Abstract
The emergence of microphysiologic epithelial lung models using human cells in a physiologically relevant microenvironment has the potential to be a powerful tool for preclinical drug development and to improve predictive power regarding in vivo drug clearance. In this study, an in vitro model of the airway comprising human primary lung epithelial cells cultured in a microfluidic platform was used to establish a physiologic state and to observe metabolic changes as a function of glucocorticoid exposure. Evaluation of mucus production rate and barrier function, along with lung-specific markers, demonstrated that the lungs maintained a differentiated phenotype. Initial concentrations of 100 nM hydrocortisone (HC) and 30 nM cortisone (C) were used to evaluate drug clearance and metabolite production. Measurements made using ultra-high-performance liquid chromatography and high-mass-accuracy mass spectrometry indicated that HC metabolism resulted in the production of C and dihydrocortisone (diHC). When the airway model was exposed to C, diHC was identified; however, no conversion to HC was observed. Multicompartmental modeling was used to characterize the lung bioreactor data, and pharmacokinetic parameters, including elimination clearance and elimination half-life, were estimated. Polymerse chain reaction data confirmed overexpression of 11-β hydroxysteroid dehydrogenase 2 (11βHSD2) over 11βHSD1, which is biologically relevant to human lung. Faster metabolism was observed relative to a static model on elevated rates of C and diHC formation. Overall, our results demonstrate that this lung airway model has been successfully developed and could interact with other human tissues in vitro to better predict in vivo drug behavior.

Introduction
Lung disease is a significant worldwide health problem in which mortality rates continue to rise despite significant efforts to address underlying causes (Kiley, 2011). Approximately 235 million people suffer from asthma, chronic obstructive pulmonary disease leads to more than three million deaths per year, and lung cancer is responsible for approximately 1.6 million deaths, being the third leading cause of death in the United States (Dela Cruz et al., 2011). With few effective treatment options for many of these conditions, there is an urgent need for improved therapeutic approaches.
Detailed studies of lung pathology in patients suffering from lung disease are limited. Historically, information regarding lung-specific responses to therapeutic compounds has been derived from the study of lung tissue at autopsy or from animal models (Craig et al., 1975; Elliott et al., 1987; Yokose et al., 2000; Pemberton et al., 2006). Consequently, many researchers are now turning to in vitro human-cell–based culture models to answer basic questions related to cellular responses in the lung.
One of the earliest laboratory models used to study lung development was focused on the formation of alveolar-like tissue structures (Yu et al., 2007). More recent efforts have focused on models of the pulmonary-alveolar-capillary barrier (Bhattacharya and Matthay, 2013; Jaworski and Redlarski, 2014). These simple culture models comprising one or two cell types were not able to recapitulate organ-level functionalities that are required for the development of meaningful disease models. An emerging approach to mimicking organ-level functionality in a culture model is the development of human “organs-on-chips” (Li et al., 2012; Bhatia and Ingber, 2014; Esch et al., 2015). These organ-mimetic microdevices enable the study of complex human physiology in an organ-specific context; more importantly, they provide the opportunity to develop specialized in vitro human disease models capable of impacting drug development.
Significant efforts have been aimed at the development of in vitro models for the human airway (Huang et al., 2013; Nesmith et al., 2014). Progress in basic mechanisms of important airway functions (Thompson et al., 2006; Pohl et al., 2009; Prytherch et al., 2011; Sellgren et al., 2014; Farsani et al., 2015) has been obtained with these models; however, many airway models rely on cell lines rather than human primary epithelial cells and are often cultured in laboratory systems that are difficult to scale or implement in an automated fashion.
We report a human airway model and its interaction with glucocorticoids in an air-liquid-interface (ALI) culture system that can be easily multiplexed. The model is demonstrated in a microfluidic platform that offers a flexible format capable of accepting a wide variety of module types and sizes. Further, the platform enables circulation of the cell culture media in a manner that permits dynamic control over mixing and sampling; this feature also permits interactions between the airway model and other compartments and organ models. This platform model adds a new dimension in functional capability over static culture systems, namely, the ability to manipulate the mixing times of drugs introduced into the module and any concentration gradients that are present for the drugs, metabolites, or other chemical species in the reactor system. In this regard, dynamic platform conditions more closely represent physiologic conditions, in which vascular and interstitial flow and drainage into the lymphatic system can strongly influence the transport of drugs and other species. Successful exploitation of this human airway model requires attention to a number of factors that affect the ability of lung cells to adapt to in vitro conditions. For example, glucocorticoids promote differentiation and prolong the life of human cells in cultures (Smith et al., 1973). Therefore, for this study, hydrocortisone (HC) and cortisone (C) were used to support relevant physiologic functions of the lung.
