Abstract
The present study aimed to screen potential genes associated with pituitary adenomas to obtain further understanding with regard to the pathogenesis of pituitary adenomas. The microarray GSE23207 dataset, containing 16 pituitary adenoma samples from multiple endocrine neoplasia syndrome-associated rats and 5 normal pituitary tissue samples, was downloaded from Gene Expression Omnibus. The Linear Models for Microarray Data package was used to identify the differentially-expressed genes (DEGs) with the cut-off criteria of a |log2fold change (FC)|>1 and adjusted P-values of <0.05. The potential functions of the DEGs were predicted by functional and pathway enrichment analysis with the Database for Annotation, Visualization and Integrated Discovery. Furthermore, the interaction associations of the up- and downregulated DEGs obtained from the Search Tool for the Retrieval of Interacting Genes database were respectively revealed by the protein-protein interaction networks visualized with Cytoscape. A total of 391 upregulated and 238 downregulated DEGs in were screened in the pituitary adenoma samples. The upregulated DEGs with a higher degree in the protein-protein interaction network (e.g., CCNA2, CCNB1 and CDC20) were significantly involved in cell cycle and cell division. Notably, PTTG1 was enriched in every functional term. These DEGs interacted with each other. The downregulated DEGs (e.g., GABRA1, GABRA4 and GABRB1) also interacted with each other, and were relevant to neuroactive ligand-receptor interaction; the DEG POU1F1, interacting with POMC, was correlated with the development of the pituitary gland, adenohypophysis and endocrine system. Certain DEGs, including CCNB1, CCNA2, CDC20, GABRA1, GABRA4, GABRB1, POU1F1 and POMC, and particularly PTTG1, were shown to be closely involved in the pathogenesis of pituitary adenomas.
Keywords: pituitary adenomas, differentially-expressed genes, pathway, protein-protein interaction
Introduction
Pituitary adenomas, accounting for ~15% of all diagnosed intracranial tumors, are benign monoclonal adenomas that originate from cells of the anterior pituitary gland (1). Surgical resection, with or without adjuvant radiotherapy, is always the first line of treatment for the majority of pituitary adenomas, with the exception of prolactinomas (2). However, these treatments cannot usually control invasive pituitary adenomas due to the limited understanding of the underlying molecular mechanisms. Thereby, further research into the tumorigenesis will contribute to identifying novel therapeutic targets, which will be conductive to the development of novel therapeutic approaches for pituitary adenomas.
In past years, considerable progress has been made in identifying the key players in pituitary adenomas. A previous study has shown that the phosphoinositide 3-kinase/AKT signaling pathway is activated and enhanced in pituitary adenomas, which may be due to the mutation and amplification of an oncogene, PIK3CA (3). Mutation in another oncogene, GNAS, which encodes the guanine nucleotide-activating α subunit has also been suggested to be involved in pituitary hyperplasia (4). Meanwhile, a tumor suppressor aryl hydrocarbon receptor-interacting protein has been demonstrated to function in modulating cellular signaling and cAMP signaling pathways via regulation of the localization of the aryl hydrocarbon receptor (5). Also, the absence of expression of another two tumor suppressors, growth arrest and DNA-damage-inducible β (GADD45β) and γ (GADD45γ), has been observed in human pituitary adenomas (6,7). Aberrant methylation of a number of genes, such as DAPK (8) and FGFR2 (9) has been confirmed to have a momentous role in pituitary tumorigenesis. Additionally, certain cell cycle regulators, such as p16, p21, p27, cyclin D1 and cyclin E, have also been demonstrated to function in pituitary tumorigenesis (8,10). Recently, certain microRNAs (miRNA/miR) have been found to be crucial in pituitary adenomas. For instance, the expression levels of miR-431 and miR-770-5p have been found to be slightly higher in non-functioning pituitary adenomas compared to their levels in the normal pituitary gland (11). Recently, another study has shown that miRNA-dependent impairment of the HMGA/E2F1 pathway functions as pro-oncogene signaling in pituitary adenomas. Several miRNAs targeting HMGA2 (miR-326, miR-570 and miR-432) or E2F1 (miR-326 and miR-603) could inhibit the growth of pituitary cell lines (HP75 and GH3) (12).
Lee et al demonstrated that gonadotroph adenomas in MENX-affected rats closely resemble their human counterparts (13). The study further found that CYP11A1 and NUSAP1, two commonly dysregulated differentially-expressed genes (DEGs) in the gonadotroph adenomas of rats and humans, are upregulated in 77 and 95% of human gonadotroph adenomas, respectively. Using the microarray data deposited by Lee et al, the present study aimed to further identify genes that were differentially expressed between pituitary adenomas samples and normal controls. Following Gene Ontology (GO) functional and pathway enrichment analysis of the screened DEGs, Protein-Protein Interaction (PPI) networks were constructed for the up- and downregulated DEGs, respectively, in order to learn more about the interaction of proteins encoded by DEGs, which may aid in our understanding of the molecular mechanisms of pituitary adenomas. The results are expected to assist in elucidating the etiology of pituitary adenomas, and provide novel insights for the clinical diagnosis of this disease.
