Abstract
Ovarian cancer is the most common cause of gynecological cancer-related mortality. Serine/threonine protein phosphatase 5 (PP5, PPP5C) has been recognized to be involved in the regulation of multiple cellular signaling cascades that control diverse cellular processes, including cell growth, differentiation, proliferation, motility and apoptosis. In this study, to evaluate the functional role of PP5 in ovarian cancer cells, lentivirus-mediated RNA interference (RNAi) was applied to silence PPP5C in the human ovarian cancer cell line CAOV-3. Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell colony forming ability was measured by colony formation. Cell cycle progression was determined by propidium iodide staining and flow cytometry. The results demonstrated that lentivirus-mediated RNAi specifically suppressed the expression of PPP5C at the mRNA and protein levels in CAOV-3 cells. Further investigations revealed that PP5 knockdown significantly inhibited the proliferation and colony formation of CAOV-3 cells. Moreover, the cell cycle of CAOV-3 cells was arrested at the G0/G1 phase following PP5 knockdown. This study highlights the crucial role of PP5 in promoting ovarian cancer cell proliferation, and provides a foundation for further study into the clinical potential of lentiviral-mediated delivery of PP5 RNAi therapy for the treatment of ovarian cancer.
Keywords: ovarian cancer, protein phosphatase 5, lentivirus, proliferation, RNA interference
Introduction
Ovarian cancer is the most common invasive malignancy of the female genital tract in the USA, with an estimated 22,240 cases diagnosed annually. Approximately 14,030 females succumb every year to ovarian cancer, representing the most common cause of mortality among females with gynecological malignancies (1). Surgical resection and platinum-based combination regimens offer a modest but significant survival advantage in ovarian cancer patients with advanced or metastatic disease, although most patients eventually experience disease progression (2). These data highlight the need to identify new approaches that, along with the current treatments, may assist in bringing about a better outcome for ovarian cancer patients.
Protein kinases and phosphatases work together to control cellular processes and signaling pathways (3,4). Although much more is known about protein kinases and their relevant substrates compared with protein phosphatases (5–7), the significance of studying protein phosphatase enzymes and their targets has been demonstrated for disease states attributed in part to malfunctioning protein phosphatase enzymes (8). Protein phosphatase 5 (PP5; gene name, PPP5C) is a ubiquitously expressed serine/threonine protein phosphatase related to PP1, PP2A and PP2B (9). Structural analysis has revealed that PP5 contains a C-terminal catalytic domain and three N-terminal tetratricopeptide repeats (TPRs) that are unique in the phosphoprotein phosphatase family (10). PP5 is auto-inhibited by intramolecular interactions with its TPR domain (11).
PP5 has been implicated in numerous cellular processes, including MAPK-mediated growth and differentiation (12), cell cycle arrest and DNA damage repair via the p53, ataxia telangiectasia mutated (ATM) and ATM and Rad3-related (ATR) pathways (13,14), regulation of ion channels via the membrane receptor for atrial natriuretic peptide (15), cellular heat shock response as mediated by the heat shock transcription factor, and steroid receptor signaling, in particular glucocorticoid receptor (16,17). Notably, another steroid, estradiol, upregulates PP5 expression in MCF-7 breast cancer cells (18). PP5 was observed to promote proliferation of breast cancer cells and growth of tumors in a mouse xenograph model (19). Until now, the issue of whether PP5 is involved in other types of gynecological cancer, including ovarian cancer, has been unclear. Therefore, this study was designed to investigate the the biological role of PP5 in human ovarian cancer. Herein, we successfully silenced PPP5C mRNA and protein expression in CAOV-3 ovarian cancer cells using RNA interference (RNAi) technology. Functional analysis revealed that PP5 knockdown significantly inhibited the proliferation and colony formation of CAOV-3 ovarian cancer cells, as well as G0/G1 phase cell cycle arrest. This study provides new evidence that PP5 plays a significant role in ovarian cancer development.