Here we describe a microfluidic platform on which primary human lung cells were seeded into compartments coated with collagen, forming an ALI, and flow-through channels underneath the ALI provided a fluid path for mixing and stirring of media between media changes. Cell differentiation was evaluated by immunohistochemistry, transepithelial electrical resistance (TEER), and mucus secretion values. In vitro glucocorticoid concentration versus time data were characterized using multicompartmental pharmacokinetic modeling, and real-time polymerase chain reaction (PCR) was performed to evaluate 11βHSD1 and 11βHSD2 in the presence of glucocorticoids.
Materials and Methods
Chemicals and Reagents.
Deuterated HC, used as internal standard, was obtained from Cambridge Isotope Laboratories (Tewksbury, MA). High-performance liquid chromatography (HPLC) grade (≥99.9%) methanol, chloroform, formic acid (FA), molecular biology–grade dimethyl sulfoxide, HC, and C were purchased from Sigma-Aldrich (St. Louis, MO). The standard solution used to tune and calibrate the quadruple time-of-flight (QTOF) mass spectrometer was acquired from Agilent Technologies (Santa Clara, CA). HPLC-grade acetonitrile (ACN) was from EMD Millipore Corporation (Billerica, MA). Distilled water was prepared inhouse with double distillation.
Cell Culture.
Cryopreserved normal human bronchial epithelial (NHBE) cells were purchased from Lonza (lot 307177 Lonza, Walkersville, MD). After expansion with bronchial epithelial growth medium (BEGM; Lonza), cells were seeded onto human collagen-IV (Sigma Aldrich) coated 6.5-mm transwell inserts (Costar, Corning, NY) at a density of 1 × 105 cells per insert. After 3 days, cultures were exposed to ALI. Differentiation medium was made according to published specifications (Fulcher et al., 2005) and changed every other day. Cells were maintained at an ALI for up to 3 to 4 weeks. After the differentiation period, cells were placed in dynamic flow conditions (on-platform, described below) with either HC or C.
Lung cells were plated in duplicate to evaluate the biologic variation within the wells. For the HC pharmacokinetic experiments, each well was treated with 100 nM HC, and culture media samples were collected from a corner region of the basal compartment at 0, 1, 4, 24, and 48 hours. To assess the reversibility of the conversion of HC to C mediated by the enzymes 11βHSD1 and 11βHSD2, a similar experiment was conducted in which two new human airway models were dosed with C. These experiments were done using the same procedure as that used with HC, except that the drug dose was 30 nM C. These concentrations were chosen because, although most serum cortisol is bound to proteins, with both free and total concentrations varying diurnally, free concentrations are between 25 and 86 nM (Davidson et al., 2006; Levine et al., 2007).
Microfluidic Platform.
These transwell devices were placed into a custom microfluidic platform comprising four locations, so that four replicates of the airway model could be maintained simultaneously (Fig. 1). Each location in the platform permits insertion of the transwell into a precision-machined slot and a fluid network path in which flow is circulated in a controlled manner through the lower chamber of the transwell module underlying the tracheobronchial culture model at ALI. The platform is precision-machined from polysulfone and has an integrated set of flow-control pumps that are pneumatically driven; each pump contains a solenoid valve (The Lee Co., Westbrook, CT ) that addresses a fluid displacement chamber, enabling precise one-way flow through the circuit. In the experiments described here, the dynamic flow protocol comprised 10 seconds of the flow of media (800 μl) out of the lower transwell chamber, 10 seconds of an equal volume of flow into the chamber, and 1060 seconds of a no-flow condition. This resulted in an 18-minute cycle time, such that every 18 minutes, approximately one-third of the media underlying the tracheobronchial epithelial layer was cycled in and out of the chamber. This process enabled fluid mixing of media components and glucocorticoids in a manner not possible in conventional static lung cell culture, in which the media is changed every 2 days, but between media changes, there is no convective fluid motion within the chamber and no opportunity to control or manipulate chemical gradients that are established in the culture system.
Fig. 1.
Human airway epithelium model. This device is a precision-machined from polysulfone and has an integrated set of flow-control pumps that are pneumatically driven; each pump contains a solenoid valve (The Lee Co.) that addresses a fluid displacement chamber, enabling precise one-way flow through the circuit.
Immunohistochemistry.
Differentiation of NHBE cells was validated by immunofluorescent staining. First, NHBE cells were fixed with 4% paraformaldehyde. Before antibody incubation, cells were washed, permeabilized, and blocked with 2% donkey serum (Jackson ImmunoResearch, West Grove, PA) for 1 hour. Primary antibodies were incubated overnight at 4°C for identification of goblet cells (mouse anti-muc5AC) and ciliated cells (mouse antiacetylated tubulin). Cells were then incubated for 1 hour with fluorescent secondaries from Molecular Probes (Eugene, OR): Alexa Fluor 488 anti-mouse IgG, Alexa Fluor 546 anti-rabbit IgG or a phalloidin F-actin probe. Cell nuclei were stained with Hoescht (Molecular Probes). Samples were mounted on glass cover slides using Fluoro-gel (EMS, Hatfield, PA). Fluorescent images were captured with an Orca-Flash 4.0 LT camera (Hamamatsu, Bridgewater, NJ) attached to an inverted AxioObserver Z.1 microscope (Carl Zeiss, Oberkochen, Germany) using an EC Plan-Neofluar 40x/1.3 Oil objective (Carl Zeiss) and 10× eyepiece.
