Abstract
AIM: To study the morphological and serum hyaluronic acid (HA), laminin (LN), and type IV collagen changes in hepatic fibrosis of rats induced by dimethylnitrosamine (DMN).
METHODS: The rat model of liver fibrosis was induced by DMN. Serum HA, type IV collagen, and LN were measured by ELISA. The liver/weight index and morphological changes were examined under electron microscope on d 7, 14, 21, and 28 by immunohistochemical alpha smooth muscle actin α-SMA staining as well as Sirius-red and HE staining.
RESULTS: The levels of serum HA, type IV collagen and LN significantly increased from d 7 to d 28 (P = 0.043). The liver/weight index increased on d 7 and decreased on d 28. In the model group, the rat liver stained with HE and Sirius-red showed evident hemorrhage and necrosis in the central vein of hepatic 10 lobules on d 7. Thin fibrotic septa were formed joining central areas of the liver on d 14. The number of α-SMA positive cells was markedly increased in the model group. Transitional hepatic stellate cells were observed under electron microscope. All rats in the model group showed micronodular fibrosis in the hepatic parenchyma and a network of α-SMA positive cells. Typical myofibroblasts were embedded in the core of a fibrous septum. Compared to the control group, the area-density percentage of collagen fibrosis and pathologic grading were significantly different in the model group (P<0.05) on different d (7, 14, and 28). The area-density percentage of collagen fibrosis in hepatic tissue had a positive correlation with the levels of serum HA, LN, and type IV collagen.
CONCLUSION: The morphological and serum HA, type IV collagen, and LN are changed in DMN-induced liver fibrosis in rats.
Keywords: Rat, Hepatic fibrosis, DMN, Morphological change, Serum, Experimental studies
INTRODUCTION
In China, the incidence of liver cirrhosis is still high[1]. Hepatic cirrhosis results from fibrosis[2-4]. Many factors can lead to chronic liver disease and hepatic fibrosis[5-9]. Hepatic fibrosis is associated with a number of morphological and biochemical changes leading to structural and metabolic abnormalities in the liver. Hepatic stellate cells (HSCs) play a major role in various types of liver fibrosis through initial myofibroblast transformation. Transformed HSCs can actively synthesize extracellular matrix and then change morphology and function of the liver. Dimethylnitrosamine (DMN)-induced hepatic fibrosis in rats appears to be a good and reproducible model with decompensating features of human disease[10,11]. This study was to observe the morphological and serum hyaluronic acid (HA), laminin (LN), and type IV collagen changes in DMN-induced hepatic fibrosis of rats.
MATERIALS AND METHODS
Animals and experiment protocol
Male Wistar rats weighing 175-200 g were obtained from the Experimental Animal Center of Yanbian University College of Medicine. The rats were divided into two groups. The model group (n = 40) received 1% DMN (10 μL/kg body weight, i.p.) thrice a week for 4 wk, while the control group (n = 12) received an equivalent amount of saline. The animals were killed on d 7, 14, 21, and 28 (10 treated with DMN at each time interval). Blood was taken from the left ventricle. Serum samples obtained from all study subjects were frozen at -70 °C and aliquots were thawed when needed for specific tests. The liver was examined by light and electron microscopy.
Serum HA, type IV collagen, and LN levels testing
A quantitative ELISA was used to determine serum HA, type IV collagen, and LN levels according to the manufacturer’s instructions.
Sirius-red and HE staining
Formalin-fixed tissues were paraffin-embedded and cut into 4-μm thick sections from the right lobe of rat’s liver for HE and Sirius-red staining. HE staining was used to observe liver pathologic structures, Sirius-red staining was used to grade liver fibrosis from 0 to 4[16]: grade 0 = no fibrosis, grade 1 = portal area fibrosis, grade 2 = fibrotic septa between portal tracts, grade 3 = fibrosis septa and structure disturbance of hepatic lobule, grade 4 = cirrhosis. At the same time, Sirius-red staining and CMIAS image analysis system (Beijing, China) were used to determine the area-density percentage of collagen fibrosis in hepatic tissue. At least five high-power (×400 field) fields were chosen and positive collagen fibrosis (red staining) was determined. Area-density percentage of collagen fibrosis was calculated by dividing the number of positive collagen fibroses (positive optical density) over the total number of collagen fibroses (integrated optical density).
