Abstract
AIM: To define the prevalence of gastroesophageal reflux disease (GERD) in mild persistent asthma and to value the effect of pantoprazole therapy on asthmatic symptoms.
METHODS: Seven of thirty-four asthmatic patients without GERD served as the non-GERD control group. Twenty-seven of thirty-four asthmatic patients had GERD (7/27 also had erosive esophagitis, sixteen of them presented GERD symptoms. An upper gastrointestinal endoscopy was performed in all the subjects to obtain five biopsy specimens from the lower 5 cm of the esophagus. Patients were considered to have GERD when they had a dilation of intercellular space (DIS)>0.74 μm at transmission electron microscopy. Patients with GERD were treated with pantoprazole, 80 mg/day. Forced expiratory volume in one second (FEV1) was performed at entry and after 6 mo of treatment. Asthmatic symptoms were recorded. The required frequency of inhaling rapid acting β2-agonists was self-recorded in the patients’ diaries.
RESULTS: Seven symptomatic patients presented erosive esophagitis. Among the 18 asymptomatic patients, 11 presented DIS, while all symptomatic patients showed ultrastructural esophageal damage. Seven asymptomatic patients did not present DIS. At entry the mean of FEV1 was 1.91 L in symptomatic GERD patients and 1.88 L in asymptomatic GERD patients. After the treatment, 25 patients had a complete recovery of DIS and reflux symptoms. Twenty-three patients presented a regression of asthmatic symptoms with normalization of FEV1. Four patients reported a significant improvement of symptoms and their FEV1 was over 80%.
CONCLUSION: GERD is a highly prevalent condition in asthma patients. Treatment with pantoprazole (80 mg/day) determines their improvement and complete regression.
Keywords: Asthma, Gastroesophageal reflux disease, Pantoprazole, NERD, ERD, Dilated intercellular spaces, TEM
INTRODUCTION
The association of asthma with GERD has attracted particular attention because about half of the patients with asthma also have GERD[1-3]. Mechanisms by which esophageal reflux triggers asthma include acid aspiration, direct acid stimulation of the esophagus, or stimulation of vagal nerves which heightens bronchial responsiveness to extrinsic allergens. Clinicians are advised to treat GERD to improve and control asthma[1]. Endoscopic findings of the esophageal erosions (ERD) confirm the diagnosis of GERD. However, absence of macroscopic signs of damage does not rule out an endoscopy negative esophagitis (NERD) that may also be associated with asthma[4]. Recently, we have shown the presence of a highly sensitive and specific marker of damage, the dilation of intercellular spaces (DIS) in GERD with or without erosions, which permits us to define NERD with a strong accuracy[5].
The prevalence of GERD and effects of proton pump inhibitor (PPI) treatment on the decors of asthma are still uncertain and results obtained are often conflicting[6,7]. Bias in the selection of asthma patients or during PPI treatment and absence of highly sensitive parameters of morphology to define the presence of esophageal mucosa damage, may affect the reported results of studies. Moreover, several studies have a non-randomized poor quality design which leads to a further potential error on definition of treatment effects[8,9].
For this reason, the aim of the present study was to define the prevalence of GERD in patients with mild persistent intrinsic asthma and to estimate the effect of pantoprazole in relation with GERD, asthmatic symptoms and respiratory function in this subset of patients.
MATERIALS AND METHODS
Among the 301 asthma patients, 34 consecutive asthma patients with intrinsic, mild persistent asthma[10] were enrolled and their diagnosis was made according to the diagnostic criteria recommended by American Thoracic Society (ATS). Patients were excluded if they had any of the following: past or present smoker, unequivocally extrinsic and/or occupational asthma, acids suppression therapy within 4 wk prior to recruitment, previous gastroesophageal surgery, professional voice users, previous glottal surgery or radiotherapy or malignancy, immune suppression therapy, age above 50 years.
For evaluation of ventilation function, FEV1 was performed using the Jaeger Masterlab spirometer based on the guidelines of ATS[14]. Symptoms were recorded in each patient to rate the frequency and severity of asthmatic episodes. Symptom severity was rated on a scale of 0 (none) to 6 (severe). The required frequency of inhaling rapid acting β2-agonists was self-recorded in the patients’ diaries. All the patients did not use systemic bronchodilators or corticosteroids.
For the diagnosis of GERD, the patients underwent gastrointestinal endoscopic (GE) examination and interviews according to the QUEST questionnaire[11]. Reflux esophagitis was graded according to the Los Angeles classification[12,13]. During endoscopy, five biopsy specimens were taken from the lower 5 cm of esophagus for ultrastructural evaluation. At transmission electron microscopy (TEM), ultrastructural signs of mucosal damage were considered to be the DIS>0.74 µm[5]. To obtain this measure, 10 photomicrographs of biopsy specimens of the supra-basal layer of the esophageal mucosa from each patient were taken. At least 10 randomly selected perpendicular trans-sections to adjacent membranes were drawn and measured in each image for a total of 100 measurements in each case. Measurements obtained were used to calculate the mean DIS score.