Mucus and TEER Evaluation.
Lungs were evaluated for TEER and mucus secretion after exposure to dynamic conditions. Mucus was harvested apically and quantified using an Alcian Blue (Richard Allan Scientific, Kalamazoo, MI) colorimetric assay. Briefly, Alcian Blue was added to samples and equilibrated for 2 hours. Next, samples were centrifuged, washed in a resuspension buffer, followed by dissociation with 10% SDS (Sigma-Aldrich) solution. Absorbance was read with a microplate reader (Molecular Devices) at 620 nm. Mucin standard was prepared from bovine submaxillary glands (Sigma-Aldrich). For detailed methods on collection and quantification of mucus, see Lever et al. (2015).
We measured TEER using a 24-well EndOhm chamber and an ohmmeter (WPI, Sarasota, FL). Transwell inserts were placed into the EndOhm chamber with phosphate-buffered saline buffer in both the apical and basal compartments.
Liquid Chromatography-Mass Spectroscopy–Based Metabolite Profiling.
Metabolite profiling was performed as previously described (Sarkar et al., 2015). Briefly, media samples were spiked with deuterium-labeled HC (d4-HC) to a final concentration of 50 nM, extracted with cold methanol and chloroform at a 1:4 ratio, and kept in ice for 2 minutes. The samples were vortexed for 20 seconds, kept at −20°C for 30 minutes, vortexed, and centrifuged. The organic layer was collected, dried, and resuspended in 2% ACN containing 0.1% formic acid.
Liquid chromatography-mass spectrometry (LC-MS) analysis using an Agilent 6530 Accurate-Mass LC-QTOF mass spectrometer with an Agilent jet stream electrospray ionization source coupled to an Agilent 1290 ultra-high-performance liquid chromatography (UHPLC) system (Agilent Technologies) was performed on the resuspended samples. Parameters and conditions can be found in detail in Sarkar et al. (2015). In summary, the column was an Agilent Extend-C18 (2.1 × 50 mm, 1.8 μm; Agilent Technologies); the column temperature was 40°C; mass spectra were collected in positive ion mode-between m/z 70 and 1000 at two scans/s; ion spray voltage was 3800 V; heated capillary temperature was 350°C; drying gas was 8 liters/min; nebulizer was 30 psi; sheath gas temp was 380°C; sheath gas flow was 12 liters/min. Mass calibration was maintained by infusing two reference masses (m/z 121.0509: C5H4N4; m/z 922.0098: C18H18O6N3P3F24). Distilled water containing 0.1% FA (A) and ACN containing 0.1% FA (B) were used in a linear gradient from 2% to 95% B over 12 minutes at 0.4 ml/min.
The mass spectral data were processed using Agilent Mass Hunter qualitative analysis software (version B.06, Agilent Technologies). Extracted ion chromatograms were used to obtain the peak areas for the internal standard and the different glucocorticoids. The Agilent molecular feature extractor and manual interpretation were used to characterize the different metabolites. Microsoft Excel 2011 (Redmond, WA) and GraphPad PRISM software (GraphPad Software, San Diego, CA) were used to further process and plot the data. Metabolite identification was based on targeted tandem mass spectrometry (MS/MS) analysis and metabolic pathway analysis with the KEGG database (http://www.genome.jp/kegg/; http://metlin.scripps.edu/index.php).
Each of the two biologic well replicates was extracted twice for technical variation assessment, and each sample was run twice in positive ion mode in the RP-UHPLC-QTOF-MS for instrumental variation evaluation (Supplemental Fig. 1). The analytical validation of this LC-MS method was previously described (Sarkar et al., 2015), and parameters have been summarized in Supplemental Table 1.
In Vitro Characterization of Parent Glucocorticoid Disposition: Pharmacokinetic Modeling.
HC-to-C and C-to-dihydrocortisone (diHC) lung bioreactor data were modeled separately with two-compartment pharmacokinetic models for the parent drugs HC and C, respectively, and a single compartment for the metabolites C and diHC, respectively, using the SAAM II software system (SAAM Institute, Seattle, WA) (Barrett et al., 1998; Meissner et al., 2013). To model these data, the parent drug dose was a molar amount, and the plasma parent drug and metabolite concentrations were molar concentrations. The pharmacokinetic parameters describing parent drug disposition were determined first. After finalizing the fit of the pharmacokinetic model to parent drug data, the parameters of the model were fixed, and a model was fit to the metabolite data, with the formation of metabolite modeled as a fraction of the elimination clearance of the parent drug in those models (i.e., parent drug elimination clearance was set equal to the sum of clearance by metabolism to the measured metabolite plus the clearance by any other mechanisms). After finalizing the fit of the pharmacokinetic model to metabolite data, parameters were released and the fit of the combined parent drug to metabolite model was finalized. The SAAM II objective function used was the extended least-squares maximum likelihood function using data weighted with the inverse of the model-based variance of the data at the observation times. Model misspecification was sought by visual inspection of the measured and predicted drug concentrations versus time relationships. Model fits to the data were assessed on the basis of the coefficients of variation associated with the adjustable parameters for each model (Metzler, 1986).
