Abstract
Commonly used staging procedures often cannot predict the absence of cervical metastases (CM) in squamous cell carcinomas (SCCs) of the oral cavity. Due to the high incidence of occult CM in numerous N0 cases in the clinic, an elective neck dissection (ND) is performed. The sentinel lymph node biopsy (SNB) is a common concept in the modern surgical therapy of malignancies. The present study evaluates the applicability of this concept for T1/T2-SCC of the tongue. In a prospective clinical study, 10 consecutive patients with T1/T2-SCC of the tongue and cN0 necks, were enrolled. Following sentinel lymph node (SLN) scintigraphy, all patients underwent SNB with a γ-probe and a subsequent ND. SNB specimens were compared with histopathological assessments of surgical specimens from the ND. A total of 5 female and 5 male patients (mean age, 52 years; women, 62 years; men, 42 years), with a median follow-up time of 33.5 months (range, 10–40 months), were treated. All patients presented with detectable SLNs. In 7 cases, the SLN(s) and the residual ND were negative for CM. In 3 cases, the SLN(s) were positive without further CM in the other neck nodes. Furthermore, 1 patient showed additional CMs after 10 months in the contralateral neck and lung metastasis after 18 months, but none at the time of the initial treatment. The concept of an SNB appears to be applicable to the management of the cN0 neck in small SCC of the tongue. The role of SNB in the management of SCC requires further investigation by prospective trials with larger patient numbers.
Keywords: cervical metastases, oral cancer, squamous cell carcinoma of the tongue, sentinel lymph node
Introduction
Oral cancer is responsible for 200,000–350,000 cancer-associated fatalities per year worldwide and is thus ranked sixth with regard to the cause of mortality due to tumors (1,2).
Beside the time of diagnosis and the consequent size of the tumor (3), the presence of lymph node metastasis in the neck is the most important prognostic indicator (4,5). Oral SCC is disseminated preferentially by the lymphatic system and mainly the cervical lymph nodes at levels I and II are affected (6–8). The high incidence of occult cervical lymph node metastases of ~25% in N0 cases in the clinic underscores the clinical significance and the resulting therapeutic difficulties (9,10).
The commonly used staging procedures often cannot predict the absence of CM. Clinical and radiological examination have approximate false-negative and false-positive rates of 30% in the determination of CM (11). The most precise method and the gold standard for the correct N-staging is the histopathological examination of the surgical specimen following elective neck dissection (END) (12).
The management of the clinically and radiologically negative neck, particularly in patients with early oral SCC, remains a matter of debate, although the majority of centers favor END for staging of the neck and the removal of occult disease (11).
In the modern surgical treatment of melanoma or breast cancer, the presence of regional lymph node metastases is evaluated by the identification and examination of the sentinel lymph node (SLN). Radiolabeled colloid solution is injected around the primary tumor, which drains to the next lymph nodes and predominantly to the SLN, which may contain metastatic deposits of the primary tumor. The combination of pre-operative lymphoscintigraphy and the intraoperative detection of the SLN with a γ-probe allows the radioactive tracer in the lymph nodes to be precisely located during the surgery (11,13).
In the past decade, the SLN-technique has been increasingly used for other malignancies, including head and neck carcinomas. Technical developments and a gain in experience have led to a wider use of SNB, even in the complex lymphatic system of the head and neck region (14). Multiple small patient series have been published evaluating the application of SLN biopsy for head and neck cancers, with a sensitivity of at least 75% for the identification of CM (Table I) (11,15,16). But the majority of these studies included higher stage SCC and did not focus on a specific region and a clinical N0 neck.
Table I.
