) were cultured with psoralidin (0, 5, 10 and 20 µM) at 37°C in a humidified atmosphere containing 5% CO2 for 48 h in 6-well plates. Caspase-3 activity was detected by measuring fluorescence at a wavelength of 405 nm using a microplate reader (SN11693; Bio-Rad Laboratories, Inc., Hercules, CA, USA) with caspase-3 colorimetric assay kits.
Measurement of NF-κB activity
SW480 cells (1×106 cells/well) were cultured with psoralidin (0, 5, 10 and 20 µM) at 37°C in a humidified atmosphere containing 5% CO2 for 0, 24, 48 and 72 h in 6-well plates. Working solution (100 µl; 1% biotin labeling, 99% antibody) was added into every well and incubated at 37°C for 1 h. Following incubation, the supernatant was dried and discarded, and 350 µl wash buffer was added to each well and washed for 1 min. Next, 3.90 µl tetramethylbenzidine substrate was added to each well, followed by incubation at 37°C for 30 min in darkness. Then, 50 µl stop solution was added into every well. NF-κB activity was analyzed using ELISA assay kits (DRE20177; Shanghai Bioleaf Biotech Co., Ltd., Shanghai, China) using a microplate reader (SN11693; Bio-Rad Laboratories, Inc.) at 450 nm, as described previously (20).
Western blot analysis
SW480 cells (1×106 cells/well) were cultured with psoralidin (0, 5, 10 and 20 µM) at 37°C in a humidified atmosphere containing 5% CO2 for 0, 24, 48 and 72 h in 6-well plates. SW480 cells were subsequently incubated with ice-cold lysis buffer (Beyotime Institute of Biotechnology) for 30 min on ice and then centrifuged at 12,000 × g for 10 min at 4°C. The total protein concentration of soluble materials was determined using a bicinchoninic acid protein assay (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal quantities of protein were separated using 12% SDS-PAGE (Invitrogen Life Technologies) and then transferred onto a polyvinylidene difluoride membrane (0.22 mm; GE Healthcare Life Sciences, Piscataway, NJ, USA). Following blocking with Tris-buffered saline (Beyotime Institute of Biotechnology) containing 5% non-fat milk for 2 h, the membranes were incubated with monoclonal mouse anti-human Bcl-2 (1:1,500; cat. no. sc-7382; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), monoclonal mouse anti-human Bax (1:2,000; cat. no. sc-8432; Santa Cruz Biotechnology, Inc.) and monoclonal mouse anti-human β-actin (1:500; cat. no. sc-8432; Tiangen Biotech Co., Ltd., Beijing, China) overnight at 4°C. The membrane was washed twice with Tris-buffered saline with Tween-20 (Sigma-Aldrich) for 2 h and then incubated with horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G (1:1,000; cat. no. sc-358922; Santa Cruz Biotechnology, Inc.) at room temperature for 2 h. Proteins were subsequently detected using enhanced chemiluminescence (DAB Horseradish Peroxidase Color Development Kit; Beyotime Institute of Biotechnology).
Effects of Bcl-2 inhibition
To further investigate the potential association between Bcl-2 inhibition and the effect of psoralidin on SW480 cells, SW480 cells were incubated with 1 µM Bcl-2 inhibitor (ABT-737; 99% purity; Sigma-Aldrich) and 10 µM psoralidin for 48 h at 37°C in a humidified atmosphere containing 5% CO2. The aforementioned MTT assay and western blotting protocols were then performed to determine cell viability and Bcl-2 protein expression levels, respectively.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation. Data were analyzed using Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Psoralidin treatment reduces the viability of SW480 cells
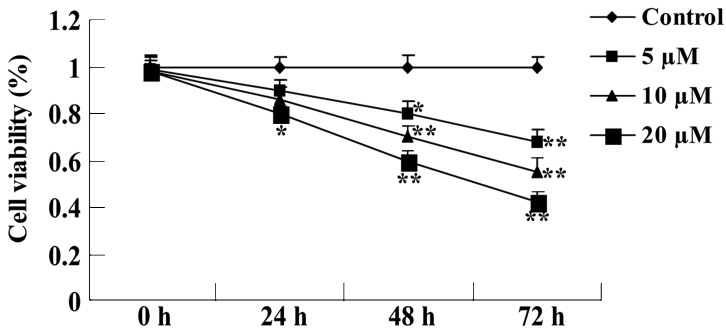
The anti-proliferative effect of psoralidin (0, 5, 10 and 20 µM) on the viability of SW480 cells was detected by MTT assay. As demonstrated in Fig. 2, psoralidin (5, 10 and 20 µM) treatment reduced the viability of SW480 cells in a dose- and time-dependent manner. This effect was particularly evident at a concentration of 20 µM, at which the proliferation of SW480 cells was significantly inhibited at 24, 48 and 72 h (P<0.01; Fig. 2). Additionally, treatment with 5 µM psoralidin for 72 h and 10 µM psoralidin for 48 and 72 h also significantly reduced SW480 cell viability (P<0.01; Fig. 2).