Immunohistochemical staining
α-SMA for the detection of activated HSCs was studied by immunohistochemical staining. Sections (5-μm) were deparaffinized, rehydrated and incubated with 0.3% hydrogen peroxidase in methanol for 15 min at room temperature to block endogenous peroxidase activity. After being washed twice with phosphate-buffered saline (PBS) for 5 min, tissue sections were incubated at 37 °C for 20 min with blocking solution, then incubated at 37 °C for 2 h with rabbit anti-rat α-SMA antibody (Dako, Denmark) at dilution 1:100. After being washed twice with PBS (0.01 mol/L, PH 7.4) for 10 min, tissue sections were incubated at 37 °C for 30 min with biotin-anti-rabbit IgG. After being washed twice with PBS for 5 min, the sections were incubated with streptavidin-HRP for 30 min. Then the sections were washed twice with PBS for 5 min and incubated with metal-enhanced 3, 3-diaminobenzidine solution for 15 min, washed twice in distilled waster and counterstained with hematoxylin. Negative control sections were incubated with normal rabbit serum instead of primary antibody. The positive staining for α-SMA positive cells was expressed as red brown granules and photomicrographed (Olympus PM-10AD).
Electron microscopy
Fresh fragments of 1 mm3 liver tissue were fixed in 10% paraffin, dehydrated and embedded in Epon-812 resin. Sections were stained with uranyl acetate for 15 min and then lead citrate for 15 min. Transitional HSCs were observed under JEM-1200EX, 80 kV electron microscope (JEOL, Japan).
Statistical analysis
Data were expressed as mean±SD. The two-tailed χ2 test was used to examine the correlation between the area-density percentage of collagen fibrosis in hepatic tissue and serum HA, LN, and type IV collagen levels. Statistical significance was estimated by t-test. P<0.05 was considered statistically significant. All calculations were made by SPSS 11.0 for Windows.
RESULTS
Serum HA, type IV collagen, and LN levels change
When compared to control values, a significant increase (P<0.05) was observed in serum levels of HA, LN, and type IV collagen on d 7, 14, 21, and 28 after administration of DMN. The maximum increase in serum levels of HA, LN, and type IV collagen was observed on d 28 after DMN treatment (Figure 1).
Figure 1.
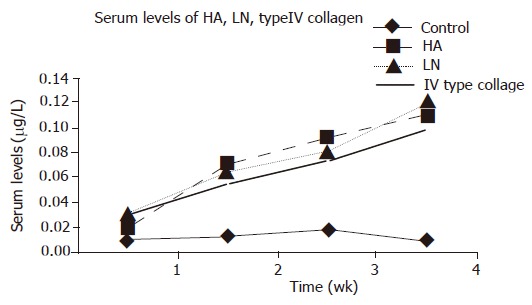
Serum levels of HA, LN, and Type IV collagen in DMN-induced fibrosis of rats and control, P<0.05 vs control.
Change in the weight of body and liver as well as liver and body ratio
An increase in liver weight was observed on d 7 after DMN treatment, with a decreased liver weight on d 28. The maximum liver and body weight ratio increased on d 14 and decreased on d 28 (Table 1).
Table 1.