Materials and methods
Affymetrix microarray data
The gene expression profile data of GSE23207 (13) were acquired from Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/), which was based on the platform of the GPL6247 [RaGene-1_0-st] Affymetrix Rat Gene 1.0 ST Array. This dataset contains 16 samples of pituitary homozygous mutants (p27Kip1/Cdknb1) from MENX-associated rats, aged 7–8 months, with large tumors 1–2 mm in size, and 5 samples of normal pituitary tissues purchased from BioChain Inc. (Hayward, CA, USA).
Data preprocessing and screening of DEGs
CEL files and probe annotation files were downloaded, and the gene expression data of all the samples were preprocessed via the Robust Multichip Averaging background correction (14), quantile normalization and probe summarization methods using the Oligo package (15). The Linear Models for Microarray Data package (16) of R was used for the identification of genes that were significantly differentially expressed in pituitary adenomas samples. The raw P-value was adjusted by the Benjamin and Hochberg method (17), and only the genes meeting the cut-off criteria of a |log2fold change (FC)| of >1 and an adjusted P-value of <0.05 were selected as DEGs.
GO and pathway enrichment analysis
The Database for Annotation, Visualization and Integrated Discovery (DAVID) gene functional classification tool now provides a comprehensive set of novel and powerful tools for researchers to understand the biological meaning behind abundant genes (18). Pathway enrichment analysis was conducted to identify the significant metabolic pathways for the DEGs (19). P<0.05 and a count number of >2 were used as the cut-off criteria for GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses by DAVID.
PPI network construction
The Search Tool for the Retrieval of Interacting Genes database was used to analyze the PPIs for DEGs by calculating the combined scores (20), and a score >0.4 was chosen as the cut-off. Next, PPI networks for up- and downregulated DEGs were visualized using Cytoscape (http://cytoscape.org/) (21). The highly connected nodes (hub proteins) were detected by calculating the degree of each node protein based on the scale-free property of interaction networks (22).
Results
Identification of DEGs
Based on the cut-off criteria, a total of 629 DEGs were screened from the pituitary adenomas samples, including 391 upregulated and 238 downregulated DEGs.
Enrichment analysis of up- and downregulated DEGs
According to GO functional annotation, the upregulated DEGs were mainly enriched in GO terms associated with the cell cycle and cell division. For example, DEGs such as CCNA2, NUSAP1, CCNB1, CENPF, CDC20 and SPC25 were significantly involved in the cell cycle (P=1.08×10−12); NUSAP1, CENPF, SPC25, CDC20 and CCNB1 were involved in the M phase (P=3.09×10−11); and DEGs such as CCNB1, SPC25, TOP2A, CDC20 and CCNA2 were correlated with cell division (P=1.25×10−7). Notably, PTTG1 was found to be enriched in every GO term (Table I). The downregulated DEGs, such as KCND3, GABRA1, GABRA4 and GABRB1, were markedly associated with ion transport (P=6.05×10−7); DEGs such as KCNJ5, KCND3, KCNJ6 and KCNT2 were relevant to metal ion transport (P=3.87×10−6) and potassium ion transport (P=7.01×10−5); DEGs such as DRD2, POU1F1 and GHRHR were distinctly associated with the positive regulation of multicellular organism growth (P=2.47×10−4), pituitary gland development (P=5.70×10−4), adenohypophysis development (P=8.52×10−4), diencephalon development (P=1.24×10−3) and endocrine system development (P=6.09×10−3); and DEGs such as NOTCH2, ERBB4 and POU1F1 were involved in cell fate commitment (P=3.22×10−2) and the regulation of cell proliferation (P=4.55×10−2) (Table II).
Table I.
Top two enriched GO biological process term clusters with the highest enrichment score for the upregulated differentially-expressed genes.