Gene Expression.
Total RNA was isolated with miRNeasy mini kit (Qiagen, Valencia, CA). RNA was quantified with a microplate reader (BioTek, Epoch, Take 3 adapter, Winooski, VT) at 260 nm. Reverse transcription was performed with the high capacity reverse transcription kit (Life Technologies, Carlsbad, CA). For quantitative PCR, cDNA was diluted with TaqMan gene expression Universal Master Mix and the following inventoried probes (Life Technologies, Carlsbad, CA): 11βHSD1 (Hs01547870_m1), 11βHSD2 (Hs00388669_m1), and GAPDH served as endogenous control.
Results
Presence of Lung-Specific Cell Subtypes.
NHBE cells form a pseudostratified epithelium with a mucociliary phenotype when differentiated at an ALI (Mathis et al., 2013). Immunohistochemistry was used to validate the presence of essential cell types, specifically, ciliated cells and goblet cells, after exposure to dynamic culture conditions. An antibody specific to acetylated tubulin was used for ciliated cell determination. Figure 2A depicts a population of ciliated cells (green) located on the apical surface of cultures exposed to flow, and the expression was maintained. F-actin staining (red), also shown in Fig. 2A, reveals a tight, cobblestone morphology typical of well-differentiated NHBE cells in vitro. As shown in Fig. 2B, positive staining for Muc5AC (green) confirmed the presence of goblet or mucus-producing cells.
Fig. 2.
Expression of lung-specific cell types for cultures exposed to HC under dynamic conditions. Dynamic lungs were stained and imaged to validate the presence of essential cell types: (A) ciliated cells (green) and F-actin (red); (B) goblet cells (green) and nuclei (blue). Images are representative of total field of view for lungs (n = 5).
TEER and Mucus Production.
For well-differentiated cultures, baseline TEER values were determined as a measure of barrier function. TEER increased as the cells differentiated, reaching a value >1000 Ω *cm2 more than 20 days of differentiation. Lungs were placed under circulating media conditions before TEER evaluation. No change in TEER was observed for HC- or C-treated cultures under flow conditions (1268 ± 140 Ω*cm2 for HC, 1131 ± 401 Ω*cm2 for C; Table 1).
TABLE 1.
TEER and mucus production rate for lungs cultured under dynamic conditions in the presence of glucocorticoids
Barrier function and mucus secretion were maintained for lungs across all culture conditions. Data are presented as the average ± S.E.M. for TEER and mucus.
| HC | C | |
|---|---|---|
| TEER (Ω * cm2) | 1268 ± 140 | 1131 ± 401 |
| Mucus production (μg/day) | 8 ± 1 | 7 ± 1 |
Mucus secretion was also evaluated as a baseline function. Mucus production remained stable for all cultures maintained in dynamic conditions with either HC or C (8 ± 1 μg/day and 7 ± 1 μg/day, respectively; Table 1). These results indicate that baseline functions, such as epithelial resistance and mucus secretion, were maintained.
Sample Extraction.
Figure 3 shows the workflow for the quantification of glucocorticoids and metabolite profiling. The aqueous layer was kept during the extraction process and analyzed for residual glucocorticoids. Supplemental Fig. 2 shows a representative chromatogram corresponding to the aqueous layer extracted from media sample after dosing the airway with 100 nM HC. An extracted ion chromatogram for the HC [M + H]+ ion at m/z 363.2171 confirmed that there was no loss of HC during the extraction method.
Fig. 3.
Experimental workflow used for the quantification of HC and metabolite profiling. The method consists of spiking culture media samples with internal standard after drug administration, liquid extraction, and MS analysis.
Glucocorticoid Disposition in a Human Airway Epithelium Lung Model.