Figure 2.
Psoralidin treatment reduces the viability of SW480 cells. Values are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01 compared with the control group.
Psoralidin treatment promotes the apoptosis of SW480 cells
To determine whether apoptosis mediated the anti-proliferative effect of psoralidin (0, 5, 10 and 20 µM), apoptosis of SW480 cells was analyzed using flow cytometry and a DAPI staining assay. As demonstrated in Fig. 3A, psoralidin (0, 5, 10 and 20 µM) treatment promoted the apoptosis of SW480 cells in a dose-dependent manner. This effect of psoralidin was particularly evident at concentrations of 10 and 20 µM, at which the apoptosis of SW480 cells at 48 h was significantly increased (P<0.01; Fig. 3A). In addition, treatment with 5 µM psoralidin for 72 h significantly enhanced the apoptosis of SW480 cells at 48 h (P<0.05; Fig. 3A). Notably, the results of the DAPI staining assay revealed that the apoptosis of SW480 cells was augmented in cells treated with psoralidin (5, 10 and 20 µM) at 48 h, compared with the control group (Fig. 3B).
Figure 3.
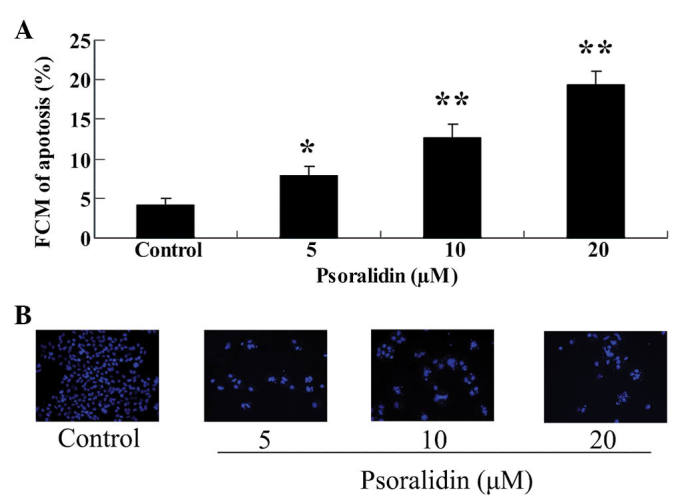
Psoralidin enhances cellular apoptosis of SW480 cells. Effect of psoralidin on the cellular apoptosis of SW480 cells examined by (A) flow cytometry and (B) DAPI staining assay (magnification, ×200). Values are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01 compared with control group. FCM, flow cytometry.