Epidemiological and clinical data.
| Patient | Gender | Age, years | Risk factors | No. of SLNs detected during surgery | No. of CMs | Follow-up time, months | Relapse |
|---|---|---|---|---|---|---|---|
| 1 | Male | 21 | No | 2 | 0 | 33 | No |
| 2 | Male | 28 | Yes | 2 | 0 | 39 | No |
| 3 | Male | 32 | No | 4 | 1 | 18 | After 10 months |
| 4 | Female | 33 | Yes | 2 | 0 | 22 | No |
| 5 | Female | 59 | Yes | 2 | 1 | 28 | No |
| 6 | Male | 62 | Yes | 3 | 2 | 40 | No |
| 7 | Female | 63 | No | 2 | 0 | 38 | No |
| 8 | Male | 69 | Yes | 2 | 0 | 40 | No |
| 9 | Female | 75 | Yes | 3 | 0 | 10 | No |
| 10 | Female | 82 | Yes | 2 | 0 | 34 | No |
SLN, sentinel lymph node; CM, cervical metastases.
The aim of the present study was to analyze and evaluate the applicability of the SLN concept for T1/T2 SCC of the tongue with a clinical N0 situation.
Patients and methods
Patients
Between 2010 and 2012, 10 patients with SCC of the tongue were selected from the Department of Oral and Maxillofacial of the University Medical Center (Johannes Gutenberg-University of Mainz, Mainz, Germany) to take part in the study. The criteria for inclusion were: SCC of the tongue, a tumor size <T3 and a clinical N0 situation. All tumors were classified and staged according to the 2003 tumor-node-metastasis (TNM) staging system of the Union for International Cancer Control, and special attention was paid to the CM (17).
The study protocol was approved by the internal institutional review board and informed consent was obtained from all the patients involved in the study. Computed tomography and ultrasonography of the head and neck region were performed on all patients prior to the treatment.
Treatment
All patients received peritumorous injections of technetium-99m-labeled colloidal human serum albumin (0.2 ml; 50 MBq) in an attempt to completely surround the tumor in its deep and lateral aspects. Injection was performed 1 day prior to surgery. The pre-operative lymphoscintigraphy was performed 30 min after the injection. Static images were accomplished in lateral and antero-posterior projections, and the radioactive lymph nodes were marked on the skin and controlled by B-mode sonography.
Beside the resection of the tongue tumor, all patients received an END at levels I–III. Using a hand-held γ-probe (Gamma Finder® II, World Of Medicine USA, Inc., Orlando, Florida, USA), the SLN was identified in vivo and dissected separately. Next, the remaining neck was re-evaluated for the absence of radioactivity. All lymph nodes with radioactivity were dissected and considered as SLNs. Afterwards, the proposed END was performed. The SLNs and all neck specimens from the subsequent END were sent for histopathological examination.
Results
The cohort consisted of 5 (50%) female and 5 (50%) male patients, with an average age of 52 years and a range of 21–82 years (female: Mean, 62 years; range, 33–82 years; male: Mean, 42 years; range, 21–69 years). The majority of the patients (70%) showed a risk profile regarding smoking and alcohol consumption (Table I).
SCC was evenly spread in the tongue without a preference for a side, however, 70% was located in the front and middle section of the tongue (Fig. 1).
Figure 1.
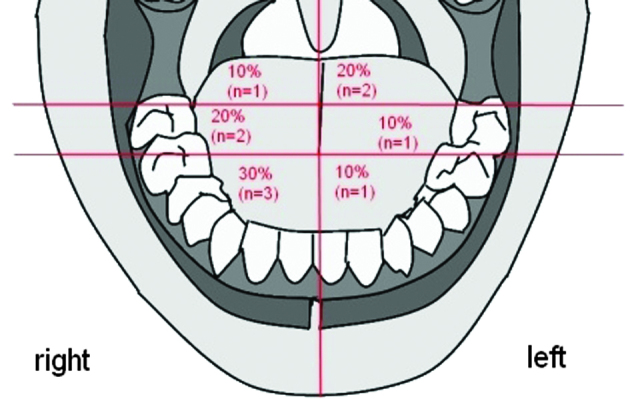
Classification and distribution of the primary tumor location.