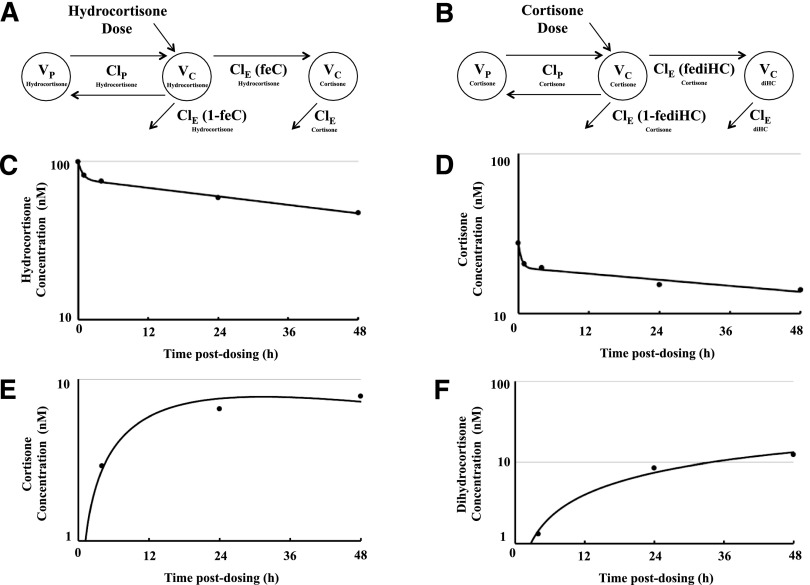
The distribution and elimination of the parent drugs HC and C were well characterized by two compartmental pharmacokinetic models (Fig. 4, A and B). After 48 hours, the initial 100 nM fluid HC concentration had decreased to 47 nM under flow conditions (Fig. 4C). To determine the reversibility of the conversion of HC to C mediated by the enzymes 11βHSD1 and 11βHSD2, a similar experiment was conducted in which two new human airway models were dosed with C. After 48 hours, the initial 30 nM C fluid concentration had decreased to 14 nM under flow conditions (Fig. 4D).
Fig. 4.
Pharmacokinetic modeling. A two-compartment pharmacokinetic model was used to characterize the distribution and elimination of the parent drugs hydrocortisone and cortisone, and single compartment was used to characterize the disposition of the metabolites cortisone and dihydrocortisone: (A) hydrocortisone dose and (B) cortisone dose. The distribution and elimination of the parent drugs, hydrocortisone and cortisone, were well characterized by the models (C and D, respectively), in which the average measured concentrations are represented by circles, and the fit of the model to the data by the solid lines) as were the dispositions of the metabolites cortisone and dihydrocortisone (E and F, respectively).
In drug pharmacokinetic studies, it is necessary to distinguish between decreases in drug concentrations owing to cellular distribution and metabolism and that owing to adsorption to the materials of the apparatus. To evaluate this, two other wells without cells were used as negative controls for nonspecific binding experiments using the same drug doses and two of the same collection times (i.e., 1 and 48 hours). The same sample preparation and analysis described for HC and C were used. Supplemental Fig. 3, A and B, shows the concentration of HC and C respectively over a period of 48 hours during which the cell-free human lung airway models were exposed to the glucocorticoids. There was no observed drop in fluid HC or C concentration over the 48-hour period, which is consistent with a lack of significant adsorption to the culture system or platform fluidic circuit. Therefore, any decrease in the concentration of HC or C observed in the pharmacokinetic studies is due to cellular distribution and metabolism.
Metabolite Formation.
Hydrocortisone is metabolized by a series of oxidation and/or reductions by either bioreductive or oxidoreductive enzymes such as 5β/α-reductases, 20-β/α oxidoreductases, 11βHSD1, and 11βHSD2. High mass accuracy, retention time, database search engines, and manual interpretation of MS/MS spectra were used to elucidate the putative structures of the metabolites generated in the human airway model.
After dosing with 100 nM HC, we observed that HC was metabolized and resulted in the formation of two metabolites, which were identified as C (m/z 361.2015) (Fig. 4E) and diHC (m/z 363.2171). A representative MS/MS spectrum of the metabolite identified as diHC is shown in Supplemental Fig. 4, together with the MS/MS spectrum corresponding to C. The MS/MS spectrum of diHC revealed an intense peak m/z 163.1119, which is presumably a signature for metabolites derived from C. When the airway was dosed with 30 nM C, a single metabolite was identified: diHC (Fig. 4F). Figure 5, A and B, shows plots of concentration (nM) versus time postdosing (h) in which the formation of each metabolite is monitored for HC and C, respectively.
Fig. 5.
Product formation over time after administration of glucocorticoids. (A) 100 nM hydrocortisone; (B) 30 nM cortisone. Taking in consideration high accuracy mass, retention time, database search engines, and the characteristic ions found in the MS/MS pattern, the metabolites were identified as cortisone and dihydrocortisone. n = 2 for biologic and technical replicates. Values are represented as mean ± S.D.
To assess the contribution of fluid mixing of media components and glucocorticoids into an ALI trachobronchial model, the rate of metabolite formation in the dynamic platform was compared with a platform under static conditions. In the latter case, media were changed every 2 days, but between media changes there was no convective fluid motion within the chamber and no opportunity to control or manipulate chemical gradients that were established in the culture system. Under dynamic circulating conditions, product formation was at least four times faster than for static controls (Fig. 6)
Fig. 6.
Comparison of metabolite formation rate between dynamic and static platforms. (A) hydrocortisone dose; (B) cortisone dose. Under dynamic circulating conditions, product formation occurs more quickly than for static controls. n = 2 for biologic and technical replicates. Values are represented as mean ± S.D.