The endoscopists, pneumologists and pathologists were unaware of the clinical history of the patients. Data were collected separately by another physician (DZ) who assigned patients to different groups and established the therapy. Symptomatic and asymptomatic patients with or without endoscopic signs, were considered to have GERD when they had ultrastructural evidence of esophageal damage. Patients so defined with GERD were treated with pantoprazole, 80 mg once daily for 6 mo. After 6 mo, a new endoscopy with biopsies was performed and DIS was evaluated. Improvements in GERD symptoms according to the QUEST questionnaire were recorded. FEV1 was valued.
Asthma patients without GERD were followed up for 6 mo and the use of anti-asthmatic treatment (inhaled glucocorticosteroid 200–1 000 µg BDP or rapid acting β2-agonists) and symptoms were recorded. We considered them as the control group for the evaluation of asthmatic symptoms, respiratory function and drug assumption with time.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki. The local research ethical committee approved the study protocol in 2002. The objective of the study was explained to each patient and written informed consent was obtained from each one.
For statistical analysis, one-way analysis of variance, paired and unpaired t test were performed. The results of the treatment were compared by χ2 test or Fisher’s exact test. All statistical analyses were two-tailed. Data were analyzed with SPSS software. P<0.05 was considered statistically significant.
RESULTS
Among the 34 patients evaluated, 16 presented GERD symptoms (heartburn and/or acid regurgitation) and 18 were asymptomatic for reflux disease. At endoscopy, all asymptomatic subjects had no macroscopic signs of esophagitis. Seven symptomatic patients (43.75%) presented erosive esophagitis (ERD).
Among the 18 asymptomatic patients, 11 (61%) presented DIS, while all symptomatic patients showed ultrastructural esophageal damage. Seven asymptomatic patients (38.9%) did not present DIS (Table 1). At entry the mean of FEV1 was 1.91 L in symptomatic GERD patients and 1.88 L in asymptomatic GERD patients. The two groups at baseline were not significantly different (P = NS) (Table 2).
Table 1.
Demographic data of 34 asthmatic patients with or without GERD symptoms
| Symptomatic |
Asymptomatic |
||
| GERD | Non-GERD | ||
| Number | 16 | 11 | 7 |
| Male/female | 6/10 | 5/6 | 4/3 |
| ERD | 7 | 0 | 0 |
| NERD | 9 | 11 | 7 |
| Age (mean±SD) (yrs) | 33.75±10 | 38.27±5.68 | 36.14±8.76 |
| DIS (mean±SD) (µm) | 2.105±0.262 | 2.08±0.24 | 0.5±0.08 |
| FEV1 (mean±SD) (L) | 1.87±0.05 | 1.95±0.04 | 1.88±0.02 |
Table 2.
Comparison of background characteristics of asthmatic patients with GERD and controls
| GERD (27) | Controls (7) | t-Test | |
| Age (yrs) (mean±SD) | 35.59±8.67 | 36.14±8.76 | NS |
| Male/female | 11/16 | 4/3 | NS |
| QUEST score | 8.8±4.5 | 0.12±0.8 | P<0.0001 |
| Erosive esophagitis | 7 (4B, 2C, 1D) | 0 | P<0.05 |
| DIS (mean±SD) (µm) | 2.09±0.24 | 0.5±0.08 | P<0.001 |
| FEV1 (L) | 1.91±0.045 | 1.88±0.02 | NS |
All the 27 patients with GERD (79.4%) completed the study. They were treated for 6 mo with pantoprazole. At the end of this period erosive esophagitis was healed in seven patients with ERD. Among the 25 patients (92.6%), 6 with ERD and 19 with NERD, had a complete recovery of DIS and reflux symptoms (Table 3 and Figure 1A).
Table 3.
Effect of treatment on dilation of intercellular space (DIS) in 25 responders to pantoprazole (mean±SD)
| Value (μm) | t-Test | ||
| Baseline | 2.105±0.261 | ||
| Symptomatic | P<0.001 | ||
| After therapy | 0.592±0.194 | ||
| Baseline | 2.081±0.242 | ||
| Asymptomatic | P<0.001 | ||
| After therapy | 0.517±0.072 |
Figure 1.