The pathological TNM stage of the patients is shown in Table II; 80% of the patients presented with a T1 tumor and 20% with a T2 tumor. No distant metastases were detected following primary staging. The majority of the patients (90%) presented with a tumor of grade G1-G2.
Table II.
pTNM classification following surgical therapy.
| pTNM stage | Patients, n (%) |
|---|---|
| T-Stage | |
| pT1 | 8 (80) |
| pT2 | 2 (20) |
| N-stage | |
| pN0 | 7 (70) |
| pN1 | 2 (20) |
| pN2 | 1 (10) |
| M-stage | |
| cM0 | 10 (100) |
| Grade | |
| G1 | 4 (40) |
| G2 | 5 (50) |
| G3 | 1 (10) |
pTNM, pathological tumor-node-metastasis.
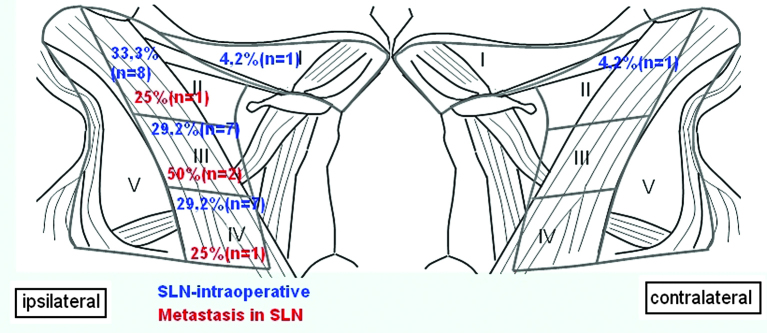
In all patients, SLN could be detected intraoperatively. On average, 2.4 SLNs per patient were found. Fig. 2 shows the distribution of SLNs and CMs at the neck level. A total of 2 SLNs were found in 7 patients, 3 SLNs in 2 patients and 1 SLN in 1 patient were detected. In 7 cases, the SLNs and the residual neck dissection were negative for cervical lymph node metastasis.
Figure 2.
Distribution of the sentinel lymph nodes (SLNs) detected during the surgery (blue) and distribution of the cervical metastases following pathological examination according to the neck level (red).
In total, 30% (n=3) of the patients exhibited lymph node metastases, which were detected by the SLN biopsy, without further CM in the other neck nodes. One patient exhibited skip metastasis; the patient presented with a CM in a SLN at level IV, which had bypassed the common upper neck level I–III.
Additional CMs were developed in 1 patient after 10 months in the contralateral neck, with lung metastasis after 18 months.
The median follow-up time for the patients was 32 months (range, 8–39 months). During the follow-up, none of the other 9 patients experienced local or cervical recurrence.
If the case with the contralateral CM recurrence after 10 months is defined as a false-negative result, then the sensitivity and specificity of the SLN biopsy in the patient group were 75% (3/4 patients with CM were detected) and 100% (6/6 patient without CN were detected), respectively, and the false negative rate was 25%.
Discussion
The demographic data of the present SCC patients, with a mean age of 52 years and the high presence of risk factors, are comparable with the international literature (15–20). SCC was evenly spread in the tongue and was identical to the distribution pattern in the literature (21).
The management of patients with early oral SCC with a clinically negative neck remains controversial. The majority of clinics prefer the END instead of a wait-and-see strategy due to the high rate of occult metastases. However, 70–80% of this patient group are ultimately pN0 and are theoretically overtreated with a selective neck dissection (SND) (22,23). Although an SND is less invasive than a modified radical dissection, measurable morbidity does exist, including shoulder dysfunction, contour changes, pain and lower lip paresis (24–27). Although the SND has proven reliability and worldwide acceptance, it is an extended surgery compared with the SLN biopsy, meaning a longer surgical time, higher costs and greater morbidity. Functional outcome and post-operative complications following an SLN biopsy are also significantly better than after an SND (28,29).