Materials and methods
Cell lines and reagents
The human ovarian mucinous adenocarcinoma cancer cell line CAOV-3 and the human embryonic kidney cell line 293T were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China). The CAOV-3 cell line was cultured in RPMI-1640 (Hyclone, Pittsburgh, PA, USA) supplemented with 10% fetal bovine serum (FBS; Biowest, Nuaillé, France) at 37°C with 5% CO2. The HEK293T cell line was cultured in Dulbecco's modified Eagle's medium (Hyclone) with 10% FBS at 37°C with 5% CO2. Short hairpin RNA (shRNA) expression vector pFH-L and helper plasmids pVSVG-I and pCMVΔR8.92 were purchased from Shanghai Hollybio (Shanghai, China). Lipofectamine 2000 and TRIzol were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). M-MLV reverse transcriptase was purchased from Promega (Madison, WI, USA). AgeI, EcoRI and SYBR-Green master mix kits were purchased from Takara (Dalian, China). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). The antibodies used were as follows: anti-PP5 (1:3,000 dilution; Abcam, Cambridge, UK), anti-GAPDH (1:10,000 dilution; Proteintech Group, Inc., Chicago, IL, USA), and anti-rabbit horseradish peroxidase (HRP; 1:5,000 dilution; Santa Cruz Biotechnology, Inc. Dallas, TX, USA).
Construction of PPP5C shRNA containing lentivirus and transduction into ovarian cancer cells
CAOV-3 cells were transduced with PPP5C shRNA following the manufacturer's instructions. To create a PPP5C shRNA-silenced sub-cell line, we used the following shRNA sequence designed against the PPP5C gene: 5′-GAGACAGAGAAGATTACAGTACTCGAG TACTGTAATCTTCTCTGTCTCTTTTT-3′. The control shRNA sequence was 5′-GCGGAGGGTTTGAAAGAATAT CTCGAGATATTCTTTCAAACCCTCCGCTTTTTT-3′. Each nucleotide sequence was inserted into the pFH-L shRNA expressing vector. Lentiviruses were generated by triple transfection of 80% confluent 293T cells with modified pFH-L plasmid and pVSVG-I and pCMVΔR8.92 helper plasmids using Lipofectamine 2000 according to the manufacturer's instructions. Then the lentiviral particles were harvested by ultra-centrifugation (4,000 × g at 4°C) for 10 min, filtered through a 45-µm filter and centrifuged again (4,000 × g at 4°C) for 15 min.
For cell infection, CAOV-3 cells were seeded in a volume of 2 ml at a density of 5×104 cells/well in six-well plates and transduced with the constructed lentiviruses containing PP5 shRNA (shPPP5C) and non-silencing shRNA (shCon) at a multiplicity of infection of 20. The infection efficiency was observed after 96 h through a fluorescence microscope for the green fluorescence protein expression.
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from cells using TRIzol reagent and synthesized into cDNA by M-MLV reverse transcriptase according to the manufacturer's instructions. qPCR was performed on a Connect real-time PCR platform (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using a SYBR-Green master mix kit. In brief, each PCR reaction mixture containing 10 µl 2X SYBR Premix Ex Taq, 0.8 µl sense and antisense primers (2.5 µM), 5 µl cDNA and 4.2 µl ddH2O was run for 40 cycles with initial denaturation at 95°C for 1 min, denaturation at 95°C for 5 sec and extension at 60°C for 20 sec. β-actin was used as an internal control. Relative gene expression levels were calculated using 2−ΔΔCT analysis. The primers were as follows: PPP5C (forward), 5′-CCCAACTACTGCGACCAGAT-3′; PPP5C (reverse), 5′-CCCGTCACCTCACATCATTC-3′; β-actin (forward), 5′-GTGGACATCCGCAAAGAC-3′; β-actin (reverse), 5′-AAAGGGTGTAACGCAACTA-3′.