Psoralidin treatment enhances caspase-3 activity of SW480 cells
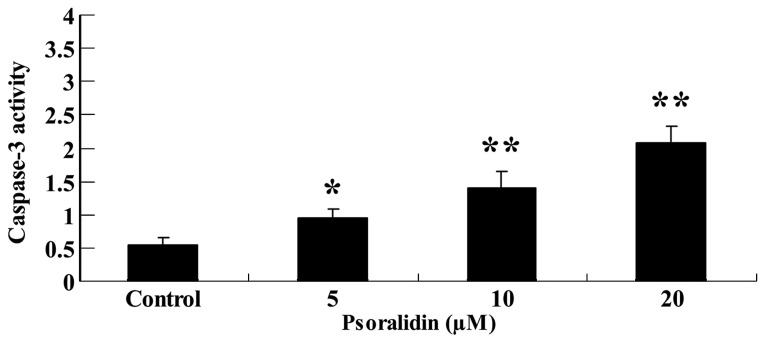
To further investigate the anti-proliferative effect of psoralidin (0, 5, 10 and 20 µM) on the caspase-3 activity of SW480 cells, caspase-3 activity was measured using colorimetric assay kits. As demonstrated in Fig. 4, psoralidin (5, 10 and 20 µM) treatment increased the caspase-3 activity of SW480 cells in a dose-dependent manner. Following psoralidin (10 and 20 µM) treatment for 48 h, the caspase-3 activity of SW480 cells was significantly augmented (P<0.01), and following psoralidin (5 µM) treatment for 48 h, the caspase-3 activity of SW480 cells was also augmented (P<0.05; Fig. 4A and B).
Figure 4.
Psoralidin treatment enhances caspase-3 activity of SW480 cells. Values are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01 compared with control group.
Psoralidin treatment reduces the NF-κB activity of SW480 cells
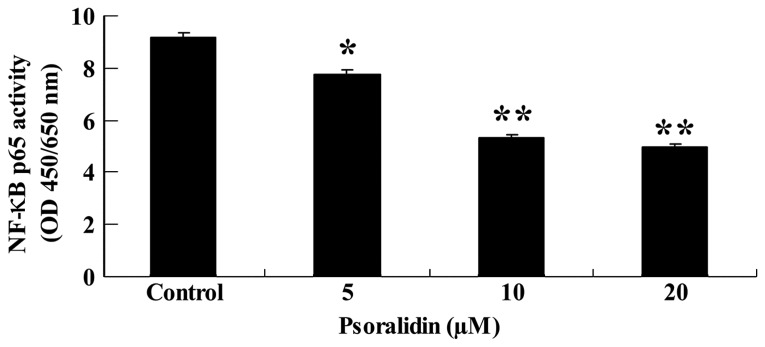
The potential association between the anti-proliferative effect of psoralidin (0, 5, 10 and 20 µM) and NF-κB activity in SW480 cells was evaluated. NF-κB p65 activity was investigated using an ELISA assay kit. As demonstrated in Fig. 5, NF-κB p65 activity was reduced by psoralidin (5, 10 and 20 µM) treatment in a dose-dependent manner. NF-κB p65 activity was significantly decreased following psoralidin (10 and 20 µM) treatment for 48 h (P<0.01), and markedly decreased following psoralidin (5 µM) treatment for 48 h (P<0.05; Fig. 5).
Figure 5.
Psoralidin reduces the NF-κB activity of SW480 cells. Values are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01 compared with control group. NF-κB, nuclear factor-κB; OD, optical density.
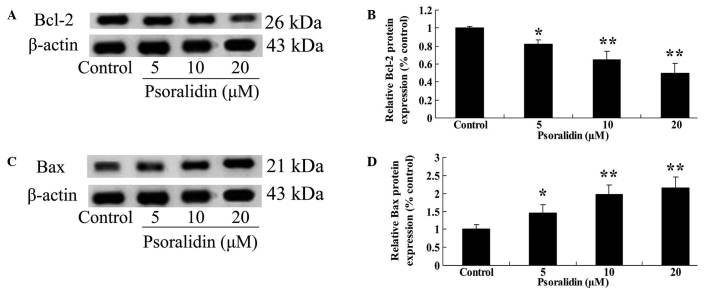
Psoralidin treatment inhibits Bcl-2 and enhances Bax protein expression in SW480 cells
To determine whether there was an association between the anti-proliferative effect of psoralidin (0, 5, 10 and 20 µM) treatment on SW480 cells, and the Bcl-2/Bax signaling pathway, Bcl-2 and Bax protein expression in SW480 cells was detected by western blot analysis. As demonstrated in Fig. 6A and B, Bcl-2 protein expression was significantly inhibited by psoralidin (10 and 20 µM) treatment for 48 h (P<0.01), and markedly decreased following psoralidin (5 µM) treatment for 48 h (P<0.05; Fig. 6A and B). By contrast, Bax protein expression in SW480 cells was markedly promoted by psoralidin (5, 10 and 20 µM) treatment: Bax protein expression was significantly increased following psoralidin (10 and 20 µM) treatment for 48 h (P<0.01), and markedly increased following psoralidin (5 µM) treatment for 48 h (P<0.05; Fig. 6C and D).