| Enrichment score | Term | Description | Count | P-value | Genes |
|---|---|---|---|---|---|
| 7.432 | GO:0007049 | Cell cycle | 31 | 1.08×10−12 | SPC25, CCNA2, CENPF, NUSAP1, CDC20, NDC80, CCNB1, PTTG1, CCNB2, BUB1B… |
| GO:0022403 | Cell cycle phase | 23 | 1.10×10−12 | NUSAP1, CENPF, NDC80, CDC20, CCNB1, PTTG1, SPC25, CDKN1A, PLK1, BUB1B… | |
| GO:0022402 | Cell cycle process | 27 | 3.26×10−12 | PTTG1, SPC25, CDKN2C, CENPF, NUSAP1, CDC20, NDC80, CCNB1, CDKN1C, BUB1B… | |
| GO:0000279 | M phase | 19 | 3.09×10−11 | NUSAP1, CENPF, NDC80, CDC20, PTTG1, CCNB1, SPC25, PLK1, BUB1B, SKA3… | |
| GO:0051301 | Cell division | 14 | 1.25×10−7 | NUSAP1, CDC20, PTTG1, CCNB1, SPC25, CCNB2, PLK1, BUB1B, TOP2A, CCNA2… | |
| GO:0000087 | M phase of mitotic cell cycle | 12 | 2.16×10−7 | CCNB1, SPC25, PLK1, NUF2, NUSAP1, BUB1B, SKA3, CENPF, CDC20, PTTG1… | |
| GO:0000278 | Mitotic cell cycle | 16 | 5.40×10−7 | NUSAP1, CENPF, NDC80, CDC20, PTTG1, CCNB1, SPC25, CDKN1A, CDKN2C, BUB1B… | |
| GO:0000280 | Nuclear division | 10 | 1.09×10−5 | CCNB1, SPC25, KIF11, PLK1, NUF2, NUSAP1, BUB1B, SKA3, CDC20, PTTG1 | |
| GO:0007067 | Mitosis | 10 | 1.09×10−5 | CCNB1, SPC25, KIF11, PLK1, NUF2, NUSAP1, BUB1B, SKA3, CDC20, PTTG1 | |
| GO:0048285 | Organelle fission | 10 | 1.74×10−5 | CCNB1, SPC25, KIF11, PLK1, NUF2, NUSAP1, BUB1B, SKA3, CDC20, PTTG1 | |
| GO:0051439 | Regulation of ubiquitin-protein ligase activity during mitotic cell cycle | 4 | 4.86×10−2 | CCNB1, PLK1, BUB1B, CDC20 | |
| 5.335 | GO:0000279 | M phase | 19 | 3.09×10−11 | NUSAP1, CENPF, NDC80, CDC20, PTTG1, CCNB1, SPC25, PLK1, BUB1B, SKA3… |
| GO:0051327 | M phase of meiotic cell cycle | 7 | 2.32×10−4 | ADCY3, KIF2C, MKI67, PLK1, SGOL2, PTTG1, RAD51 | |
| GO:0007126 | Meiosis | 7 | 2.32×10−4 | ADCY3, KIF2C, MKI67, PLK1, SGOL2, PTTG1, RAD51 | |
| GO:0051321 | Meiotic cell cycle | 7 | 2.76×10−4 | ADCY3, KIF2C, MKI67, PLK1, SGOL2, PTTG1, RAD51 |
GO, gene ontology.
Table II.
Top two enriched GO biological process term clusters with the highest enrichment score for the downregulated differentially-expressed genes.
| Enrichment score | Term | Description | Count | P-value | Genes |
|---|---|---|---|---|---|
| 4.727 | GO:0006811 | Ion transport | 16 | 6.05×10−7 | KCND3, GABRA1, GABRA4, GABRB2, GABRB1, CACNG6, ATP2B4, KCNT2, KCNH7, CACNA1A…… |
| GO:0030001 | Metal ion transport | 12 | 3.87×10−6 | KCNJ5, KCND3, KCNJ6, ATP2B4, CACNG6, KCNH7, KCNH8, SCNN1G, KCNJ3, CACNA1A…… | |
| GO:0006812 | Cation transport | 12 | 2.54×10−5 | KCNJ5, KCND3, KCNJ6, ATP2B4, CACNG6, KCNH7, KCNH8, SCNN1G, KCNJ3, CACNA1A…… | |
| GO:0006813 | Potassium ion transport | 7 | 7.01×10−5 | KCNJ5, KCND3, KCNJ6, KCNT2, KCNH7, KCNH8, KCNJ3 | |
| GO:0015672 | Monovalent inorganic cation transport | 8 | 5.52×10−4 | KCNJ5, KCND3, KCNJ6, KCNT2, KCNH7, KCNH8, SCNN1G, KCNJ3 | |
| 2.321 | GO:0040018 | Positive regulation of multicellular organism growth | 4 | 2.47×10−4 | GH1, DRD2, POU1F1, GHRHR |
| GO:0021983 | Pituitary gland development | 4 | 5.70×10−4 | DRD2, POU1F1, GHRHR, TBX19 | |
| GO:0021984 | Adenohypophysis development | 3 | 8.52×10−4 | DRD2, POU1F1, GHRHR | |
| GO:0021536 | Diencephalon development | 4 | 1.24×10−3 | DRD2, POU1F1, GHRHR, TBX19 | |
| GO:0043567 | Regulation of insulin-like growth factor receptor signaling pathway | 3 | 1.82×10−3 | GH1, POU1F1, GHRHR | |
| GO:0040014 | Regulation of multicellular organism growth | 4 | 4.82×10−3 | GH1, DRD2, POU1F1, GHRHR | |
| GO:0035270 | Endocrine system development | 4 | 6.09×10−3 | DRD2, POU1F1, GHRHR, TBX19 | |
| GO:0030900 | Forebrain development | 5 | 9.58×10−3 | ERBB4, DRD2, POU1F1, GHRHR, TBX19 | |
| GO:0045927 | Positive regulation of growth | 4 | 9.75×10−3 | GH1, DRD2, POU1F1, GHRHR | |
| GO:0048732 | Gland development | 5 | 1.75×10−2 | ERBB4, DRD2, POU1F1, GHRHR, TBX19 | |
| GO:0045165 | Cell fate commitment | 4 | 3.22×10−2 | NOTCH2, ERBB4, POU1F1, TBX19 | |
| GO:0051240 | Positive regulation of multicellular organismal process | 5 | 3.49×10−2 | GH1, ERBB4, DRD2, POU1F1, GHRHR | |
| GO:0042127 | Regulation of cell proliferation | 8 | 4.55×10−2 | NOTCH2, ERBB4, DRD2, NR3C2, CDK6, POU1F1, GHRHR, TBX19 |
GO, gene ontology.