Western blot analysis
CAOV-3 cells were collected 7 days after lentivirus infection and lysed in 2X sodium dodecyl sulphate (SDS) sample buffer [100 mM Tris-HCl (pH 6.8), 10 mM EDTA, 4% SDS and 10% glycine). The protein content was measured using the Lowry method. To detect target proteins, equal amounts of protein samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidenedifluoride membranes. The membranes were incubated with Tris-buffered saline and Tween-20 (TBST; 25 mM Tris, pH 7.4, 150 mM NaCl and 0.1% Tween-20) containing 5% nonfat dry milk at room temperature for 1 h. After washing with TBST three times, the membranes were probed with the primary antibody (anti-PP5 rabbit mAb or anti-GAPDH rabbit mAb) at 4°C overnight followed by incubation with goat anti-rabbit IgG, HRP-linked antibody for 1 h at room temperature. The blots were detected with an enhanced chemiluminescence detection kit (Pierce Biotechnology, Inc., Rockford, IL, USA) according to the manufacturer's instructions. GAPDH was used as the reference control.
Cell viability assay
Following lentivirus infection, CAOV-3 cells were seeded in a volume of 200 µl at a density of 4×103 cells/well in 96-well plates. Following incubation for 1, 2, 3, 4 and 5 days, respectively, 20 µl 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5.0 mg/ml) was added into each well and incubated with cells for 4 h. Then 100 µl acidic isopropanol (10% SDS, 5% isopropanol and 0.01 mol/l HCl) was added to each well after removing the medium and MTT from the wells. The absorbance was measured using a microplate reader (BioTek, Winooski, VT, USA) at 595 nm.
Colony formation assay
Following lentivirus infection, CAOV-3 cells were were seeded in a volume of 2 ml at a density of 1.5×103 cells/well in six-well plates. The medium was changed every three days. After nine days of culture, the cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. The fixed cells were stained purple with freshly prepared crystal violet staining (Sigma-Aldrich) for 20 min. The colony formation was observed through a light/fluorescence microscope to obtain the colony numbers.
Cell cycle analysis
The cell cycle distribution was analyzed by flow cytometry using propidium iodide (PI) staining. Following lentivirus infection for 6 days, CAOV-3 cells were seeded in a volume of 5 ml at a density of 2×105 cells/well in 6-cm dishes. Cells were harvested and fixed in 70% ice-cold ethanol for 12 h at 4°C. After washing with PBS three times, cells were stained for DNA content by use of 300 µl PBS containing 50 µg/ml PI and 50 µg/ml preboiled RNase A. The suspension was incubated in the dark at room temperature for 30 min and then subjected to FACSCalibur flow cytometry (BD Biosciences, San Jose, CA, USA). Data were analyzed with the ModFit DNA analysis program (Verity Software House, Maine, USA).
Statistical analysis
Data were presented as the mean ± standard deviation from at least three independent experiments. Statistical analysis was performed using Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Lentivirus-mediated RNAi suppresses PP5 expression in CAOV-3 ovarian cancer cells
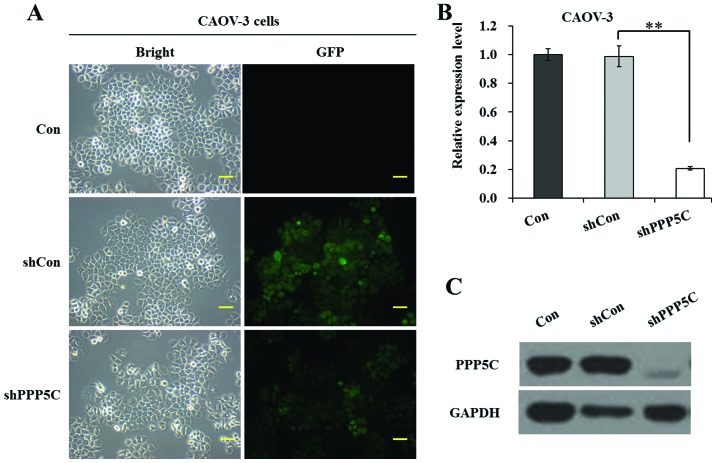
To examine the function of PP5 in ovarian cancer cells, we firstly applied lentivirus-mediated RNAi to specifically suppress PPP5C in the ovarian cancer cell line CAOV-3 long term. As shown in Fig. 1A, the ratio of cells with green fluorescence protein (GFP) expression to shRNA-treated cells was >80%, indicating a satisfactory infection efficiency. As shown in Fig. 1B, the mRNA level of PPP5C was significantly (P<0.01) reduced in shPPP5C-treated cells compared with non-treated and shCon-treated cells. The knockdown efficiency of PP5 was calculated as 79.0% in CAOV-3 cells. Moreover, the protein level of PP5 was significantly downregulated in shPPP5C-treated cells compared with non-treated and shCon-treated cells (Fig. 1C). The data indicated that the lentivirus-mediated shRNA silencing efficiently suppressed the expression of endogenous PP5 in CAOV-3 ovarian cancer cells.