Changes in the weight of body and liver as well as liver/body ratio of rats during DMN treatment (mean±SD)
| Group | n | Body weight | Liver weight | Liver/body ratio |
| Control | 10 (0) | 192.50±9.0446 | 6.2260±0.3848 | 3.2191±0.2829 |
| Day 7 | 6 (4) | 207.85±5.3293 | 7.8114±0.3869 | 3.7492±0.1195 |
| Day 14 | 9 (1) | 167.27±11.9157a | 6.9164±0.6229a | 4.1059±0.1636a,c |
| Day 21 | 9 (1) | 181.11±13.3536a | 6.5122±0.6200a | 3.5671±0.1437a |
| Day 28 | 10 (0) | 186.42±10.7301a | 6.3657±0.5306a | 3.3903±0.1096a,c |
aP<0.05 vs control; cP<0.05 vs day 7; ( ): number of deaths.
Changes after DMN treatment
The rat liver stained with Sirius-red and HE showed an extensive accumulation of collagens. Fibrotic septum increased from port to port and from port to central vein in some parts of lobules. The livers of the rats in the control group showed normal lobular architecture with central veins and radiating hepatic cords (Figure 2A). After 7 d of DMN treatment, extensive necrosis occurred in portal area and hemorrhage was prominent (Figure 2B). After 14 d, hemorrhagic necrosis and formation of thin fibrotic septa joining central areas were found (Figure 2C). After 21 d, thick intralobular septa were evident (Figure 2D). After 28 d, the pattern was similar with that after 21 d. At the same time, fibrotic septum increased the area-density percentage of collagen fibrosis in hepatic fibrosis. Compared to control values, a significant increase (P<0.05) was observed in area-density percentage of collagen fibrosis on d 7, 14, 21, and 28 after administration of DMN. The maximum increase in the levels of the area-density percentage of collagen fibrosis was observed on d 28 after DMN treatment (Figure 3). Liver fibrosis was graded from 0 to 4 (Table 2)
Figure 2.
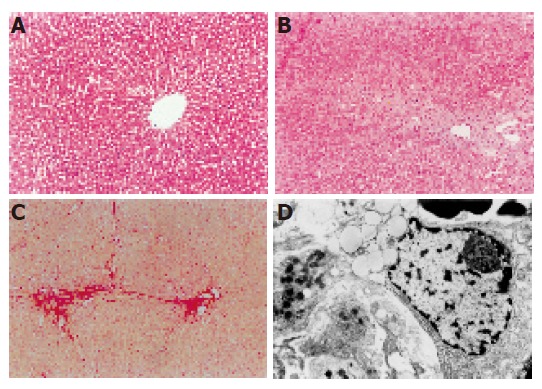
Changes after DMN treatment. A: Livers in the control group showed normal lobular architecture with central veins; B: after 7 d of DMN treatment, extensive necrosis was observed in portal areas; C: after 14 d of DMN treatment, thin fibrotic septa were formed joining central areas; D: after 21 or 28 d of DMN treatment, thick intralobular septa were evident.
Figure 3.
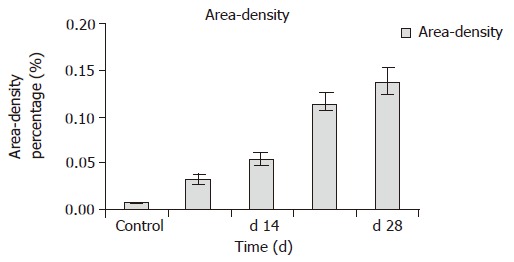
Area–density percentage of collagen fibrosis in hepatic tissue. P<0.05 vs control; P<0.05 vs d 7.
Table 2.
Pathologic grading of DMN-induced hepatic fibrosis in rats (mean±SD)
| Groups | n |
Grading of hepatic fibrosis |
||||
| – | + | ++ | +++ | ++++ | ||
| Control | 10 (0) | 10 | 0 | 0 | 0 | 0 |
| Day 7 | 6 (4) | 0 | 3 | 3 | 0 | 0 |
| Day 14 | 9 (1) | 0 | 2 | 4 | 3 | 3 |
| Day 21 | 9 (1) | 0 | 0 | 2 | 4 | 3 |
| Day 28 | 10 (0) | 0 | 0 | 2 | 4 | 4 |
P<0.001 vs control; P<0.001 vs d 7; ( ): number of deaths.