The concept of an SLN biopsy provides the possibility of accurate pathological cervical node staging, whilst minimizing the invasiveness of the procedure and its associated morbidity. In addition, pre-operative lymphoscintigraphy and intraoperative detection with a hand-held γ-probe have the additional advantage of identifying aberrant drainage pathways (22,23). In the present study, a contralateral SLN could be detected in 1 patient and a CM was found at level IV, which had bypassed the common upper neck level I–III (skip metastasis).
The SLN biopsy has the benefit of concentrating only on the relevant nodes for pathological examination. This selection allows a more in-depth evaluation of the small number of sentinel nodes, using step serial sections and immunohistochemistry (22,30,31). However, if there are multiple SLNS at different levels, the number of SLNs that should be removed for the examination remains unknown. The majority of studies recommend the removal of at least 2–3 SLNs to reduce the possibility of false-negative results (32–34). In the present study, an average of 2.4 SLNs were detected per patient.
There are a number of studies focusing on the use of SLN in SCC (Table III) (11,15,16,26,33,36,38–44). But only few studies do have a homogenous clientele with only small tumors and a clinical N0 neck in which the SLN is of importance. In addition the majority of these studies did not focus on a specific region (oral cavity vs. oropharynx). The sensitivity of the SLN biopsy for head and neck cancer varies in the literature between 75 and 100%.
Table III.
Published sensitivity rates for SLN biopsy.
| First author (ref.) | Year | No. of patients with oral SCC/no. of patients | Cancer | T-Stage | No. of detected CM by SLN/no. of all CM | Sensitivity, % |
|---|---|---|---|---|---|---|
| Hyde et al (38) | 2003 | 19/19 | Oral cancer | 1–4 | 3/4 | 75 |
| Gallegos et al (33) | 2005 | 48/48 | Oral cancer | 1–2 | 13/17 | 77 |
| Frerich et al (39) | 2007 | 28/40 | Oropharyngeal cancer | 1–2 | 8/10 | 80 |
| Chone et al (11) | 2008 | 24/35 | Oropharyngeal cancer | 1–3 | 9/11 | 82 |
| Werner et al (30) | 2004 | 11/90 | Oropharyngeal cancer | 1–3 | 20/23 | 87 |
| Civantos et al (23) | 2010 | 140/140 | Oral cancer | 1–2 | 34/40 | 90 |
| Tschopp et al (40) | 2005 | 25/31 | Oropharyngeal cancer | 1–3 | 14/15 | 93 |
| Shoaib et al (41) | 2001 | 37/37 | Oral cancer | 1–4 | 16/17 | 94 |
| Ionna et al (42) | 2002 | 40/40 | Oral cancer | 1–2 | 4/4 | 100 |
| Höft et al (43) | 2004 | 22/50 | Oropharyngeal cancer | 1–4 | 12/12 | 100 |
| Bilde et al (44) | 2008 | 51/51 | Oral cancer | 1–2 | 11/11 | 100 |
| Current study | 2015 | 10/10 | Oral cancer | 1–2 | 4/5 | 80 |
SLN, sentinel lymph node; CM, cervical metastases; T, tumor.
The sensitivity of the SLN biopsy for head and neck cancer varies in the literature between 75 and 100%. This has to be compared with the rate of regional recurrence after SND, which is recorded as between 6–30% in the literature (35–37). In the present patient group, the sensitivity of the SLN biopsy was 75% when defining the contralateral CM recurrence after 10 months in 1 patient as a false-negative result.
Although further studies are necessary to confirm the results, patients with cN0 and early-stage oral SCC may benefit from an SLN biopsy by avoiding the morbidity of a neck dissection.