Figure 1.
Effect of lentivirus-mediated short hairpin RNA silencing on protein phosphatase 5 (PP5) expression in CAOV-3 cells. (A) Green fluorescence protein (GFP) expression recorded under a fluorescence microscope. Representative images are from one of three independent experiments. Scale bar, 100 µm. (B) Relative expression levels of PPP5C in non-treated cells (Con), and cells treated with lentiviruses containing non-silencing shRNA (shCon) and PP5 shRNA (shPPP5C) were determined by quantitative polymerase chain reaction analysis. (C) Expression levels of PP5 in non-treated, shCon-treated and shPPP5C-treated cells were determined by western blot analysis. Data are presented as the means ± standard deviation of three independent experiments performed in triplicate. **P<0.01 vs. shCon.
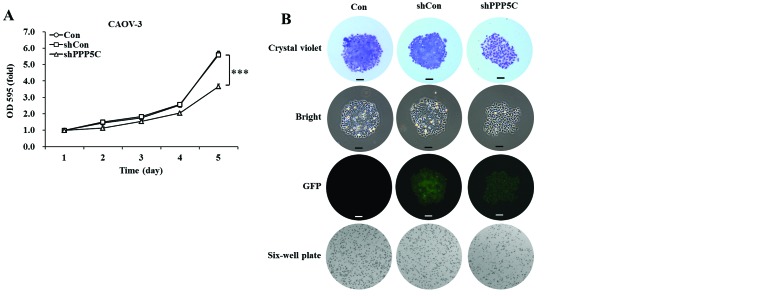
PP5 knockdown inhibits the proliferation and colony formation of CAOV-3 ovarian cancer cells. The effect of PP5 silencing on the proliferation of CAOV-3 cells was determined by MTT assay. The cell viability was observed for 5 days in non-treated, shCon-treated and shPPP5C-treated cells. As shown in Fig. 2A, the growth curve of shPPP5C-treated cells started to drop from the second day, compared with that of non-treated and shCon-treated cells. The decline reached 34.4% (P<0.001) on the fifth day, compared with shCon-treated cells. There was no difference with regard to cell viability between non-treated and shCon-treated cells. The data indicated that PP5 knockdown significantly inhibited the proliferation of CAOV-3 ovarian cancer cells.
Figure 2.
Effects of protein phosphatase 5 (PP5) knockdown on the proliferation and colony formation of CAOV-3 cells. (A) Cell proliferation was measured using 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (B) Size and number of colonies in CAOV-3 cells recorded under a fluorescence microscope. Representative images are from one of three independent experiments. Scale bar, 250 µm. (C) Statistical analysis of colony numbers in non-treated cells (Con), and cells treated with lentiviruses containing non-silencing shRNA (shCon) and PP5 shRNA (shPPP5C). Data are presented as the means ± standard deviation of three independent experiments performed in triplicate. ***P<0.001 vs. shCon. GFP, green fluorescence protein.