Figure 6.
Psoralidin inhibits Bcl-2 and enhances Bax protein expression in SW480 cells. (A and B) Effect of psoralidin on Bcl-2 protein expression of SW480 cells by western blotting assay and statistical analysis of Bcl-2 protein expression of SW480 cells. (C and D) Effects of psoralidin on Bax protein expression of SW480 cells by western blotting assay and statistical analysis of Bax proteins expression of SW480 cells. Values are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01 compared with control group. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein.
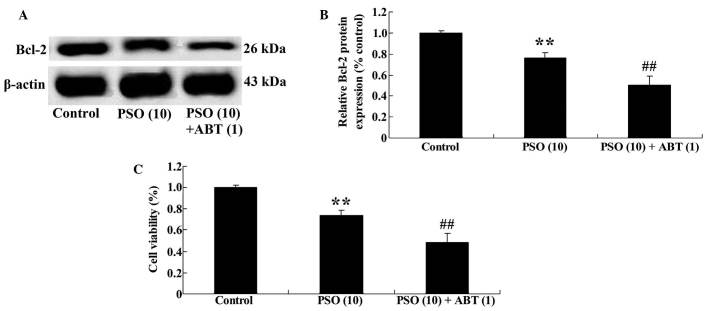
Bcl-2 inhibitor is able to enhance the anti-proliferative effect of psoralidin on SW480 cells
To further investigate the potential association between Bcl-2 inhibition and the effect of psoralidin on SW480 cells, psoralidin-treated SW480 cells were incubated with a Bcl-2 inhibitor (ABT-737) for 48 h. ABT-737 inhibited Bcl-2 protein expression (Fig. 7A and B) and decreased the viability of SW480 cells (Fig. 7C), compared with the psoralidin (10 µM) treatment control group.
Figure 7.
Bcl-2 inhibitor enhances the effect of psoralidin on SW480 cells. (A) Effect of Bcl-2 inhibitor on Bcl-2 protein expression of SW480 cells by western blotting assays; (B) statistical analysis of Bcl-2 protein expression of SW480 cells; and (C) Bcl-2 inhibitor enhances the effect of psoralidin on viability of SW480 cells. Values are expressed as the mean ± standard deviation. **P<0.01 compared with control group; ##P<0.01 compared with PSO (10) group. Bcl-2, B-cell lymphoma-2; PSO, psoralidin; ABT, ABT-737.
Discussion
Worldwide, colon cancer is the third most common cancer in terms of incidence, following lung and breast cancer (21). There are differences in the geographical distribution of colon cancer (22). In developed countries or regions, including Australia, New Zealand, Europe and North America, there is a higher prevalence of colon cancer, whereas in African and South Asian countries, incidence is reduced, and may be up to ten times lower (23). Colon cancer mortality rates are highest in Central and Eastern Europe, and lowest in Central Africa (24). In the present study, psoralidin treatment reduced the viability and promoted the apoptosis of SW480 cells in a dose-dependent manner. Furthermore, Hao et al (18) reported that psoralidin inhibited the proliferation of A549 cells by inducing ROS production. Szliszka et al (25) identified that psoralidin inhibited cell proliferation of prostate cancer cells by enhancement of tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis. Additionally in the present study, caspase-3 activity of SW480 cells was significantly enhanced following psoralidin treatment. Das et al (26) suggested that psoralidin may promote growth arrest in prostate cancer cells by activation of caspase-3 and caspase-9.