Compartmental Pharmacokinetic Modeling.
Although their initial distribution volume was approximately equal to the fluid volume of the microfluidic platform, the total volume of distribution was slightly larger because of distribution into the cultured cells (Tables 2 and 3). The elimination clearance of the parent drugs HC and C were completely accounted for by metabolism to the metabolites C and diHC, respectively. The elimination half-lives of the parent drugs HC and C were 67 hours and 95 hours, respectively.
TABLE 2.
Pharmacokinetic parameters for the conversion of hydrocortisone to cortisone
| Central Volume of Distribution | Peripheral Volume of Distribution | Total Volume of Distribution | Intercom-partmental Clearance | Elimination Clearance | Fraction of Dose Eliminated | Elimination Half-Life | |
|---|---|---|---|---|---|---|---|
| Hydrocortisone | ml 3.03 | ml 0.86 | ml 3.89 | ml/h 0.93 | ml/h 0.04 | 1 | h 67.3 |
| Cortisone | N/A | N/A | 4.0 | N/A | 0.29 | N/A | N/A |
N/A, not applicable.
TABLE 3.
Pharmacokinetic parameters for the conversion of cortisone to dihydrocortisone
| Central Volume of Distribution | Peripheral Volume of Distribution | Total Volume of Distribution | Intercom-partmental Clearance | Elimination Clearance | Fraction of Dose Eliminated | Elimination Half-Life | |
|---|---|---|---|---|---|---|---|
| Cortisone | ml 3.01 | 1.26 | ml 4.27 | ml/h 1.80 | ml/h 0.03 | 1 | h 95.3 |
| Dihydrocortisone | N/A | N/A | 1.74 | N/A | 0 | N/A | N/A |
N/A, not applicable.
Gene Expression of 11βHSD.
Expression of 11βHSD1 and 11βHSD2 was evaluated after treatment with glucocorticoids under dynamic and static conditions (Fig. 7). 11βHSD1 mRNA expression for cultures treated with HC under dynamic flow was essentially equal to that obtained under static conditions. On the other hand, results for 11βHSD2 under dynamic conditions were approximately twice that under static conditions. For C, 11βHSD1 mRNA expression for cultures under dynamic conditions was nearly identical to expression under static conditions. For 11βHSD2 expression, dynamic conditions led to expression that was approximately 1.3 times higher than that obtained under static conditions.
Fig. 7.
11βHSD gene expression data. Expression of 11βHSD1 and 11βHSD2 was evaluated after treatment with glucocorticoids under dynamic and static conditions: (A) hydrocortisone dose; (B) cortisone dose. n = 2 for biologic and technical replicates. Values are represented as mean ± S.D.
Discussion
NHBE cells differentiated at an ALI recapitulate human airway physiology and function, such as epithelial integrity, mucus secretion, and representative cell types such as goblet and ciliated cells (Lever et al., 2015). In the model described here, metrics to evaluate these functions were used to confirm that the lung cultures maintained in vivo–like physiology and baseline functions after exposure to dynamic flow conditions and glucocorticoid treatment. We chose to evaluate these components, as they are essential for normal lung function and critical for defending against inhaled pathogens and particulates.
Evaluation of barrier function, as measured by TEER, indicated that the epithelial integrity of our lung model was not adversely affected after exposure to dynamic flow and different glucocorticoids. Maintenance of this function is essential, as the epithelium provides a protective barrier that maintains airway homeostasis (Vareille et al., 2011). The baseline mucus production rate also remained stable for all culture conditions, indicating that cells were functioning and responding normally (Table 1). Along with TEER and mucus, we also stained for lung-specific markers, specifically ciliated cells and goblet cells. In vivo, these cell subtypes are required for mucociliary transport, a process in which inhaled pathogens are trapped and cleared from the airway (Vareille et al., 2011). In our study, ciliated cell expression was abundant (Fig. 2A), whereas expression of Muc5AC was observed for all lung cultures (Fig. 2B). Together, these results demonstrate that the cultures maintained a differentiated phenotype after exposure to dynamic flow conditions and regardless of glucocorticoid treatment.
It is common to have glucocorticoids in the media used to seed the cells as they promote differentiation and prolong the life of human cells in culture (Hauner et al., 1989). In the Randall medium cocultures, glucocorticoids are maintained in human physiologic concentrations. Here we described a system that demonstrated not only that the NHBE cells can be maintained in glucocorticoids, but also that further metabolites are formed after glucocorticoid metabolism.
To exemplify the metabolic capability of the airway model for complex drug metabolism studies, a detailed characterization of glucocorticoid clearance and metabolite profiling has been completed using concentrations ranging from 100 nM down to normal physiologic concentrations (i.e., 30 nM) to observe changes in the lung epithelial cultures. We found after administration of 100 nM HC that C was a metabolite leading to the major metabolite (diHC) as a result of further C metabolism. When dosing the cultures with 30 nM C, only diHC was identified, and no HC was found, suggesting that there is conversion from HC to C but not vice versa, presumably owing to the dominance of 11βHSD2. To confirm these results, real-time PCR was performed to compare the gene expression levels of both 11βHSD enzymes (Fig. 7), and, in fact, 11βHSD2 is more highly expressed.