The long-term effect of PP5 silencing on the colony forming ability of CAOV-3 cells was determined by colony formation assay. As shown in Fig. 2B, the size of independent colonies was much smaller in shPPP5C-treated cells compared with non-treated and shCon-treated cells. Moreover, the number of colonies formed in CAOV-3 cells was significantly (P<0.001) decreased following PP5 knockdown (Fig. 2C). The data indicated that PP5 knockdown also significantly inhibited the colony formation of CAOV-3 ovarian cancer cells.
PP5 knockdown arrests CAOV-3 cells at the G0/G1 phase
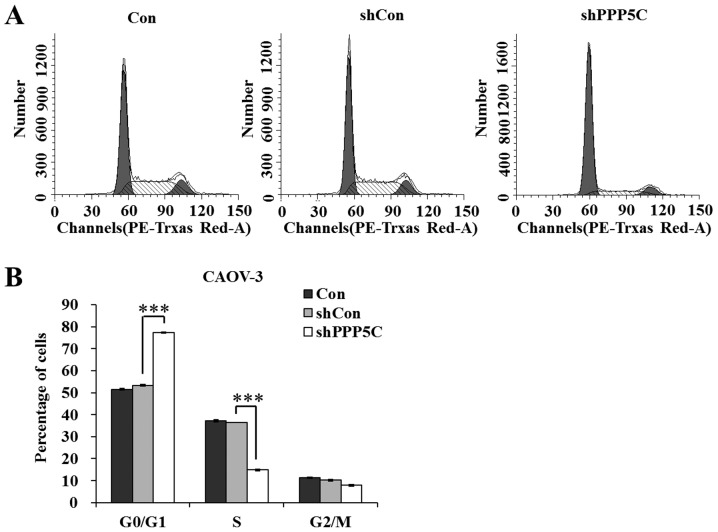
To investigate the mechanisms underlying the growth suppression effect of PP5 knockdown, the cell cycle distribution of CAOV-3 cells was analyzed using a flow cytometer. The results shown in Fig. 3 indicate that shPPP5C-treated cells presented an increased G0/G1-phase population and a decreased S-phase population (P<0.001), compared with non-treated and shCon-treated cells. The data revealed that PP5 knockdown arrested the cell cycle at the G0/G1 phase. Taken together, we suggest that PP5 knockdown suppresses ovarian cancer cell growth via the blockade of cell cycle progression.
Figure 3.
Effect of protein phosphatase 5 (PP5) knockdown on cell cycle of CAOV-3 cells. (A) Flow cytometric analysis of cell cycle. Representative images are from one of three independent experiments. (B) Statistical analysis of G0/G1-phase, S-phase and G2/M-phase populations in non-treated cells (Con), and cells treated with lentiviruses containing non-silencing shRNA (shCon) and PP5 shRNA (shPPP5C). Data are presented as the means ± standard deviation of three independent experiments performed in triplicate. ***P<0.001 vs. shCon.
Discussion
Ovarian cancer is the leading cause of mortality from gynecological cancers and the fifth most common cause of cancer mortality among females (20). An increased understanding of the molecular and genetic changes causing ovarian cancer progression is likely to produce strategies to predict and prevent the occurrence of refractory disease. Targeted therapy is a type of medication that blocks the growth of cancer cells by interfering with the specific molecules needed for carcinogenesis and tumor growth, rather than interfering with all rapidly dividing cells. In this study, PP5 was identified as a specific molecule that drives ovarian cancer progression. Using lentivirus-mediated shRNA silencing, we potently suppressed the expression of PPP5C at the mRNA and protein levels in CAOV-3 ovarian cancer cells. PP5 knockdown significantly inhibited the proliferation and colony formation of CAOV-3 cells.