A number of studies have revealed that NF-κB is highly expressed within tumor cells, and is involved in cell proliferation, invasion, angiogenesis and inhibition of apoptosis (27). Activation of the NF-κB signal transduction pathway may weaken the antitumor effects of chemoradiation therapy (28). The Hut-78 cell line, which highly expresses NF-κB, is resistant to the anticancer drug Taxol; however, Jurket cells, which do not express NF-κB, are sensitive to Taxol. This suggests that NF-κB participates in regulation of the sensitivity of tumor cells to anticancer therapies (29). In the present study, it was observed that psoralidin treatment markedly reduced the NF-κB p65 activity of SW480 cells. Chiou et al (30) observed that psoralidin inhibited lipopolysaccharide-induced inducible nitric oxide synthase expression by mediating NF-κB signaling. Yang et al (17) reported that psoralidin regulated ionizing radiation-induced pulmonary inflammation by regulation of the phosphoinositide 3-kinase/Akt and NF-κB pathways.
Apoptosis is the primary mechanism for removal of unwanted cells during embryonic development and tissue repair, and disorders of the apoptotic mechanism are closely associated with tumorigenesis. Apoptosis is a process of active cell death that is controlled by genes (31). In particular, Bcl-2 family genes are significant for apoptosis and have been extensively studied. Bcl-2 family proteins may act on the apoptotic pathway and exert regulatory effect on apoptosis via various routes (32). The levels of and interaction between Bcl-2 and Bax gene products may also be the regulatory pathway center of Bcl-2 apoptosis, while the Bcl-2/Bax protein ratio is a significant switch in apoptosis regulation (33). It is known that psoralidin may decrease Bcl-2 protein expression and increase Bax protein expression in SW480 cells (34). Based on these findings, the present study demonstrated that Bcl-2 protein expression was markedly decreased following psoralidin treatment. It was previously revealed that psoralidin inhibited androgen-independent prostate cancer cells via Bcl-2/Bax signaling pathway inhibition (17). In the present study, it was demonstrated that inhibition of Bcl-2 protein expression was capable of enhancing the anticancer effects of psoralidin on SW480 cells. Srinivasan et al (35) suggested that psoralidin inhibited cell proliferation and induced apoptosis of prostate cancer cells by mediation of NF-κB and Bcl-2 signaling pathways.
In conclusion, psoralidin decreased the expression levels of NF-κB and Bcl-2, increased the expression levels of Bax, promoted caspase-3 activity and subsequently induced apoptosis in human colon cancer SW480 cells. The results of the present study demonstrate the potential benefits of psoralidin in clinical practice.
References
- 1.Isik A, Peker K, Firat D, Yilmaz B, Sayar I, Idiz O, Cakir C, Demiryilmaz I, Yilmaz I. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–1375. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu ZJ, Lu LG, Tao KZ, Chen DF, Xia Q, Weng JJ, Zhu F, Wang XP, Zheng P. MicroRNA-185 suppresses growth and invasion of colon cancer cells through inhibition of the hypoxia-inducible factor-2α pathway in vitro and in vivo. Mol Med Rep. 2014;10:2401–2408. doi: 10.3892/mmr.2014.2562. [DOI] [PubMed] [Google Scholar]