The observation that metabolism was faster under dynamic conditions than for static controls is significant for drug discovery. Drug metabolism can result in the formation of highly reactive metabolites that are known to play a role in toxicity, resulting in attrition during drug development and clinical use. Thus, the earlier such reactivity is detected, the better.
Some limitations to the pharmacokinetic modeling merit mention. Although the pharmacokinetic models of the parent drugs HC and C (Fig. 4, A and B) were designed to allow characterization of their elimination clearance not only to the metabolites C and diHC, respectively, but also to other possible metabolites, other metabolites were not identified in the coculture media, and the clearances of HC and C were completely accounted for by metabolism to C and diHC, respectively. The validity of this modeling approach is supported by the observation that the total volume of distribution of C as a parent drug, 4.27 ml (Table 3), is similar to that of C as metabolite, 4.0 ml (Table 2). In addition, sampling was not conducted long enough to allow full and accurate characterization of the pharmacokinetics of the metabolites C and diHC, which is why the elimination clearance of C is 0.03 ml/h as a parent drug but 0.29 ml/h as a metabolite. Finally, although diHC concentrations were measured after dosing the parent drug, HC, it was not possible to model its formation from the metabolite C because the relatively few sampling times did not provide enough information to support such model complexity.
In summary, we have presented the results of an investigation of glucocorticoid fate in an in vitro system comprising primary human airway epithelial cells cultured in a microfluidic platform with circulating flow. The in vitro airway model exhibits physiologically relevant behavior including barrier function, mucus formation, and ciliated subpopulations. Clearance, metabolism, and pharmacokinetics of HC and C have been evaluated in this platform. Product formation is shown to be at least four times faster in the microfluidic-circulating platform than in static controls, an important finding given the potential role of this platform in evaluating drug toxicity. The microfluidic-circulating platform represents a powerful tool for investigating drug fate in preclinical studies, and the dynamic behavior of this system may enable the construction of a multiorgan human platform to correlate more accurately in vitro data to in vivo clearance.
Supplementary Material
Acknowledgments
The authors thank the Defense Advance Research Project Agency Microphysiological (DARPA MPS) Barrier-Immune-Organ: Microphysiology, Microenvironment Engineered Tissue Construct Systems (BIO-MIMETICS) team for general technical advice and Dr. Rachelle Prantil-Baun for technical guidance on the lung airway experimental design.
Abbreviations
- 11βHSD
11-β-hydroxysteroid dehydrogenase
- ACN
acetonitrile
- ALI
air-liquid-interface
- C
cortisone
- diHC
dihydrocortisone
- FA
formic acid
- HPLC
high-performance liquid chromatography
- HC
hydrocortisone
- LC-MS
liquid chromatography-mass spectrometry
- NHBE
normal human bronchial epithelial
- PCR
polymerase chain reaction
- QTOF
quadrupole time of flight
- RP
reversed phase
- MS/MS
tandem mass spectrometry
- TEER
transepithelial electrical resistance
- UHPLC
ultra-high-performance liquid chromatography
Authorship Contributions
Participated in research design: Rivera-Burgos, Sarkar, Lever, Coppeta, Wishnok, Borenstein, Tannenbaum.
Conducted experiments: Rivera-Burgos, Sarkar, Lever.
Performed data analysis: Rivera-Burgos, Sarkar, Lever, Avram.
Wrote or contributed to the writing of the manuscript: Rivera-Burgos, Sarkar, Lever, Wishnok, Avram, Borenstein.