Distribution of α-SMA positive cells
Activated HSCs characterized by the expression of α-SMA increased in the liver of rats that received DMN. The distribution of α-SMA positive cells was similar to that of collagen in the liver. After 14 d, linear immunoreaction for α-SMA was scattered along the sinusoidal wall (Figure 4A). After 21 d, a network of α-SMA cells was evident (Figure 4B). After 28 d, a dense network of α-SMA cells was evident (Figure 4C). Transitional HSCs were observed under electron microscope, showing features of lipid-containing myofibroblasts and bundles of connective tissue after 14 d of DMN treatment (Figure 4D). Typical myofibroblasts were embedded in the core of fibrous septa after 21–28 d of DMN treatment. The elongated cell body contained a nucleus and numerous microfilaments outlined by a lamina-like structure. Collagen fibers of variable size were seen around the myofibroblasts (Figure 4E).
Figure 4.
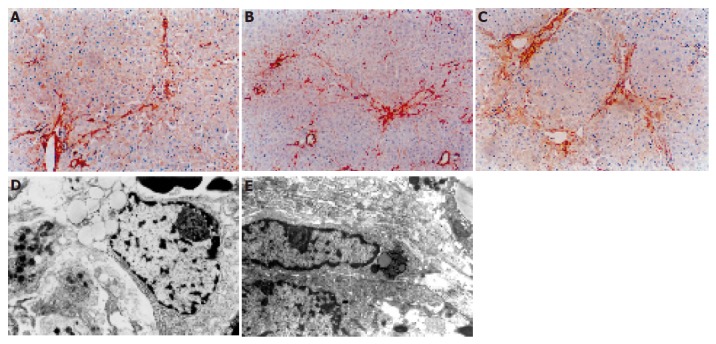
Distribution of α-SMA positive cells after DMN treatment. A: After 14 d, linear immunoreaction for α-SMA was scattered along the sinusoidal wall; B: after 21 d, a network of α-SMA cells was evident; C: after 28 d, a dense network of α-SMA cells was evident; D: after 14 d, transitional hepatic stellate cells were observed under electron microscope; E: after 21 or 28 d, typical myofibroblasts were embedded in the core of fibrous septa.
Relationship between area-density percentage of collagen fibrosis and serum HA, type IV collagen, and LN levels
A positive correlation (r = 0.707, P<0.01) was noticed between the area-density percentage of collagen fibrosis and serum levels of HA, LN, and type IV collagen during the course of DMN administration.
DISCUSSION
Liver fibrosis is common in most chronic liver diseases regardless of their etiology[12-15]. The incidence rate of chronic liver disease in China is high[16]. Hepatic fibrosis is the intermediate and crucial stage of cirrhosis. If treated properly in this stage, cirrhosis could be successfully prevented[17], but it remains a problem to prevent cirrhosis or to control its progression in patients with chronic liver disease[18]. HSCs play a central role in the pathogenesis of liver fibrosis. After liver injury, HSCs become activated and express a wide variety of extracellular matrixes. It was reported that hepatic fibrosis is induced in rats by low doses of DMN and morphological changes of hepatic fibrosis are associated with cells bearing ‘transitional’ features of HSCs, myofibroblasts and fibroblasts[19]. Activated but not quiescent HSCs have a high level of collagen and express α-SMA. HSCs play a key role in the pathogenesis of hepatic fibrosis[20]. To evaluate the distribution of α-SMA positive cells in various liver diseases, Yu et al[21]. undertook an immunohistochemical study of liver diseases including chronic persistent hepatitis, chronic active hepatitis, liver cirrhosis, intrahepatic cholelithiasis and hepatocellular carcinoma, and found that expression of α-SMA may be related to the fibrotic process[22]. DMN-induced experimental model may contribute to the understanding of the relationship between liver injury and hepatic fibrosis[23] and is used to detect different degrees of hepatic fibrosis[24,25]. In our study, serum levels of HA, LN, and type IV collagen increased significantly in hepatic fibrosis, suggesting that detection of HA, LN, and type IV collagen level is an optimal choice[26]. In this study, a positive correlation was noticed between the area-density percentage of collagen fibrosis and serum levels of LN, HA, and type IV collagen during the course of DMN administration, indicating that DMN is a potent hepatotoxin that can cause fibrosis of the liver. DMN can be administered to adult male albino rats in order to document sequential pathological and biochemical alterations[27].