- 3.Su CC. Tanshinone IIA potentiates the efficacy of 5-FU in Colo205 colon cancer cells in vivo through downregulation of P-gp and LC3-II. Exp Ther Med. 2012;3:555–559. doi: 10.3892/etm.2011.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grijelmo C, Rodrigue C, Svrcek M, Bruyneel E, Hendrix A, de Wever O, Gespach C. Proinvasive activity of BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent signaling pathways in colon cancer cells. Cell Signal. 2007;19:1722–1732. doi: 10.1016/j.cellsig.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Miskolci V, Rollins J, Vu HY, Ghosh CC, Davidson D, Vancurova I. NFkappaB is persistently activated in continuously stimulated human neutrophils. Mol Med. 2007;13:134–142. doi: 10.2119/2006-00072.Miskolci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo W, Liu Y, Zhang J, Luo X, Lin C, Guo J. Andrographolide inhibits the activation of NF-κB and MMP-9 activity in H3255 lung cancer cells. Exp Ther Med. 2013;6:743–746. doi: 10.3892/etm.2013.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 9.Yu M, Tong X, Qi B, Qu H, Dong S, Yu B, Zhang N, Tang N, Wang L, Zhang C. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NF-κB. Mol Med Rep. 2014;9:249–254. doi: 10.3892/mmr.2013.1762. [DOI] [PubMed] [Google Scholar]
- 10.Tanwar L, Vaish V, Sanyal SN. Chemoprevention of 1,2-dimethylhydrazine-induced colon carcinogenesis by a non-steroidal anti-inflammatory drug, etoricoxib, in rats: Inhibition of nuclear factor kappaB. Asian Pac J Cancer Prev. 2009;10:1141–1146. [PubMed] [Google Scholar]
- 11.Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise LH, Thompson CB, Golemis E, Fong L, Wang HG, et al. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Yu N, Guo T, Hou Y, Zeng Z, Yang X, Hu P, Tang X, Wang J, Liu M. Tissue biomarkers for prognosis of prostate cancer: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1047–1054. doi: 10.1158/1055-9965.EPI-13-0696. [DOI] [PubMed] [Google Scholar]
- 13.Giatromanolaki A, Stathopoulos GP, Koukourakis MI, Rigatos S, Vrettou E, Kittas C, Fountzilas G, Sivridis E. Angiogenesis and apoptosis-related protein (p53, bcl-2, and bax) expression versus response of gastric adenocarcinomas to paclitaxel and carboplatin chemotherapy. Am J Clin Oncol. 2001;24:222–226. doi: 10.1097/00000421-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mirjolet JF, Barberi-Heyob M, Didelot C, Peyrat JP, Abecassis J, Millon R, Merlin JL. Bcl-2/Bax protein ratio predicts 5-fluorouracil sensitivity independently of p53 status. Br J Cancer. 2000;83:1380–1386. doi: 10.1054/bjoc.2000.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko YJ, Jeong JW, Choi YH, Ryu CH. Soy soluble polysaccharide induces apoptosis in HCT-116 human colon cancer cells via reactive oxygen species generation. Mol Med Rep. 2013;8:1767–1772. doi: 10.3892/mmr.2013.1725. [DOI] [PubMed] [Google Scholar]
- 16.Mao JD, Wu P, Xia XH, Hu JQ, Huang WB, Xu GQ. Correlation between expression of gastrin, somatostatin and cell apoptosis regulation gene bcl-2/bax in large intestine carcinoma. World J Gastroenterol. 2005;11:721–725. doi: 10.3748/wjg.v11.i5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang HJ, Youn H, Seong KM, Yun YJ, Kim W, Kim YH, Lee JY, Kim CS, Jin YW, Youn B. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem Pharmacol. 2011;82:524–534. doi: 10.1016/j.bcp.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Hao W, Zhang X, Zhao W, Chen X. Psoralidin induces autophagy through ROS generation which inhibits the proliferation of human lung cancer A549 cells. PeerJ. 2014;2:e555. doi: 10.7717/peerj.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pu Z, Zhang X, Chen Q, Yuan X, Xie H. Establishment of an expression platform of OATP1B1 388GG and 521CC genetic polymorphism and the therapeutic effect of tamoxifen in MCF-7 cells. Oncol Rep. 2015;33:2420–2428. doi: 10.3892/or.2015.3864. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Liu K, Seneviratne CJ, Li X, Cheung GS, Jin L, Chu CH, Zhang C. Lipoteichoic acid from an Enterococcus faecalis clinical strain promotes TNF-α expression through the NF-κB and p38 MAPK signaling pathways in differentiated THP-1 macrophages. Biomed Rep. 2015;3:697–702. doi: 10.3892/br.2015.