According to the KEGG pathway enrichment analysis, the upregulated DEGs were mainly enriched in 10 pathways
For example, CDC20, CCNB1, CCNB2, BUB1, CDKN1A and MCM3 were enriched in the pathway of the cell cycle (P=5.01×10−7); CDC20, CCNB1, CCNB2, BUB1 and PLK1 were distinctly enriched in the pathway of oocyte meiosis (P=1.058×10−3); CCNB1, CCNB2, CCNA2, BUB1 and PLK1 were significantly enriched in the pathway of progesterone-mediated oocyte maturation (P=1.150×10−3); and CDKN1A, CCNB1, CCNB2 and CASP8 were markedly enriched in the p53 signaling pathway (P=3.4841×10−2) (Table III). Meanwhile, the downregulated DEGs were mainly enriched in 7 pathways. GH1, GABRA1, GABRA4 and GABRB1 were enriched in the pathway of neuroactive ligand-receptor interaction (P=4.35×10−4); MAOB, ALDH2 and ALDH1A7 were mainly enriched in the pathways of histidine metabolism (P=1.2476×10−2) and tryptophan metabolism (P=3.7487×10−2); ATP2B4, ERBB4 and PLCG2 were enriched in the calcium signaling pathway (P=3.9919×10−2); and GSTA4, MGST1, GSTM7 were significantly enriched in the pathways of drug metabolism (P=1.4397×10−2) and glutathione metabolism (P=4.9294×10−2) (Table III).
Table III.
Results of pathway enrichment analysis of the up- and downregulated differentially-expressed genes.
| Category | Term | Description | Count | P-value | Genes |
|---|---|---|---|---|---|
| Upregulated | rno04110 | Cell cycle | 14 | 5.01×10−7 | CDC20, MCM3, CDKN1C, CCNB1, CDKN1A, CCNB2, CDKN2C, BUB1, BUB1B, CCNA2…… |
| rno04114 | Oocyte meiosis | 9 | 1.06×10−3 | CCNB1, ADCY3, AR, CCNB2, MAPK12, PLK1, BUB1, CDC20, PTTG1 | |
| rno00601 | Glycosphingolipid biosynthesis | 5 | 1.10×10−3 | B4GALT1, B3GALT2, B3GALT5, FUT4, B4GALT4 | |
| rno04914 | Progesterone-mediated oocyte maturation | 8 | 1.15×10−3 | CCNB1, ADCY3, CCNB2, KRAS, MAPK12, PLK1, BUB1, CCNA2 | |
| rno04062 | Chemokine signaling pathway | 9 | 1.46×10−2 | ADCY3, KRAS, LYN, ARRB1, PREX1, GRK5, PRKCD, CCL6, SHC4 | |
| rno00330 | Arginine and proline metabolism | 5 | 1.70×10−2 | ARG1, GOT1, NOS1, ASS1, GAMT | |
| rno05219 | Bladder cancer | 4 | 2.94×10−2 | CDKN1A, KRAS, PGF, VEGFA | |
| rno04115 | p53 signaling pathway | 5 | 3.48×10−2 | CCNB1, CDKN1A, CCNB2, CASP8, IGFBP3 | |
| rno04610 | Complement and coagulation cascades | 5 | 4.19×10−2 | C1QA, A2M, C1S, C1QC, F2R | |
| rno00510 | N-glycan biosynthesis | 4 | 4.89×10−2 | B4GALT1, MAN2A1, ALG5, MAN1A1 | |
| Downregulated | rno04080 | Neuroactive ligand-receptor interaction | 9 | 4.35×10−4 | GH1, GABRA1, GABRA4, GABRB2, DRD2, GABRB1, TSHB, LHB, GHRHR |
| rno00410 | β-alanine metabolism | 3 | 1.05×10−2 | ALDH2, ALDH1A7, DPYD | |
| rno00340 | Histidine metabolism | 3 | 1.25×10−2 | MAOB, ALDH2, ALDH1A7 | |