Footnotes
This research was supported by the United States Defense Advanced Research Projects Agency [Grant W911NF-12-2-0039], the National Institutes of Health [Grant 5-UH2-TR000496], and the Massachusetts Institute of Technology Center for Environmental Health Sciences [Grant P30-ES002109].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Barrett PHR, Bell BM, Cobelli C, Golde H, Schumitzky A, Vicini P, Foster DM. (1998) SAAM II: Simulation, Analysis, and Modeling Software for tracer and pharmacokinetic studies. Metabolism 47:484–492. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Ingber DE. (2014) Microfluidic organs-on-chips. Nat Biotechnol 32:760–772. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Matthay MA. (2013) Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75:593–615. [DOI] [PubMed] [Google Scholar]
- Craig JR, Dunn AE, Peters RL. (1975) Cirrhosis associated with partial deficiency of alpha-1-antitrypsin: a clinical and autopsy study. Hum Pathol 6:113–120. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Bolland MJ, Croxson MS, Chiu W, Lewis JG. (2006) A case of low cortisol-binding globulin: use of plasma free cortisol in interpretation of hypothalamic-pituitary-adrenal axis tests. Ann Clin Biochem 43:237–239. [DOI] [PubMed] [Google Scholar]
- Dela Cruz CS, Tanoue LT, Matthay RA. (2011) Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 32:605–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JA, Osterlind K, Hirsch FR, Hansen HH. (1987) Metastatic patterns in small-cell lung cancer: correlation of autopsy findings with clinical parameters in 537 patients. J Clin Oncol 5:246–254. [DOI] [PubMed] [Google Scholar]
- Esch EW, Bahinski A, Huh D. (2015) Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsani SM, Deijs M, Dijkman R, Molenkamp R, Jeeninga RE, Ieven M, Goossens H, van der Hoek L. (2015) Culturing of respiratory viruses in well-differentiated pseudostratified human airway epithelium as a tool to detect unknown viruses. Influenza Other Respi Viruses 9:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. (2005) Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107:183–206. [DOI] [PubMed] [Google Scholar]
- Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. (1989) Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 84:1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Wiszniewski L, Constant S, Roggen E. (2013) Potential of in vitro reconstituted 3D human airway epithelia (MucilAir™) to assess respiratory sensitizers. Toxicol In Vitro 27:1151–1156. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Redlarski G. (2014) A compartment model of alveolar-capillary oxygen diffusion with ventilation-perfusion gradient and dynamics of air transport through the respiratory tract. Comput Biol Med 51:159–170. [DOI] [PubMed] [Google Scholar]
- Kiley JP. (2011) Advancing respiratory research. Chest 140:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever AR, Park H, Mulhern TJ, Jackson GR, Comolli JC, Borenstein JT, Hayden PJ, Prantil-Baun R. (2015) Comprehensive evaluation of poly(I:C) induced inflammatory response in an airway epithelial model. Physiol Rep 3:e12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. (2007) Measuring cortisol in human psychobiological studies. Physiol Behav 90:43–53. [DOI] [PubMed] [Google Scholar]
- Li XJ, Valadez AV, Zuo P, Nie Z. (2012) Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis 4:1509–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C, Poussin C, Weisensee D, Gebel S, Hengstermann A, Sewer A, Belcastro V, Xiang Y, Ansari S, Wagner S, et al. (2013) Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol 304:L489–L503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Avram MJ, Yermolenka V, Francis AM, Blood J, Kharasch ED. (2013) Cyclosporine-inhibitable blood-brain barrier drug transport influences clinical morphine pharmacodynamics. Anesthesiology 119:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler CM (1981) Estimation of pharmacokinetic parameters: Statistical considerations. Pharmacol Ther 13:543–556. [DOI] [PubMed] [Google Scholar]
- Nesmith AP, Agarwal A, McCain ML, Parker KK. (2014) Human airway musculature on a chip: an in vitro model of allergic asthmatic bronchoconstriction and bronchodilation. Lab Chip 14:3925–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton PA, Kobayashi D, Wilk BJ, Henstrand JM, Shapiro SD, Barr PJ. (2006) Inhaled recombinant alpha 1-antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse. COPD 3:101–108. [DOI] [PubMed] [Google Scholar]
- Pohl C, Hermanns MI, Uboldi C, Bock M, Fuchs S, Dei-Anang J, Mayer E, Kehe K, Kummer W, Kirkpatrick CJ. (2009) Barrier functions and paracellular integrity in human cell culture models of the proximal respiratory unit. Eur J Pharm Biopharm 72:339–349. [DOI] [PubMed] [Google Scholar]
- Prytherch Z, Job C, Marshall H, Oreffo V, Foster M, BéruBé K. (2011) Tissue-specific stem cell differentiation in an in vitro airway model. Macromol Biosci 11:1467–1477. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Rivera-Burgos D, Large EM, Hughes DJ, Ravindra KC, Dyer RL, Ebrahimkhani MR, Wishnok JS, Griffith LG, Tannenbaum SR. (2015) Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab Dispos 43:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren KL, Butala EJ, Gilmour BP, Randell SH, Grego S. (2014) A biomimetic multicellular model of the airways using primary human cells. Lab Chip 14:3349–3358. [DOI] [PubMed] [Google Scholar]
- Smith BT, Torday JS, Giroud CJ. (1973) The growth promoting effect of cortisol on human fetal lung cells. Steroids 22:515–524. [DOI] [PubMed] [Google Scholar]
- Thompson CI, Barclay WS, Zambon MC, Pickles RJ. (2006) Infection of human airway epithelium by human and avian strains of influenza a virus. J Virol 80:8060–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareille M, Kieninger E, Edwards MR, Regamey N. (2011) The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev 24:210–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokose T, Ito Y, Ochiai A. (2000) High prevalence of atypical adenomatous hyperplasia of the lung in autopsy specimens from elderly patients with malignant neoplasms. Lung Cancer 29:125–130. [DOI] [PubMed] [Google Scholar]
- Yu W, Fang X, Ewald A, Wong K, Hunt CA, Werb Z, Matthay MA, Mostov K. (2007) Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis. Mol Biol Cell 18:1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.