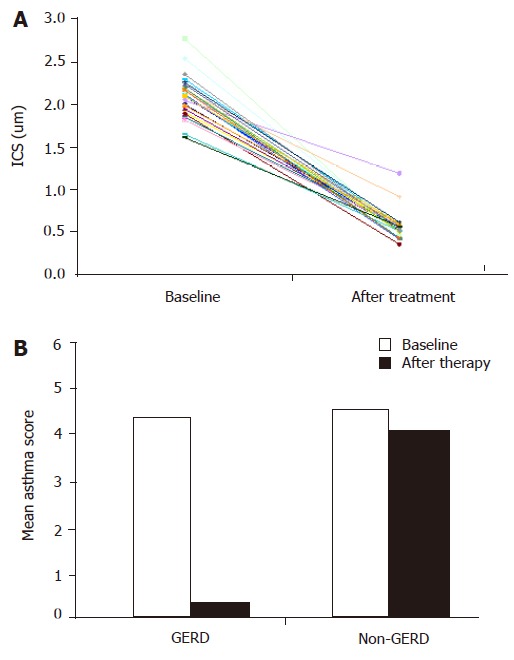
Effect of pantoprazole treatment on dilation of DIS (A) and asthma symptom scores (B) in GERD patients. The dashed line in Figure 1A corresponds to the mean score of DIS; its cut-off (0.74 μm) represents the presence of damage. Graphic is based on the data analyzed as mean value.
Twenty-three patients (85.18%) presented a regression of asthmatic symptoms (Table 4 and Figure 1B), including nocturnal asthma and FEV1 (2.75 L, P<0.01), but there was no statistical difference in respiratory parameters between patients with ERD and those with NERD. After 3 wk of treatment no more asthmatic symptoms occurred and no inhaler was needed.
Table 4.
Effect of therapy on asthma symptom score in GERD patients (mean±SD)
| Baseline | After therapy | t-test | ||
| GERD | 4.37±0.97 | 0.33±0.83 | P<0.001 | |
| Patients | ||||
| Controls | 4.57±0.79 | 4.14±0.38 | P = NS |
Four patients (14.8%) reported a significant improvement in symptoms, including dyspnea, cough, wheeze, and expectoration with a significant reduction in the consumption of inhalers and FEV1 over 80%.
DISCUSSION
Physicians mostly focus on the effects induced by gastroesophageal reflux into the esophagus. The anatomic proximity of the esophagus to the respiratory tract in some patients also leads to his involvement. Thus, the spectrum of symptoms of GERD includes also respiratory complications like chronic cough, laryngeal disorders, chest pain, and asthma. In this contest, GERD gains an additional fraction of significance and has been reported to occur in 30-89% of asthma patients[13,15-18].
Treatment with PPI can relieve the symptoms of GERD in up to 90% of patients. It has been tempted to investigate if treatment of GERD with PPI could improve also the respiratory symptoms of asthma patients[19].
It seems obvious that to achieve improvement in asthma symptoms and lung function, the treatment needs to be effective in controlling reflux as well as reducing acidity. However, this has been only objectively confirmed in few studies using high doses of omeprazole[19]. An optimal study design would establish if the esophageal damage is adequately treated as a prerequisite to assess the effects on asthma.
In this study, we analyzed the esophageal specimens from 31 consecutive asthmatic patients, 16 of them were symptomatic for GERD. We found that all symptomatic patients presented an ultrastructural pattern of damage and 73% of asymptomatic GERD patients had esophageal signs of ultrastructural damage, suggesting that GERD is a highly prevalent condition even in asymptomatic patients. Acid stress might initiate or exacerbate asthma in this contest. Electron microscopy is able to find out esophageal damage, especially in asymptomatic patients with NERD.
All patients with GERD so defined in our study were treated with a high dosage of pantoprazole for 6 mo. We observed that 93% of patients recovered DIS, 85% of them presented a regression of asthmatic symptoms with normalization of FEV1, with no statistical difference in respiratory parameters between patients with ERD or NERD. Other patients improved markedly their symptoms and pulmonary function but sporadically presented asthmatic episodes.
We believe that the strong efficacy of PPI treatment in these patients is related to the characteristics of the carefully defined population. The young age of subjects and the mild-moderate asthma let us suppose the existence of an early “action” of treatment before the chronicity of the illness or the worsening to a severe status of asthma. In other words, PPI can suppress or delay the potential development of pulmonary tissue injury.
In conclusion, GERD-related asthma complications are highly prevalent in patients with mild-moderate intrinsic asthma. Treatment with pantoprazole (80 mg once daily for 6 mo) determines the improvement and complete regression of asthmatic symptoms and respiratory function in most patients.