| rno00982 | Drug metabolism | 4 | 1.44×10−2 | GSTA4, MAOB, MGST1, GSTM7 | |
| rno00380 | Tryptophan metabolism | 3 | 3.75×10−2 | MAOB, ALDH2, ALDH1A7 | |
| rno04020 | Calcium signaling pathway | 5 | 3.99×10−2 | ATP2B4, ERBB4, PLCG2, PLCB1, CACNA1A | |
| rno00480 | Glutathione metabolism | 3 | 4.93×10−2 | GSTA4, MGST1, GSTM7 |
Analysis of PPI network
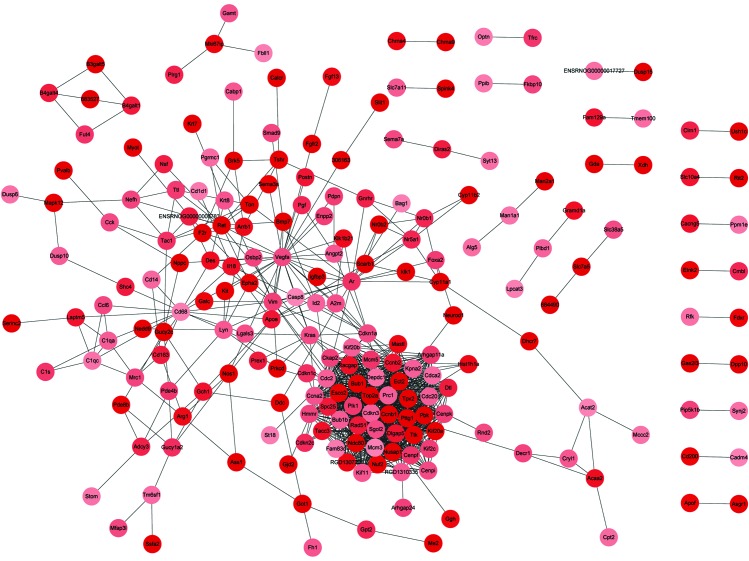
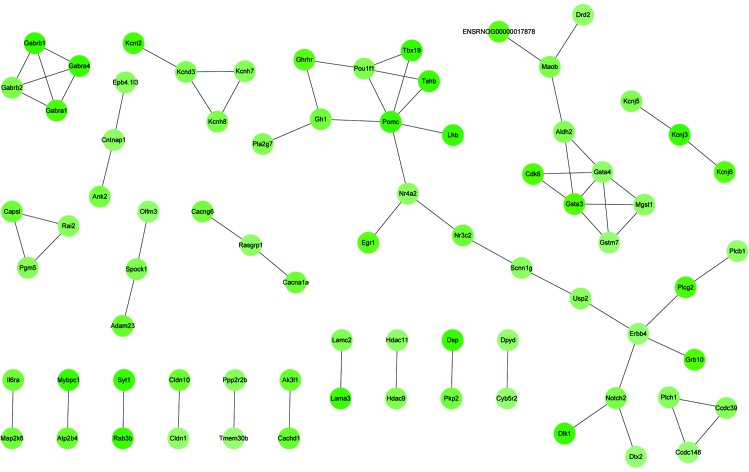
The PPI networks constructed with the up- and downregulated DEGs consisted of 1,044 and 69 PPI pairs, respectively. In the former, PTTG1, along with CCNB1, CCNA2, SPC25, CENPF, NUSAP1, CDC20, TOP2A and BUB1, were observed to interact with each other (Fig. 1). Within the PPI network built with downregulated DEGs, GABRA1, GABRA4, GABRB1 and GABRB1 were observed to interact with each other; GSTA3, GSTA4, GSTM7 and MGST1 were also found to interact with each other, and POU1F1 was observed to interact with POMC (Fig. 2). The connection degrees of the top 15% highly-connected upregulated DEGs were each >30, and those of CDK1, CCNB1, CCNA2 and BUB1 were 51, 47, 46 and 44, respectively (Table IV). The top 20% highly-connected downregulated DEGs all had connection degrees of at least 3, and the degrees of POMC, GSTA4, POU1F1, ERBB4, KCND3 and NOTCH2 were 6, 5, 4, 4, 3 and 3, respectively (Table IV).
Figure 1.
Protein-protein interaction network constructed with the upregulated differentially-expressed genes. Different shades of nodes colors represent the degree of up- or downregulation.
Figure 2.
Protein-protein interaction network constructed with the downregulated differentially-expressed genes. Different shades of nodes colors represent the degree of up- or downregulation.
Table IV.
Upregulated DEGs with connection degrees of >30 and the downregulated DEGs with connection degrees of at least 3 in the protein-protein interaction networks.
| Category | Degree |
|---|---|
| Upregulated DEGs | |
| CDK1 | 51 |
| CCNB1 | 47 |
| CCNA2 | 46 |
| BUB1 | 44 |
| ECT2 | 43 |
| TPX2 | 42 |
| NDC80 | 42 |
| PRC1 | 42 |
| NUSAP1 | 41 |
| TOP2A | 41 |
| CCNB2 | 41 |
| PBK | 41 |
| RACGAP1 | 41 |
| TTK | 41 |
| PIK1 | 40 |
| SPC25 | 40 |
| BUB1B | 40 |
| CENPF | 40 |
| CDKN3 | 39 |
| NUF2 | 38 |
| CDC20 | 38 |
| KIF11 | 38 |
| DLGAP5 | 38 |
| SGOL2 | 37 |
| DTL | 36 |
| KIF20A | 36 |
| CDCA2 | 36 |
| KIF20B | 35 |
| ESCO2 | 35 |
| RAD51 | 34 |
| ARHGAP11A | 34 |
| CKAP2 | 33 |
| HMMR | 33 |
| KIF2C | 32 |
| DEPDC1 | 32 |
| Downregulated DEGs | |
| POMC | 6 |
| GSTA4 | 5 |
| GSTA1 | 5 |
| POU1F1 | 4 |
| ERBB4 | 4 |
| GABRA1 | 3 |
| MGST1 | 3 |
| GABRA4 | 3 |
| GSTM7 | 3 |
| ALDH2 | 3 |
| GH1 | 3 |
| NR4A2 | 3 |
| MAOB | 3 |
| KCND3 | 3 |
| NOTCH2 | 3 |
| GABRB1 | 3 |
DEGs, differentially-expressed genes.