In conclusion, HSCs play a central role in the pathogenesis of liver fibrosis and can regulate degradation of matrix in the liver.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397–403. doi: 10.3748/wjg.v5.i5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantürk NZ, Cantürk Z, Ozden M, Dalçik H, Yardimoglu M, Tülübas F. Protective effect of IGF-1 on experimental liver cirrhosis-induced common bile duct ligation. Hepatogastroenterology. 2003;50:2061–2066. [PubMed] [Google Scholar]
- 3.Xu LX, Xie XC, Jin R, Ji ZH, Wu ZZ, Wang ZS. Protein deficiency and muscle damage in carbon tetrachloride induced liver cirrhosis. Food Chem Toxicol. 2003;41:1789–1797 DOI : 10.1016/S0278-6915(03)00218-7. doi: 10.1016/s0278-6915(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 4.Pan NS, Li ST, Wang Y, Li MF, Han Z. [Therapeutic effect of "anti-hepatic-fibrosis 268" on hepatic fibrosis in rats] Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35:528–531. [PubMed] [Google Scholar]
- 5.Kuroki T, Seki S, Kawakita N, Nakatani K, Hisa T, Kitada T, Sakaguchi H. Expression of antigens related to apoptosis and cell proliferation in chronic nonsuppurative destructive cholangitis in primary biliary cirrhosis. Virchows Arch. 1996;429:119–129. doi: 10.1007/BF00192434. [DOI] [PubMed] [Google Scholar]
- 6.Jia JD. [Further systematize and standardize the diagnosis and treatment of liver cirrhosis] Zhonghua GanZangBing ZaZhi. 2005;13:401–402. [PubMed] [Google Scholar]
- 7.Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer. 2004;3:26. doi: 10.1186/1476-4598-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitkopf K, Sawitza I, Gressner AM. Characterization of intracellular pathways leading to coinduction of thrombospondin-1 and TGF-beta1 expression in rat hepatic stellate cells. Growth Factors. 2005;23:77–85. doi: 10.1080/08977190500095980. [DOI] [PubMed] [Google Scholar]
- 9.Tox U, Goeser T. [Therapy of complications of hepatic cirrhosis] Schweiz Rundsch Med Prax. 2005;94:727–733. doi: 10.1024/0369-8394.94.18.727. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins SA, Grandison A, Baxter JN, Day DW, Taylor I, Shields R. A dimethylnitrosamine-induced model of cirrhosis and portal hypertension in the rat. J Hepatol. 1985;1:489–499. doi: 10.1016/s0168-8278(85)80747-9. [DOI] [PubMed] [Google Scholar]
- 11.Veal N, Auduberteau H, Lemarie C, Oberti F, Calès P. Effects of octreotide on intestinal transit and bacterial translocation in conscious rats with portal hypertension and liver fibrosis. Dig Dis Sci. 2001;46:2367–2373. doi: 10.1023/a:1012395013396. [DOI] [PubMed] [Google Scholar]
- 12.McCaughan GW, Gorrell MD, Bishop GA, Abbott CA, Shackel NA, McGuinness PH, Levy MT, Sharland AF, Bowen DG, Yu D, et al. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev. 2000;174:172–191. doi: 10.1034/j.1600-0528.2002.017420.x. [DOI] [PubMed] [Google Scholar]
- 13.Jung SA, Chung YH, Park NH, Lee SS, Kim JA, Yang SH, Song IH, Lee YS, Suh DJ, Moon IH. Experimental model of hepatic fibrosis following repeated periportal necrosis induced by allylalcohol. Scand J Gastroenterol. 2000;35:969–975. doi: 10.1080/003655200750023057. [DOI] [PubMed] [Google Scholar]