A previous study has reported that treatment of cells with PP5 antisense RNA leads to hyperphosphorylation of p53 and subsequent G1 growth arrest (21). Herein, PI staining combined with flow cytometry analysis was performed to determine whether PP5 knockdown using lentivirus-mediated RNAi blocks cell cycle progression in CAOV-3 cells. As expected, PP5 knockdown significantly arrested CAOV-3 cells at the G0/G1 phase, which was in accordance with the growth suppression effect of PP5 knockdown.
Finally, it is known that PP5 plays a crucial role in DNA damage repair and cell cycle arrest by attenuating the activities of ATM and ATR, two closely related checkpoint kinases. A more recent study utilizing cells from PP5-deficient mice has confirmed the role of PP5 in ATM signaling (22). Thus, further study is desired to examine whether PP5 attenuates the checkpoint kinases ATM and ATR in ovarian cancer cells. Furthermore, PP5 has been observed to act as a suppressor of apoptosis signal-regulating kinase 1 (23,24), p53 (25) and DNA-dependent protein kinase catalytic subunits (26). It is likely that PP5 promotes ovarian cancer cell growth by inducing cell cycle arrest, as well as cell apoptosis. Therefore, the effect of PP5 knockdown on the apoptosis of ovarian cancer cells should also be determined in a subsequent study.
In conclusion, this study highlights the crucial role of PP5 in promoting ovarian cancer cell proliferation and demonstrates that silencing PP5 with targeted shRNA delivered to tumor cells via lentivirus is an effective method to reduce PP5 activity in the long term. These results provide a foundation for further study into the clinical potential of lentiviral-mediated delivery of PP5 RNAi therapy for the treatment of ovarian cancer.
Acknowledgements
The authors are grateful for the support of the Natural Science Foundation of Ning Bo (201301A6110080 and 201301A6110087).
Glossary
Abbreviations
- PP5
protein phosphatase 5
- RNAi
RNA interference
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PI
propidium iodide
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Yu Y, Zhang M, Zhang X, Cai Q, Hong S, Jiang W, Xu C. Synergistic effects of combined platelet-activating factor receptor and epidermal growth factor receptor targeting in ovarian cancer cells. J Hematol Oncol. 2014;7:39. doi: 10.1186/1756-8722-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalmers MJ, Kolch W, Emmett MR, Marshall AG, Mischak H. Identification and analysis of phosphopeptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;803:111–120. doi: 10.1016/j.jchromb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Ballif BA, Roux PP, Gerber SA, MacKeigan JP, Blenis J, Gygi SP. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc Natl Acad Sci USA. 2005;102:667–672. doi: 10.1073/pnas.0409143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA. 2006;103:7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang F, Stenoien DL, Strittmatter EF, Wang J, Ding L, Lipton MS, Monroe ME, Nicora CD, Gristenko MA, Tang K, et al. Phosphoproteome profiling of human skin fibroblast cells in response to low- and high-dose irradiation. J Proteome Res. 2006;5:1252–1260. doi: 10.1021/pr060028v. [DOI] [PubMed] [Google Scholar]
- 8.Ham BM, Jayachandran H, Yang F, Jaitly N, Polpitiya AD, Monroe ME, Wang L, Zhao R, Purvine SO, Livesay EA, et al. Novel Ser/Thr protein phosphatase 5 (PP5) regulated targets during DNA damage identified by proteomics analysis. J Proteome Res. 2010;9:945–953. doi: 10.1021/pr9008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi F, Umeda Y, Shimamoto S, Tsuchiya M, Tokumitsu H, Tokuda M, Kobayashi R. S100 proteins modulate protein phosphatase 5 function: a link between CA2+ signal transduction and protein dephosphorylation. J Biol Chem. 2012;287:13787–13798. doi: 10.1074/jbc.M111.329771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen MX, McPartlin AE, Brown L, Chen YH, Barker HM, Cohen PT. A novel human protein serine/threonine phosphatase, which possesses four tetratricopeptide repeat motifs and localizes to the nucleus. EMBO J. 1994;13:4278–4290. doi: 10.1002/j.1460-2075.1994.tb06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connarn JN, Assimon VA, Reed RA, Tse E, Southworth DR, Zuiderweg ER, Gestwicki JE, Sun D. The molecular chaperone Hsp70 activates protein phosphatase 5 (PP5) by binding the tetratricopeptide repeat (TPR) domain. J Biol Chem. 2014;289:2908–2917. doi: 10.1074/jbc.M113.519421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Kriegsheim A, Pitt A, Grindlay GJ, Kolch W, Dhillon AS. Regulation of the Raf-MEK-ERK pathway by protein phosphatase 5. Nat Cell Biol. 2006;8:1011–1016. doi: 10.1038/ncb1465. [DOI] [PubMed] [Google Scholar]
- 13.Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon I, Honkanen RE. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–8857. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]
- 14.Zuo Z, Dean NM, Honkanen RE. Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21(WAF1/Cip1) and mediate growth arrest. J Biol Chem. 1998;273:12250–12258. doi: 10.1074/jbc.273.20.12250. [DOI] [PubMed] [Google Scholar]
- 15.Chinkers M. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc Natl Acad Sci USA. 1994;91:11075–11079. doi: 10.1073/pnas.91.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- 17.Davies TH, Ning YM, Sánchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- 18.Urban G, Golden T, Aragon IV, Scammell JG, Dean NM, Honkanen RE. Identification of an estrogen-inducible phosphatase (PP5) that converts MCF-7 human breast carcinoma cells into an estrogen-independent phenotype when expressed constitutively. J Biol Chem. 2001;276:27638–27646. doi: 10.1074/jbc.M103512200. [DOI] [PubMed] [Google Scholar]
- 19.Golden T, Aragon IV, Zhou G, Cooper SR, Dean NM, Honkanen RE. Constitutive over expression of serine/threonine protein phosphatase 5 (PP5) augments estrogen-dependent tumor growth in mice. Cancer Lett. 2004;215:95–100. doi: 10.1016/j.canlet.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Ali MW, Cacan E, Liu Y, Pierce JY, Creasman WT, Murph MM, Govindarajan R, Eblen ST, Greer SF, Hooks SB. Transcriptional suppression, DNA methylation and histone deacetylation of the regulator of G-protein signaling 10 (RGS10) gene in ovarian cancer cells. PloS one. 2013;8:e60185. doi: 10.1371/journal.pone.0060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinkers M. Protein phosphatase 5 in signal transduction. Trends Endocrinol Metab. 2001;12:28–32. doi: 10.1016/S1043-2760(00)00335-0. [DOI] [PubMed] [Google Scholar]
- 22.Yong W, Bao S, Chen H, Li D, Sánchez ER, Shou W. Mice lacking protein phosphatase 5 are defective in ataxia telangiectasia mutated (ATM)-mediated cell cycle arrest. J Biol Chem. 2007;282:14690–14694. doi: 10.1074/jbc.C700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou G, Golden T, Aragon IV, Honkanen RE. Ser/Thr protein phosphatase 5 inactivates hypoxia-induced activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK signaling cascade. J Biol Chem. 2004;279:46595–46605. doi: 10.1074/jbc.M408320200. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, Houghton PJ. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 25.Urban G, Golden T, Aragon IV, Cowsert L, Cooper SR, Dean NM, Honkanen RE. Identification of a functional link for the p53 tumor suppressor protein in dexamethasone-induced growth suppression. J Biol Chem. 2003;278:9747–9753. doi: 10.1074/jbc.M210993200. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler T, Chen BP, Harper R, Morotomi-Yano K, Huang BC, Meek K, Cleaver JE, Chen DJ, Wabl M. DNA-PKcs function regulated specifically by protein phosphatase 5. Proc Natl Acad Sci USA. 2004;101:1247–1252. doi: 10.1073/pnas.0307765100. [DOI] [PMC free article] [PubMed] [Google Scholar]