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han SA, Lee WY, Park CM, Yun SH, Chun HK. Comparison of immunologic outcomes of laparoscopic vs open approaches in clinical stage III colorectal cancer. Int J Colorectal Dis. 2010;25:631–638. doi: 10.1007/s00384-010-0882-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Cai Q, Lin J, Fang Y, Zhan Y, Shen A, Wei L, Wang L, Peng J. Chloroform fraction of Scutellaria barbata D. Don promotes apoptosis and suppresses proliferation in human colon cancer cells. Mol Med Rep. 2014;9:701–706. doi: 10.3892/mmr.2013.1864. [DOI] [PubMed] [Google Scholar]
- 23.Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo AC, Soliman AS, Khaled HM, Aboelyazid A, Greenson JK. Lifestyle, occupational, and reproductive factors and risk of colorectal cancer. Dis Colon Rectum. 2010;53:830–837. doi: 10.1007/DCR.0b013e3181d320b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szliszka E, Czuba ZP, Sędek Ł, Paradysz A, Król W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol Rep. 2011;63:139–148. doi: 10.1016/S1734-1140(11)70408-X. [DOI] [PubMed] [Google Scholar]
- 26.Das TP, Suman S, Damodaran C. Induction of reactive oxygen species generation inhibits epithelial-mesenchymal transition and promotes growth arrest in prostate cancer cells. Mol Carcinog. 2014;53:537–547. doi: 10.1002/mc.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen M, Meisser A, Haenggeli L, Bischof P. Involvement of MAPK pathway in TNF-alpha-induced MMP-9 expression in human trophoblastic cells. Mol Hum Reprod. 2006;12:225–232. doi: 10.1093/molehr/gal023. [DOI] [PubMed] [Google Scholar]
- 28.Davoudi Z, Akbarzadeh A, Rahmatiyamchi M, Movassaghpour AA, Alipour M, Nejati-Koshki K, Sadeghi Z, Dariushnejad H, Zarghami N. Molecular target therapy of AKT and NF-kB signaling pathways and multidrug resistance by specific cell penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer Prev. 2014;15:4353–4358. doi: 10.7314/APJCP.2014.15.10.4353. [DOI] [PubMed] [Google Scholar]
- 29.Ali S, El-Rayes BF, Sarkar FH, Philip PA. Simultaneous targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways for pancreatic cancer therapy. Mol Cancer Ther. 2005;4:1943–1951. doi: 10.1158/1535-7163.MCT-05-0065. [DOI] [PubMed] [Google Scholar]
- 30.Chiou WF, Don MJ, Liao JF, Wei BL. Psoralidin inhibits LPS-induced iNOS expression via repressing Syk-mediated activation of PI3K-IKK-IκB signaling pathways. Eur J Pharmacol. 2011;650:102–109. doi: 10.1016/j.ejphar.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Edwards MJ. Apoptosis, the heat shock response, hyperthermia, birth defects, disease and cancer. Where are the common links? Cell Stress Chaperones. 1998;3:213–220. doi: 10.1379/1466-1268(1998)003<0213:ATHSRH>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong Y, Ma XY, Zhang Z, Shao ZJ, Zhang YY, Zhou LM. Apoptosis induced by β,β-dimethylacrylshikonin is associated with Bcl-2 and NF-κB in human breast carcinoma MCF-7 cells. Oncol Lett. 2013;6:1789–1793. doi: 10.3892/ol.2013.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu Y, Liu SL, Ju WZ, Li CY, Cao P. Analgesic-antitumor peptide induces apoptosis and inhibits the proliferation of SW480 human colon cancer cells. Oncol Lett. 2013;5:483–488. doi: 10.3892/ol.2012.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam H, Kim MM. Ursolic acid induces apoptosis of SW480 cells via p53 activation. Food Chem Toxicol. 2013;62:579–583. doi: 10.1016/j.fct.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan S, Kumar R, Koduru S, Chandramouli A, Damodaran C. Inhibiting TNF-mediated signaling: A novel therapeutic paradigm for androgen independent prostate cancer. Apoptosis. 2010;15:153–161. doi: 10.1007/s10495-009-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]