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Kowalski LP, Carvalho AL. Influence of time delay and clinical upstaging in the prognosis of head and neck cancer. Oral Oncol. 2001;37:94–98. doi: 10.1016/S1368-8375(00)00066-X. [DOI] [PubMed] [Google Scholar]
- 4.Capote A, Escorial V, Muñoz-Guerra MF, Rodriguez-Campo FJ, Gamallo C, Naval L. Elective neck dissection in early-stage oral squamous cell carcinoma-does it influence recurrence and survival? Head Neck. 2007;29:3–11. doi: 10.1002/hed.20482. [DOI] [PubMed] [Google Scholar]
- 5.Hiratsuka H, Miyakawa A, Nakamori K, Kido Y, Sunakawa H, Kohama G. Multivariate analysis of occult lymph node metastasis as a prognostic indicator for patients with squamous cell carcinoma of the oral cavity. Cancer. 1997;80:351–356. doi: 10.1002/(SICI)1097-0142(19970801)80:3<351::AID-CNCR1>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Kowalski LP, Bagietto R, Lara JR, Santos RL, Tagawa EK, Santos IR. Factors influencing contralateral lymph node metastasis from oral carcinoma. Head Neck. 1999;21:104–110. doi: 10.1002/(SICI)1097-0347(199903)21:2<104::AID-HED2>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Pimenta Amaral TM, Da Silva Freire AR, Carvalho AL, Pinto CA, Kowalski LP. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol. 2004;40:780–786. doi: 10.1016/j.oraloncology.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Woolgar JA. The topography of cervical lymph node metastases revisited: the histological findings in 526 sides of neck dissection from 439 previously untreated patients. Int J Oral Maxillofac Surg. 2007;36:219–225. doi: 10.1016/j.ijom.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Mishra P, Sharma AK. A 3-year study of supraomohyoid neck dissection and modified radical neck dissection type I in oral cancer: with special reference to involvement of level IV node metastasis. Eur Arch Otorhinolaryngol. 2010;267:933–938. doi: 10.1007/s00405-009-1155-9. [DOI] [PubMed] [Google Scholar]
- 10.Yuen AP, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW, Wei WI, Kong CK, Book KS, Yuen WC, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31:765–772. doi: 10.1002/hed.21033. [DOI] [PubMed] [Google Scholar]
- 11.Chone CT, Magalhes RS, Etchehebere E, Camargo E, Altemani A, Crespo AN. Predictive value of sentinel node biopsy in head and neck cancer. Acta Otolaryngol. 2008;128:920–924. doi: 10.1080/00016480701760114. [DOI] [PubMed] [Google Scholar]
- 12.Woolgar JA, Beirne JC, Vaughan ED, Lewis-Jones HG, Scott J, Brown JS. Correlation of histopathologic findings with clinical and radiologic assessments of cervical lymph-node metastases in oral cancer. Int J Oral Maxillofac Surg. 1995;24:30–37. doi: 10.1016/S0901-5027(05)80853-7. [DOI] [PubMed] [Google Scholar]
- 13.Stoeckli SJ, Alkureishi LW, Ross GL. Sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2009;266:787–793. doi: 10.1007/s00405-009-0955-2. [DOI] [PubMed] [Google Scholar]
- 14.Kuriakose MA, Trivedi NP. Sentinel node biopsy in head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2009;17:100–110. doi: 10.1097/MOO.0b013e3283293631. [DOI] [PubMed] [Google Scholar]
- 15.Bertz J, Dahm S, Haberland J, Kraywinkel K, Kurth BM, Wolf U. A publication of the Centre for Cancer Registry Data at the RKI. Westkreuz-Druckerei, Berlin: 2010. Spread of cancers in Germany. Development of prevalence between 1990 and 2010. Robert Koch Institute, Berlin, 2010. [Google Scholar]