- 14.Plummer JL, Ossowicz CJ, Whibley C, Ilsley AH, Hall PD. Influence of intestinal flora on the development of fibrosis and cirrhosis in a rat model. J Gastroenterol Hepatol. 2000;15:1307–1311. [PubMed] [Google Scholar]
- 15.Ramalho F. Hepatitis C virus infection and liver steatosis. Antiviral Res. 2003;60:125–127. doi: 10.1016/j.antiviral.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Lamireau T, Desmoulière A, Bioulac-Sage P, Rosenbaum J. [Mechanisms of hepatic fibrogenesis] Arch Pediatr. 2002;9:392–405. doi: 10.1016/s0929-693x(01)00800-4. [DOI] [PubMed] [Google Scholar]
- 17.Riley TR, Bhatti AM. Preventive strategies in chronic liver disease: part II. Cirrhosis. Am Fam Physician. 2001;64:1735–1740. [PubMed] [Google Scholar]
- 18.Murphy F, Arthur M, Iredale J. Developing strategies for liver fibrosis treatment. Expert Opin Investig Drugs. 2002;11:1575–1585. doi: 10.1517/13543784.11.11.1575. [DOI] [PubMed] [Google Scholar]
- 19.Jézéquel AM, Mancini R, Rinaldesi ML, Macarri G, Venturini C, Orlandi F. A morphological study of the early stages of hepatic fibrosis induced by low doses of dimethylnitrosamine in the rat. J Hepatol. 1987;5:174–181. doi: 10.1016/s0168-8278(87)80570-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee KS, Lee SJ, Park HJ, Chung JP, Han KH, Chon CY, Lee SI, Moon YM. Oxidative stress effect on the activation of hepatic stellate cells. Yonsei Med J. 2001;42:1–8. doi: 10.3349/ymj.2001.42.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Yu E, Choe G, Gong G, Lee I. Expression of alpha-smooth muscle actin in liver diseases. J Korean Med Sci. 1993;8:367–373. doi: 10.3346/jkms.1993.8.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka Y, Nouchi T, Yamane M, Irie T, Miyakawa H, Sato C, Marumo F. Phenotypic modulation in lipocytes in experimental liver fibrosis. J Pathol. 1991;164:273–278. doi: 10.1002/path.1711640314. [DOI] [PubMed] [Google Scholar]
- 23.Jézéquel AM, Mancini R, Rinaldesi ML, Ballardini G, Fallani M, Bianchi F, Orlandi F. Dimethylnitrosamine-induced cirrhosis. Evidence for an immunological mechanism. J Hepatol. 1989;8:42–52. doi: 10.1016/0168-8278(89)90160-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee MH, Yoon S, Moon JO. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull. 2004;27:72–76. doi: 10.1248/bpb.27.72. [DOI] [PubMed] [Google Scholar]
- 25.Hsu YC, Chiu YT, Lee CY, Lin YL, Huang YT. Increases in fibrosis-related gene transcripts in livers of dimethylnitrosamine-intoxicated rats. J Biomed Sci. 2004;11:408–417. doi: 10.1007/BF02254446. [DOI] [PubMed] [Google Scholar]
- 26.Liang XH, Zheng H. [Value of simultaneous determination of serum hyaluronic acid, collagen type IV and the laminin level in diagnosing liver fibrosis] Hunan YiKe DaXue XueBao. 2002;27:67–68. [PubMed] [Google Scholar]
- 27.Madden JW, Gertman PM, Peacock EE. Dimethylnitrosamine-induced hepatic cirrhosis: a new canine model of an ancient human disease. Surgery. 1970;68:260–267; discussion 260-267;. [PubMed] [Google Scholar]