Discussion
In the present study, 391 DEGs were identified to be significantly upregulated and 238 were significantly downregulated in the pituitary adenomas samples. According to the constructed PPI network with the upregulated DEGs, PTTG1 interacted with other DEGs with higher connection degrees, such as CCNB1, CCNA2, SPC25, CENPF, NUSAP1, CDC20, TOP2A and BUB1.
PTTG1, a tumorigenic gene in vivo (23), is abundantly expressed in pituitary tumors (24). As a securin protein, PTTG1 is correlated with the mitotic checkpoint that prevents abnormal chromosome segregation (25), and peaks at the G2/M phase (26). The overexpression of PTTG1 results in cell transformation and induces aneuploidy (27), and this exists in >90% of pituitary tumors (28). PTTG1, together with CCNB1, CCNA2, BUB1, SPC25, CENPF, NUSAP1, TOP2A and CDC20, were all found to be enriched in GO terms associated with the cell cycle or cell division, which are indispensable for tumor growth. It has been reported that CDC20 is involved in the degradation of PTTG1-encoding products (29). Meanwhile, previous studies have also reported the abnormal expression of CCNB1 (30), CCNA2 (31), and BUB1 (32) in pituitary adenomas. Furthermore, CCNB1 was enriched in the p53 signaling pathway. PTTG1-encoding protein can cooperate with p53 to take part in cell apoptosis and DNA damage/repair (33,34). Altered p53 expression has been reported in pituitary carcinomas (35). Also, PTTG1 can activate β-fibroblast growth factor, cyclin D3 and c-myc to promote cell proliferation (36,37). Therefore, PTTG1 may play a crucial role in the occurrence of pituitary adenomas via interaction with CCNB1, CCNA2, CENPF, NUSAP1, CDC20, TOP2A, BUB1 and p53.
Within the PPI network constructed with downregulated DEGs, GABRA1, GABRA4 and GABRB1 had higher degrees of connection to other genes. These genes were enriched in the pathway of neuroactive ligand-receptor interaction. GABRA1, GABRA4 and GABRB1 encode γ-aminobutyric acid (GABA) receptors. GABA is the major inhibitory neurotransmitter in the mammalian brain and may act as a paracrine or autocrine regulating factor in the human pituitary gland and human pituitary growth hormone adenomas (38). It has been reported that GABA has a specific effect on the electrical activity of a tumoral line of rat pituitary cells, and that it inhibits prolactin secretion directly at the pituitary level (39). Additionally, POU1F1 was also observed to have a higher connection degree in the PPI network. This gene encoding a member of the POU family of transcription factors (40), was correlated with the development of the pituitary gland, adenohypophysis and endocrine system. In humans, POU1F1 mutation has been shown to be associated with combined pituitary hormone deficiency (41). POU1F1 is also implicated in cell growth and prevents cell apoptosis (42). In the present study, it was observed to interact with POMC, which encodes a polypeptide hormone precursor. The encoded polypeptide hormone precursor is synthesized mainly in corticotrophin cells of the anterior pituitary (43). A previous study has shown that in silent pituitary adenomas, POMC mRNA has a diffuse low level or is absent (44). Thus, GABRA1, GABRA4, GABRB1, POU1F1 and POMC may also have critical roles in pituitary adenoma occurrence via close interaction.
In conclusion, upregulated DEGs, such as those associated with the cell cycle or cell division (e.g., CCNB1, CCNA2, BUB1, CENPF, NUSAP1, CDC20, TOP2A and particularly PTTG1) and downregulated DEGs, such as those relevant to neuroactive ligand-receptor interaction (e.g., GABRA1, GABRA4 and GABRB1), as well as those correlated with the development of the pituitary gland, adenohypophysis and endocrine system (e.g., POU1F1) may have essential roles in the pathogenesis of pituitary adenomas. The present study provides novel information for the clinical diagnosis of this disease.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 81273732/H2708) and the Shanghai Educational Commission Funding Project (no. 2012JW68).