- 16.Mashberg A, Boffetta P, Winkelman R, Garfinkel L. Tobacco smoking, alcohol drinking and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer. 1993;72:1369–1375. doi: 10.1002/1097-0142(19930815)72:4<1369::AID-CNCR2820720436>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 18.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 19.Maier H, Tisch M, Conradt C, Pötschke-Langer M. Alcohol drinking and cancer of the upper aerodigestive tract in women. Dtsch Med Wochenschr. 1999;124:851–854. doi: 10.1055/s-2007-1024430. [DOI] [PubMed] [Google Scholar]
- 20.Petti S. Lifestyle risk factors for oral cancer. Oral Oncol. 2009;45:340–350. doi: 10.1016/j.oraloncology.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Cooper JS, Porter K, Mallin K, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31:748–758. doi: 10.1002/hed.21022. [DOI] [PubMed] [Google Scholar]
- 22.Alkureishi LW, Ross GL, Shoaib T, et al. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann Surg Oncol. 2010;17:2459–2464. doi: 10.1245/s10434-010-1111-3. [DOI] [PubMed] [Google Scholar]
- 23.Civantos FJ, Zitsch RP, Schuller DE, Agrawal A, Smith RB, Nason R, Petruzelli G, Gourin CG, Wong RJ, Ferris RL, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: Results of a prospective multi-institutional trial. J Clin Oncol. 2010;28:1395–1400. doi: 10.1200/JCO.2008.20.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chepeha DB, Taylor RJ, Chepeha JC, Teknos TN, Bradford CR, Sharma PK, Terrell JE, Wolf GT. Functional assessment using constant's shoulder scale after modified radical and selective neck dissection. Head Neck. 2002;24:432–436. doi: 10.1002/hed.10067.abs. [DOI] [PubMed] [Google Scholar]
- 25.Nibu K, Ebihara Y, Ebihara M, Kawabata K, Onitsuka T, Fujii T, Saikawa M. Quality of life after neck dissection: A multicenter longitudinal study by the japanese clinical study group on standardization of treatment for lymph node metastasis of head and neck cancer. Int J Clin Oncol. 2010;15:33–38. doi: 10.1007/s10147-009-0020-6. [DOI] [PubMed] [Google Scholar]
- 26.Terrell JE, Ronis DL, Fowler KE, Bradford CR, Chepeha DB, Prince ME, Teknos TN, Wolf GT, Duffy SA. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:401–408. doi: 10.1001/archotol.130.4.401. [DOI] [PubMed] [Google Scholar]
- 27.Ferlito A, Rinaldo A, Silver CE, Gourin CG, Shah JP, Clayman GL, Kowalski LP, Shaha AR, Robbins KT, Suárez C, et al. Elective and therapeutic selective neck dissection. Oral Oncol. 2006;42:14–25. doi: 10.1016/j.oraloncology.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Murer K, Huber GF, Haile SR, Stoeckli SJ. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck. 2011;33:1260–1264. doi: 10.1002/hed.21622. [DOI] [PubMed] [Google Scholar]
- 29.Schiefke F, Akdemir M, Weber A, Akdemir D, Singer S, Frerich B. Function, postoperative morbidity and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck. 2009;31:503–512. doi: 10.1002/hed.21001. [DOI] [PubMed] [Google Scholar]
- 30.Werner JA, Dünne AA, Ramaswamy A, Dalchow C, Behr T, Moll R, Folz BJ, Davis RK. The sentinel node concept in head and neck cancer: Solution for the controversies in the N0 neck? Head Neck. 2004;26:603–611. doi: 10.1002/hed.20062. [DOI] [PubMed] [Google Scholar]
- 31.Ambrosch P, Brinck U. Detection of nodal micrometastases in head and neck cancer by serial sectioning and immunostaining. Oncology (Williston Park) 1996;10:1221–1226. discussion 1226, 1229. [PubMed] [Google Scholar]