Footnotes
Supported by grants from Altana-Pharma Italia
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest. 1997;111:1389–1402. doi: 10.1378/chest.111.5.1389. [DOI] [PubMed] [Google Scholar]
- 2.Field SK, Sutherland LR. Does medical antireflux therapy improve asthma in asthmatics with gastroesophageal reflux?: a critical review of the literature. Chest. 1998;114:275–283. doi: 10.1378/chest.114.1.275. [DOI] [PubMed] [Google Scholar]
- 3.Harding SM, Guzzo MR, Richter JE. 24-h esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest. 1999;115:654–659 DOI : 10.1378/chest.115.3.654. doi: 10.1378/chest.115.3.654. [DOI] [PubMed] [Google Scholar]
- 4.Jaspersen D, Kulig M, Labenz J, Leodolter A, Lind T, Meyer-Sabellek W, Vieth M, Willich SN, Lindner D, Stolte M, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther. 2003;17:1515–1520 DOI : 10.1046/j.1365-2036.2003.01606.x. doi: 10.1046/j.1365-2036.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, Scialpi C, Miglioli M, Di Febo G. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525–532 DOI : 10.1046/j.1365-2036.2003.01713.x. doi: 10.1046/j.1365-2036.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 6.Harding SM, Sontag SJ. Asthma and gastroesophageal reflux. Am J Gastroenterol. 2000;95:S23–S32 DOI : 10.1016/S0002-9270(00)01075-3. doi: 10.1016/s0002-9270(00)01075-3. [DOI] [PubMed] [Google Scholar]
- 7.Hogan WJ, Shaker R. Medical treatment of supraesophageal complications of gastroesophageal reflux disease. Am J Med. 2001;111 Suppl 8A:197S–201S. doi: 10.1016/s0002-9343(01)00830-0. [DOI] [PubMed] [Google Scholar]
- 8.Levin TR, Sperling RM, McQuaid KR. Omeprazole improves peak expiratory flow rate and quality of life in asthmatics with gastroesophageal reflux. Am J Gastroenterol. 1998;93:1060–1063. doi: 10.1111/j.1572-0241.1998.329_q.x. [DOI] [PubMed] [Google Scholar]
- 9.Teichtahl H, Kronborg IJ, Yeomans ND, Robinson P. Adult asthma and gastro-oesophageal reflux: the effects of omeprazole therapy on asthma. Aust N Z J Med. 1996;26:671–676. doi: 10.1111/j.1445-5994.1996.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 10.National Institute of Health NHLBI. Burden of asthma. In: Global Strategy for Asthma Management and Prevention. NIH Publication; 2002. pp. 11–27. [Google Scholar]
- 11.Carlsson R, Dent J, Bolling-Sternevald E, Johnsson F, Junghard O, Lauritsen K, Riley S, Lundell L. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023–1029. doi: 10.1080/003655298750026697. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 13.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 15.Buts JP, Barudi C, Moulin D, Claus D, Cornu G, Otte JB. Prevalence and treatment of silent gastro-oesophageal reflux in children with recurrent respiratory disorders. Eur J Pediatr. 1986;145:396–400. doi: 10.1007/BF00439246. [DOI] [PubMed] [Google Scholar]
- 16.Ducoloné A, Vandevenne A, Jouin H, Grob JC, Coumaros D, Meyer C, Burghard G, Methlin G, Hollender L. Gastroesophageal reflux in patients with asthma and chronic bronchitis. Am Rev Respir Dis. 1987;135:327–332. doi: 10.1164/arrd.1987.135.2.327. [DOI] [PubMed] [Google Scholar]
- 17.Perrin-Fayolle M, Bel A, Braillon G, Lombard-Platet R, Kofman J, Harf R, Montagnon B, Pacheco Y, Perpoint B. [Asthma and gastro-esophagal reflux (GER). Results of surgical treatment of reflux in 50 patients (author's transl)] Poumon Coeur. 1980;36:231–237. [PubMed] [Google Scholar]
- 18.Sontag SJ, O'Connell S, Miller TQ, Bernsen M, Seidel J. Asthmatics have more nocturnal gasping and reflux symptoms than nonasthmatics, and they are related to bedtime eating. Am J Gastroenterol. 2004;99:789–796. doi: 10.1111/j.1572-0241.2004.04141.x. [DOI] [PubMed] [Google Scholar]
- 19.Sontag SJ, O'Connell S, Khandelwal S, Miller T, Nemchausky B, Schnell TG, Serlovsky R. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology. 1990;99:613–620. doi: 10.1016/0016-5085(90)90945-w. [DOI] [PubMed] [Google Scholar]
- 20.Kiljander TO. The role of proton pump inhibitors in the management of gastroesophageal reflux disease-related asthma and chronic cough. Am J Med. 2003;115 Suppl 3A:65S–71S. doi: 10.1016/s0002-9343(03)00196-7. [DOI] [PubMed] [Google Scholar]
- 21.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798–1810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]