References
- 1.Chesnokova V, Melmed S. Pituitary tumour-transforming gene (PTTG) and pituitary senescence. Horm Res. 2009;71(Suppl 2):82–87. doi: 10.1159/000192443. [DOI] [PubMed] [Google Scholar]
- 2.Pereira AM, Biermasz NR. Treatment of nonfunctioning pituitary adenomas: What were the contributions of the last 10 years? A critical view. Ann Endocrinol (Paris) 2012;73:111–116. doi: 10.1016/j.ando.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Jiang X, Shen Y, Li M, Ma H, Xing M, Lu Y. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer. 2009;16:301–310. doi: 10.1677/ERC-08-0167. [DOI] [PubMed] [Google Scholar]
- 4.Vortmeyer AO, Glasker S, Mehta GU, Abu-Asab MS, Smith JH, Zhuang Z, Collins MT, Oldfield EH. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97:2404–2413. doi: 10.1210/jc.2012-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandeva S, Tichomirowa MA, Zacharieva S, Daly AF, Beckers A. Genetic factors in the development of pituitary adenomas. Endocr Dev. 2010;17:121–133. doi: 10.1159/000262534. [DOI] [PubMed] [Google Scholar]
- 6.Mezzomo LC, Gonzales PH, Pesce FG, Kretzmann Filho N, Ferreira NP, Oliveira MC, Kohek MB. Expression of cell growth negative regulators MEG3 and GADD45γ is lost in most sporadic human pituitary adenomas. Pituitary. 2012;15:420–427. doi: 10.1007/s11102-011-0340-1. [DOI] [PubMed] [Google Scholar]
- 7.Michaelis KA, Knox AJ, Xu M, Kiseljak-Vassiliades K, Edwards MG, Geraci M, Kleinschmidt-DeMasters BK, Lillehei KO, Wierman ME. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology. 2011;152:3603–3613. doi: 10.1210/en.2011-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell WE. Epigenetic mechanisms of tumorigenesis. Horm Metab Res. 2005;37:361–368. doi: 10.1055/s-2005-870153. [DOI] [PubMed] [Google Scholar]
- 9.Ezzat S. Epigenetic control in pituitary tumors. Endocr J. 2008;55:951–957. doi: 10.1507/endocrj.K08E-082. [DOI] [PubMed] [Google Scholar]
- 10.Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol. 2011;7:257–266. doi: 10.1038/nrendo.2011.40. [DOI] [PubMed] [Google Scholar]
- 11.Cheunsuchon P, Zhou Y, Zhang X, Lee H, Chen W, Nakayama Y, Rice KA, Tessa Hedley-Whyte E, Swearingen B, Klibanski A. Silencing of the imprinted DLK1-MEG3 locus in human clinically nonfunctioning pituitary adenomas. Am J Pathol. 2011;179:2120–2130. doi: 10.1016/j.ajpath.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'angelo D, Palmieri D, Mussnich P, Roche M, Wierinckx A, Raverot G, Fedele M, Croce CM, Trouillas J, Fusco A, et al. Altered microRNA expression profile in human pituitary GH adenomas: Down-regulation of miRNA targeting HMGA1, HMGA2 and E2F1. J Clin Endocrinol Metab. 2012;97:2011–3482. doi: 10.1210/jc.2011-3482. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Marinoni I, Irmler M, Psaras T, Honegger JB, Beschorner R, Anastasov N, Beckers J, Theodoropoulou M, Roncaroli F, et al. Transcriptome analysis of MENX-associated rat pituitary adenomas identifies novel molecular mechanisms involved in the pathogenesis of human pituitary gonadotroph adenomas. Acta Neuropathol. 2013;126:137–150. doi: 10.1007/s00401-013-1132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 18.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 19.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–261. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohl M, Wiese S, Warscheid B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol Biol. 2013;696:291–303. doi: 10.1007/978-1-60761-987-1_18. [DOI] [PubMed] [Google Scholar]
- 22.He X, Zhang J. Why do hubs tend to be essential in protein networks? PLoS genetics. 2006;2:e88. doi: 10.1371/journal.pgen.0020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Horwitz GA, Prezant TR, Valentini A, Nakashima M, Bronstein MD, Melmed S. Structure, expression and function of human pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1999;13:156–166. doi: 10.1210/mend.13.1.0225. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Puri R, Lefkowitz EJ, Kakar SS. Identification of the human pituitary tumor transforming gene (hPTTG) family: Molecular structure, expression and chromosomal localization. Gene. 2000;248:41–50. doi: 10.1016/S0378-1119(00)00096-2. [DOI] [PubMed] [Google Scholar]
- 25.Quereda V, Malumbres M. Cell cycle control of pituitary development and disease. J Mol Endocrinol. 2009;42:75–86. doi: 10.1677/JME-08-0146. [DOI] [PubMed] [Google Scholar]
- 26.Yu R, Ren SG, Horwitz GA, Wang Z, Melmed S. Pituitary tumor transforming gene (PTTG) regulates placental JEG-3 cell division and survival: Evidence from live cell imaging. Mol Endocrinol. 2000;14:1137–1146. doi: 10.1210/mend.14.8.0501. [DOI] [PubMed] [Google Scholar]