- 32.Antonio JK, Santini S, Politi D, Sulfaro S, Spaziante R, Alberti A, Pin M, Barzan L. Sentinel lymph node biopsy in squamous cell carcinoma of the head and neck: 10 years of experience. Acta Otorhinolaryngol Ital. 2012;32:18–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Gallegos-Hernández JF, Hernández-Hernández DM, Flores-Díaz R, Sierra-Santiesteban I, Pichardo-Romero P, Arias-Ceballos H, Minauro-Muñoz G, Alvarado-Cabrero I. The number of sentinel nodes identified as prognostic factor in oral epidermoid cancer. Oral Oncol. 2005;41:947–952. doi: 10.1016/j.oraloncology.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Atula T, Shoaib T, Ross GL, Gray HW, Soutar DS. How many sentinel nodes should be harvested in oral squamous cell carcinoma? Eur Arch Otorhinolaryngol. 2008;265(Suppl 1):S19–S23. doi: 10.1007/s00405-007-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasunla AJ, Greene BH, Timmesfeld N, Wiegand S, Werner JA, Sesterhenn AM. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol. 2011;47:320–324. doi: 10.1016/j.oraloncology.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Liu TR, Chen FJ, Yang AK, Zhang GP, Song M, Liu WW, Chen WC, Chen YF, Ouyang D, Li QL. Elective neck dissection in clinical stage I squamous cell carcinoma of the tongue: Does it improve regional control or survival time? Oral Oncol. 2011;47:136–141. doi: 10.1016/j.oraloncology.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Ebrahimi A, Ashford BG, Clark JR. Improved survival with elective neck dissection in thick early-stage oral squamous cell carcinoma. Head Neck. 2012;34:709–716. doi: 10.1002/hed.21809. [DOI] [PubMed] [Google Scholar]
- 38.Hyde NC, Prvulovich E, Newman L, Waddington WA, Visvikis D, Ell P. A new approach to pre-treatment assessment of the N0 neck in oral squamous cell carcinoma: The role of sentinel node biopsy and positron emission tomography. Oral Oncol. 2003;39:350–360. doi: 10.1016/S1368-8375(02)00121-5. [DOI] [PubMed] [Google Scholar]
- 39.Frerich B, Förster M, Schiefke F, Wittekind C, Hemprich A, Sabri O. Sentinel lymph node biopsy in squamous cell carcinomas of the lips and the oral cavity-a single center experience. J Surg Oncol. 2007;95:97–105. doi: 10.1002/jso.20664. [DOI] [PubMed] [Google Scholar]
- 40.Tschopp L, Nuyens M, Stauffer E, Krause T, Zbären P. The value of frozen section analysis of the sentinel lymph node in clinically N0 squamous cell carcinoma of the oral cavity and oropharynx. Otolaryngol Head Neck Surg. 2005;132:99–102. doi: 10.1016/j.otohns.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Shoaib T, Soutar DS, MacDonald DG, Camilleri IG, Dunaway DJ, Gray HW, McCurrach GM, Bessent RG, MacLeod TI, Robertson AG. The accuracy of head and neck carcinoma sentinel lymph node biopsy in the clinically N0 neck. Cancer. 2001;91:2077–2083. doi: 10.1002/1097-0142(20010601)91:11<2077::AID-CNCR1235>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Ionna F, Chiesa F, Longo F, Manola M, Villano S, Calabrese L, Lastoria S, Mozzillo N. Prognostic value of sentinel node in oral cancer. Tumori. 2002;88(Suppl 1):S18–S19. doi: 10.1177/030089160208800327. [DOI] [PubMed] [Google Scholar]
- 43.Höft S, Maune S, Muhle C, Brenner W, Czech N, Kampen WU, Jänig U, Laudien M, Gottschlich S, Ambrosch P. Sentinel lymph-node biopsy in head and neck cancer. Br J Cancer. 2004;91:124–128. doi: 10.1038/sj.bjc.6601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilde A, von Buchwald C, Therkildsen MH, Mortensen J, Kirkegaard J, Charabi B, Specht L. Need for intensive histopathologic analysis to determine lymph node metastases when using sentinel node biopsy in oral cancer. Laryngoscope. 2008;118:408–414. doi: 10.1097/MLG.0b013e31815d8e15. [DOI] [PubMed] [Google Scholar]