- 27.Yu R, Lu W, Chen J, Mccabe CJ, Melmed S. Overexpressed pituitary tumor-transforming gene causes aneuploidy in live human cells. Endocrinology. 2003;144:4991–4998. doi: 10.1210/en.2003-0305. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Horwitz GA, Heaney AP, Nakashima M, Prezant TR, Bronstein MD, Melmed S. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocr Metab. 1999;84:761–767. doi: 10.1210/jcem.84.2.5432. [DOI] [PubMed] [Google Scholar]
- 29.Zur A, Brandeis M. Securin degradation is mediated by fzy and fzr and is required for complete chromatid separation but not for cytokinesis. EMBO J. 2001;20:792–801. doi: 10.1093/emboj/20.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raverot G, Wierinckx A, Dantony E, Auger C, Chapas G, Villeneuve L, Brue T, Figarella-Branger D, Roy P, Jouanneau E, et al. Prognostic factors in prolactin pituitary tumors: Clinical, histological and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocr Metab. 2010;95:1708–1716. doi: 10.1210/jc.2009-1191. [DOI] [PubMed] [Google Scholar]
- 31.Leone V, Langella C, D'Angelo D, Mussnich P, Wierinckx A, Terracciano L, Raverot G, Lachuer J, Rotondi S, Jaffrain-Rea ML, et al. Mir-23b and miR-130b expression is downregulated in pituitary adenomas. Mol Cell Endocrinol. 2014;390:1–7. doi: 10.1016/j.mce.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida D, Nomura R, Teramoto A. Signalling pathway mediated by CXCR7, an alternative chemokine receptor for stromal-cell derived factor-1α, in AtT20 mouse adrenocorticotrophic hormone-secreting pituitary adenoma cells. J Neuroendocrinol. 2009;21:481–488. doi: 10.1111/j.1365-2826.2009.01867.x. [DOI] [PubMed] [Google Scholar]
- 33.Yu R, Heaney AP, Lu W, Chen J, Melmed S. Pituitary tumor transforming gene causes aneuploidy and p53-dependent and p53-independent apoptosis. J Biol Chem. 2000;275:36502–36505. doi: 10.1074/jbc.C000546200. [DOI] [PubMed] [Google Scholar]
- 34.Hamid T, Kakar SS. PTTG/securin activates expression of p53 and modulates its function. Mol Cancer. 2004;3:18. doi: 10.1186/1476-4598-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER., Jr p53 expression in pituitary adenomas and carcinomas: Correlation with invasiveness and tumor growth fractions. Neurosurgery. 1996;38:765–770. doi: 10.1227/00006123-199604000-00027. [DOI] [PubMed] [Google Scholar]
- 36.Mccabe C, Khaira J, Boelaert K, Heaney AP, Tannahill LA, Hussain S, Mitchell R, Olliff J, Sheppard MC, Franklyn JA, Gittoes NJ. Expression of pituitary tumour transforming gene (PTTG) and fibroblast growth factor-2 (FGF-2) in human pituitary adenomas: Relationships to clinical tumour behaviour. Clin Endocrinol (Oxf) 2003;58:141–150. doi: 10.1046/j.1365-2265.2003.01598.x. [DOI] [PubMed] [Google Scholar]
- 37.Pei L. Identification of c-myc as a down-stream target for pituitary tumor-transforming gene. J Biol Chem. 2001;276:8484–8491. doi: 10.1074/jbc.M009654200. [DOI] [PubMed] [Google Scholar]
- 38.End K, Gamel-Didelon K, Jung H, Tolnay M, Lüdecke D, Gratzl M, Mayerhofer A. Receptors and sites of synthesis and storage of gamma-aminobutyric acid in human pituitary glands and in growth hormone adenomas. Am J Clin Pathol. 2005;124:550–558. doi: 10.1309/6RV8JFDY57DU97KT. [DOI] [PubMed] [Google Scholar]
- 39.Israel J, Dufy B, Gourdji D, Vincent J. Effects of GABA on electrical properties of cultured rat pituitary tumor cells: An intracellular recording study. Life Sci. 1981;29:351–359. doi: 10.1016/0024-3205(81)90328-3. [DOI] [PubMed] [Google Scholar]
- 40.Cohen LE, Wondisford FE, Radovick S. Role of Pit-1 in the gene expression of growth hormone, prolactin and thyrotropin. Endocrinol Metab Clin North Am. 1996;25:523–540. doi: 10.1016/S0889-8529(05)70339-X. [DOI] [PubMed] [Google Scholar]
- 41.Quentien MH, Barlier A, Franc JL, Pellegrini I, Brue T, Enjalbert A. Pituitary transcription factors: From congenital deficiencies to gene therapy. J Neuroendocrinol. 2006;18:633–642. doi: 10.1111/j.1365-2826.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 42.Pellegrini I, Roche C, Quentien MH, Ferrand M, Gunz G, Thirion S, Bagnis C, Enjalbert A, Franc JL. Involvement of the pituitary-specific transcription factor pit-1 in somatolactotrope cell growth and death: An approach using dominant-negative pit-1 mutants. Mol Endocrinol. 2006;20:3212–3227. doi: 10.1210/me.2006-0122. [DOI] [PubMed] [Google Scholar]
- 43.Millington GW. Proopiomelanocortin (POMC): The cutaneous roles of its melanocortin products and receptors. Clin Exp Dermatol. 2006;31:407–412. doi: 10.1111/j.1365-2230.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 44.Stefaneanu L, Kovacs K, Horvath E, Lloyd RV. In situ hybridization study of pro-opiomelanocortin (POMC) gene expression in human pituitary corticotrophs and their adenomas. Virchows Arch A Pathol Anat Histopathol. 1991;419:107–113. doi: 10.1007/BF01600224. [DOI] [PubMed] [Google Scholar]